Abstract
Background
The aim was to survey the specific factors, which cause to decrease blood feeding of mosquitoes important to succeed vector control.
Methods:
Larval collection was carried out from fixed and variable breeding places of Yazd County, central Iran in 2009. Autogeny-Anautogeny, Stenogamy-Eurygamy, and blood preference of Culex pipiens were studied using standard mosquito cages blood meal source for Cx. pipiens females considered as the chickens and human and fed females were kept in insectary condition (16:8 L: D, 27±3 °C and 70±10% RH). The data were analyzed using SPSS Ver. 11.5 soft ware.
Results:
Totally, 96 females’ mosquitoes were tested for Stenogamy versus Eurygamy and 122 for blood preference assay. In the small cages (20 × 20 × 20cm) and large cage (60 × 40 × 60cm), the ability of mating and insemination rates were 60.0 and 67.0%, respectively. In spite of Cx. pipiens fed from sucrose 5%, none of them laying eggs in 60 × 40 × 60 cages during the study. This finding indicated the Anautogeny behavior of this species. This species was found of low tendency to human blood and almost 4 fold fed on chicken.
Conclusion:
The occurrence of Steno-Eurygamy, Anautogeny, and Ornithophilic behaviors of Cx. Pipiens was noted. More studies need to be carried out about the bionomics of this species to gain more data about the ecophysiological and behavioral characteristics in other parts of Iran.
Keywords: Bionomics, Behavior, Mosquito, Culex pipiens, Iran
Introduction
Malaria, West Nile and Sindbis viruses, Japanese encephalitis, Rift Valley fever as well as Dirofilaria immitis (dog heart worm) and D. repens (dirofilariasis) are important medical and veterinary mosquito-borne diseases in the World (Naficy and Saidi 1970, Saidi et al. 1976, Hayes 2005, WHO 2008, Azari-Hamidian et al. 2009).
Culex pipiens Linnaeus is medically important due to bite humans and animals besides transfer D. immitis in Iran (Ataei et al. 2012). Humans and some hosts may have allergy to mosquito bite, and this allergy can sometimes be very severe (Vinogradova 2000).
Azari-Hamidian (2007) cited sixty four species, three subspecies and seven genera of mosquitoes in Iran. By now, 33 species from two Anopheles and Cellia subgenera, siblings, genotype and type forms are recorded in the Country (Moosa-Kazemi et al. 2009, Vatandoost and Hanfi-Bojd 2012). Different studies have introduced eight malaria vectors including, An. stephensi (type, intermediate and mysorensis forms (Vatandoost et al. 2006), An. superpictus as genotypes X, Y, and Z (Oshaghi et al. 2008), An. culicifacies sibling A and B (Vatandoost et al. 2011), An. fluviatilis as three cryptic species S, T and U (Mehravaran et al. 2011), An. maculipennis group (An. atroparvus, An. maculipennis, An. melanoon, An. messeae, An. sacharovi and An. persiensis (Sedaghat et al. 2003), An. dthali (Manoochehri et al. 1972), and An. pulcherrimus (Zaim et al. 1993) is detected serologically positive to malaria parasite(s) in Baluchistan malaria foci, Southeastern of the Country. Five species of Anophelinae have been previously reported in Yazd Province, central Iran, including An. dthali, An. marteri Sogdianus, An. multicolor Combouliu, An. turkhudi Liston, and An. superpictus Grassi (Shahgudian 1956, Saebi 1987). Zaim (1987) mentioned the twelve species and four genera of Culex, Culiseta, Ochlerotatus, and Uranotaenia in this area.
Culex pipiens distributed in the Europe, the tropical and subtropical regions of Asia and Africa, the middle part of North America and Southern America and Australia (Vinogradova 2000). This species is distributed in the most part of Iran (Zaim et al. 1985, Koosha 2011). Distribution of this species has close relationship with economic activities and development of new territories. The human activity on natural environment with a change in the land and underground water resources can affect the mosquito fauna and abundance in a region. Some human activities created the irrigation domains marsh, dry land irrigation, channels, irrigation water storage, and various breeding places near the industrial activities (Vinogradova 2000).
Culex pipiens complex has at least two species (Dehghan et al. 2013). Culex quinquifasciatus Say distributed in the tropical area with the wide range of hosts whereas, Cx. pipiens is found in the moderate area with the limited range of birds nest maker. Culex pipiens form molestus is Autogenous, and the first laying eggs perform without a blood feeding (Mousakazemi 2000, Vinogradova 2000). Culex pipiens was reported as Ornithophilous, and intend to blood feeding on birds. Some reports indicated this species feeding on humans. The main host of this species in rural area was reported birds, and animals, whereas in urban area was reported as human, animal, and birds. Vinogradova (2000) described Stenogamy as the mating ability of mosquitoes in small spaces whereas, Eurygamy as the mosquitoes need to large space. Mean-while Cx. pipiens form molestus, Cx. Quinquefasciatus and possibly Cx. pipiens pallens Coquillett were described as Sten-ogamous, whereas Cx. pipiens, Cx. australicus Dobrotworsky and Drummond, Cx. vagans Wiedemann, and Cx. torrentium Martini were reported as Eurygamous (Vinogradova 2000).
There are limited studies in relation to physiology, biology, feeding behavior of Cx. pipiens in Yazd. The aim of this study was to obtain new data which would be useful for future mosquito control programs.
Materials and Methods
Study area
A descriptive cross sectional study was carried out in Yazd County, Central Iran in summer 2009. The study took place in two randomly selected rural villages with 889 houses and 24358 populations in Yazd County (54°04′N 31°59′E). The study area is located in the Yazd Province and bounded by Isfahan in the west, South Khorasan in the east, Kerman and Fars in the south, Khorasan-e-Razavi and Seman Provinces in the north (Fig. 1). The maximum and minimum mean monthly temperatures were recorded as 40.6 °C in July and −4.4 °C in January In 2009. The total annual rainfall was 62 mm, the minimum of 0.3 mm in May and maximum of 18 mm in March. The mean annual relative humidity was 37%. The main occupation of the peoples is agriculture and husbandry of cow and goats. Based on available epidemiological data from Yazd Health Centre, there are no villages under the entomological survey.
Fig. 1.
Map of Iran, highlighting the position of Yazd Province and its study area
Sampling methods
The specimens were collected from three fixed villages, Abrand-Abad, Nosrat-Abad and Gerd-e-Faramarz of Shahediyeh District and two variables Villages, Elah Abad and Sarcheshmeh from Zarch District. Mosquitoes larvae were collected by dipping method (WHO 1963, WHO 1992).
Larval habitats present within 500 meter around each village were studied for mosquito larvae collection. Five larval breeding places randomly selected within a 500-m radius of the study site. Up to 10 dipper samples were taken at intervals along the edge of each larval habitat using a standard mosquito dipper (350 ml) depending on the size of the habitat (WHO 1963).
Mosquito identification
The larvae were preserved in lactophenol and the microscope slides of the preserved larvae were prepared using Liquid de Faure’s medium. All samples were sent to the School of Public Health, Tehran University of Medical Sciences, where the author identified the specimens using the keys of Shahgudian (1960), Zaim and Cranston (1986), Harbach (1988), Azari-Hamidian and Harbach (2009).
Seta 1 of abdominal segment of III–IV in larval stage and Vein venation including intersection of costa, subcosta and bifurcation of R2+3 veins used to distinguishing of adult females of Cx. pipiens complex. Male genitalia shape as dorsal and ventral arms of phalosoma used to distinguishing of adult males of Cx. pipiens complex. The mosquito name abbreviations are cited based on Reinert (2001).
Mosquito rearing
The larvae for each habitat were placed separately and transported to the laboratory where they were sorted by genus and instars counted and recorded. Culex pipiens larvae were transferred to the laboratory of Entomology and Parasitology Department, School of Medical Sciences, Tarbiat Modares University for rearing. De-chlorinate tap water used for rearing of larvae and prepared by stand the water in glass jar for at least 24 hours. Larvae were placed in the covet and fed by available diets such as fish powder and milk baby food.
Pupae in batches of 30 samples were transferred to cups container covered by paper funnel and was placed into the cages. Petri dish containing de-chlorinate tap water was placed in to the cages for egg-laying. Cotton wool soaked in sucrose 5% used for feeding of adult mosquito. Mosquitoes were checked and monitored daily.
Behavioral and Physiological study
The experiment was designed to determine the degree Stenogamy or Eurygamy behaviors in a 60 × 40 × 60cm (Fig. 2A), and 20 × 20 × 20cm (Fig. 2B) cages. The spermathecae of the females Cx. pipiens were dissected for evidence of insemination (Fig. 3). The larvae were reared in insectarium and pupae were separated and transferred into cups inside the cages. Autogeny, Anautogeny and blood preference of this species also were studied with rearing the larvae and transferred the pupae in cages. Sucrose 5% and moisture cotton wool were used for feeding of this species in each cage. Petri dishes with no chlorine water were used for oviposition. Eurygamy, and Stenogamy behaviors were studied 10 days after emerging and mating of species using the dissection of spermathecae. Anautogeny and Autogeny behaviors of non blood feeding mosquitoes were studied at a 16:8 L: D, 27±3 °C and 70±10% RH for 20 days. Adult females were fed on chickens and human forearm for blood meal source in insectary. Fifty eight female mosquitoes were used for feeding on chicken and sixty four for human blood type. (Knight and Malek 1951, WHO 1975, Vinogradova 2000). The data were analyzed using SPSS Ver. 11.5 soft ware.
Fig. 2.
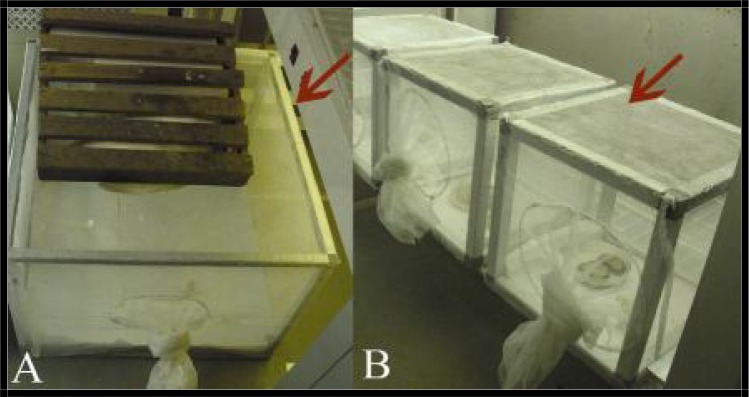
The mosquito cages including (A) large size, 60 × 40×60 cm, (B) small size, 20×20×20 cm. The pictures were taken by a digital camera in the insectary of the Department of Entomology and Parasitology, School of Medical Sciences, Tarbiat Modares University (Original photos)
Fig. 3.
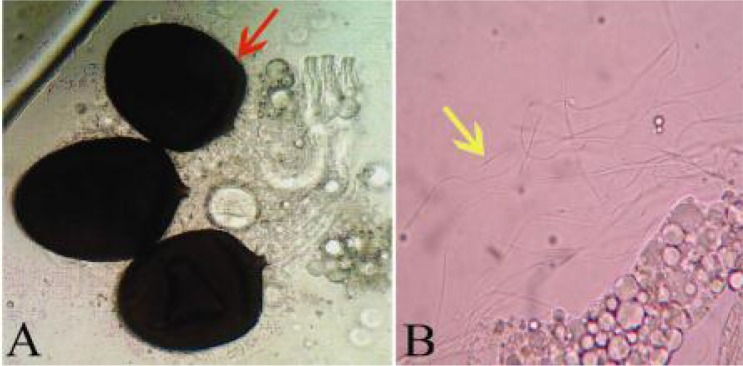
The spermatheca dissection (A) Culex pipiens spermatheca, (B) Cx. pipiens spermatozoids, The original photos of spermatheca dissection of Cx. pipiens female were taken by a digital camera in the Department of Entomology and Parasitology, School of Medical Sciences, Tarbiat Modares University
Results
Results of Stenogamy versus Eurogamy showed that sperm was active and observed in 60% of dissected Cx. pipiens. The ability of mating was found in cages in 20×20×20 cm sizes. The same result was found in large cages, 67% of mosquitoes were able to mating in cages 60×40×60 cm sizes (Table 1). In Autogeny and Anautogeny study, the mosquitoes feed on sucrose 5%, none of the mosquitoes laying eggs in 60×40×60 cm cages during the 20 days study. This finding indicated the Anautogeny behavior of this species. The mosquitoes exposed to chicken body almost 4 fold the mosquitoes were contact with the human arm. This finding indicated tendency to bird blood more than human one (Table 2).
Table 1.
Result of the Spermateca dissection of Culex pipiens to determination of Stenogamy versus Eurogamy behaviors in Department of Entomology and Parasitology insectary, School of Medical Sciences, Tarbiat Modares University, 2009
| Cage Sizes | Spermateca Dissection | |
|
| ||
| Small 20×20×20 | Positive | Negative |
|
| ||
| 1 | 9 | 4 |
| 2 | 5 | 5 |
| 3 | 7 | 4 |
| 4 | 6 | 5 |
| Total | 27 | 18 |
|
| ||
| Large 60×40×60 | - | - |
|
| ||
| 1 | 7 | 3 |
| 2 | 9 | 3 |
| 3 | 11 | 6 |
| 4 | 7 | 5 |
| Total | 34 | 17 |
Table 2.
Frequency and percentage of the blood tendency of Culex pipiens on human and chicken hosts in Department of Entomology and Parasitology insectary, School of Medical Sciences, Tarbiat Modares University, 2009
| Replicate | Culex pipiens | ||||
|
| |||||
| Host | Unfed | % | Fed | % | |
|
|
|||||
| Cage 1 | Chicken | 4 | 15.4 | 22 | 84.6 |
| Cage 2 | 8 | 25 | 24 | 75 | |
| Total | 12 | 20.2 | 46 | 79.8 | |
|
| |||||
| Cage 3 | Human | 24 | 72.7 | 9 | 27.3 |
| Cage 4 | 20 | 64.5 | 11 | 35.5 | |
| Total | 44 | 68.7 | 20 | 31.3 | |
Discussion
Culex pipiens larvae were collected from different larval habitats such as subterranean water accumulated with sewage, livestock trough, ponds in residential houses and garden ponds. The flume water contaminated by sewage was found the main breeding places of Cx. pipiens inside urban area. Similarity, Vinogradova (2000) cited Cx. pipiens larvae found in natural and artificial breeding places with high degree of contamination by organic and industrial materials. Water obtained from sewage treatment plants was created the suitable habitats of mosquito larvae outside the urban area. Culx pipiens mosquitoes can be made adaptable with the larval habitats created by human. This species can be considered as an indicator for estimating the environmental conditions in a human habitat. Development of human activity, changed the environment, and increased the abundance indicates ecological and physiological flexibility of this species.
In the present study, 60% and 58% of Cx. pipiens were able to mating in small and large cages respectively. The Eurygamy behaviors of Cx. pipiens were studied in cages in 100 × 100 × 100cm, 75 × 75 × 75cm and 60 × 40 × 60cm in Russia (Vinogradova 2000). In contrast, some researchers designed cages in 200 × 200 × 200cm sizes, and was stated a few of this species able to mating (Vinogradova 2000). Vinogradova (2000) stated the several populations of Cx. pipiens with Eurygamy behavior never did mating in cages in 20 × 20 × 30 sizes. We observed both Steno and Eurygamy behaviors. Further support to these results also came from a previous study, reported by Rioux (1965), which described Cx. pipiens with Steno-Eurygamous behaviors. It should be considered, at the present, Cx. pipiens populations were not observed with the pure form of Eurygamy or Stenogamy behavior.
It seems that there are no agreements among researchers regarding to the maintenance condition of this species in insectariums. Jupp (1987) reported that changed photoperiodic conditions of rearing of this species could be increased or decreased the mating rate.
It should be noted that, Cx. pipiens after blood meal was able to laying eggs in the 60 × 40 × 60cm cages, whereas, none of the individuals which fed only on sucrose 5% were able to lay eggs in the same cages. Our finding indicated the Anautogenous behavior of this species. Similarity, Rioux (1965) and Vinogradova (2003) cited the Anautogenous behavior in Cx. pipiens populations.
Tendency of Cx. pipiens to bird blood was more than human. Further support to this results also came from a previous study, Cx. pipiens was reported strongly Ornithophil behavior in South-central Sweden (Jaenson 1990). Also, Tempelis (1975) cited 28% of blood feeding on human and more tendency of this species to avian blood preference. Rioux (1965) expressed the Ornithophilic behavior of this species. Blood feeding of Cx. pipiens on birds and human was reported by Knight and Malek (1951), and Vinogradova (2003).
Culex pipiens has great morphological variations in all life stages and it is not easy to separate this species from Cx. quinquefasciatus and Cx. torrentium. Males of this species are distinguishable from the mentioned species using the male genitalia (Harbach 1988). Dehghan et al. (2011) reported the male genitalia was the only reliable character to identification of Cx. torrentium, Cx. quinquefasciatus and Cx. pipiens. The most important diagnostic characters of Cx. torrentium, Cx. quinquefasciatus and Cx. pipiens larvae mentioned the branch of seta 1 of abdominal segment of III–V (Harbach 1988, Dehghan et al. 2010). Based on the mentioned above, we used the recent characters to diagnosis of the Cx. pipiens samples.
Conclusion
In situations where frequent blood feeding on birds are common for Cx. pipiens, the main complication with respect to estimating the potential for arboviral parasite transmission is that vectorial capacities will be necessary. Therefore, more study about of bionomics of mosquitoes especially among the Cx. pipiens needs to be studied rigorously in the future.
Acknowledgments
The authors are grateful to Dr Azari-Hamidian, School of Health, Guilan University of Medical Sciences, Rasht, for reviewing the manuscript, Mr Kalantari, Head of Zarch City Council, Mr Rezaei, Mr Eslami, Environmental Health Director of Zarch and Shahediyeh Health Center, for supporting the investigation. We also would like to express our appreciation to the people of Abrand-Abad, Nosrat-Abad, Gerd-e-Faramarz, Elah Abad, and Sarcheshmeh Villages in Yazd County for their kind cooperation during the study. Many thanks also dedicated to the efforts of the field staff of the Yazd Health Training and Research Center. It is noteworthy that this study has been done by financial support of Research Committee in School of Medical Sciences, Tarbiat Modares University, ID No 5278604. The authors declare that there is no conflict of interests.
References
- Ataei A. Some ecological aspects of potential vectors of Dirofilariasis in the Ahar County, East Azerbaijan Province, Iran. [MSPH dissertation] School of Public Health, Tehran University of Medical Sciences; Tehran, Iran: 2012. (In Persian) [Google Scholar]
- Azari-Hamidian S. Checklist of Iranian mosquitoes (Diptera: Culicidae) J Vect Ecol. 2007;32:235–242. doi: 10.3376/1081-1710(2007)32[235:coimdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian S, Harbach RE. Keys to the adult females and forth-instar larvae of the mosquitoes of Iran. Zootaxa. 2009;2078:1–33. [Google Scholar]
- Azari-Hamidian S, Joeafshani MA, Mosslem M, Rassaei AR. Adult mosquito habitats and resting-places in Guilan Province (Diptera: Culicidae) Hakim Res J. 2003;82:12–18. (Persian with English abstract) [Google Scholar]
- Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, Harbach RE. Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 2009;23(2):111–121. doi: 10.1111/j.1365-2915.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- Dehghan H, Sadraei J, Moosa-Kazemi SH. The Morphological Variations of Culex pipiens Larvae (Diptera: Culicidae) in Yazd Province, Central Iran. Iran J Arthropod-Borne Dis. 2010;4(2):42–49. [PMC free article] [PubMed] [Google Scholar]
- Dehghan H, Sadraei J, Moosa-Kazemi SH. The morphological variations of Culex pipiens (Diptera: Culicidae) in central Iran. Asian Pac J Trop Med. 2011. pp. 412–420. [DOI] [PubMed]
- Dehghan H, Sadraei J, Moosa-Kazemi SH, Akbari Baniani N, Nowruzi F. The molecular and morphological variations of Culex pipiens complex (Diptera: Culicidae) in Iran. J Vector Borne Dis. 2013;50:111–120. [PubMed] [Google Scholar]
- Harbach RE. The mosquitoes of the subgenus Culex in southwestern Asia and Egypt (Diptera: Culicidae) Contrib Am Ent Inst. 1988;24:1–240. [Google Scholar]
- Hayes EB, Komar N, Nasci RS. Epidemiology and transmission dynamics of West Nile Virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp PG. Comparative studies on morphology and laboratory biology of Culex (Culex) pipiens Linnaeus (Diptera: Culicidae) from South Africa and England. J Ent Soc sth Afr. 1987;50:455–461. [Google Scholar]
- Jaenson TGT. Vector role of Fennoscandian mosquito attracted to mammals, birds and frogs. Med Vet Ent. 1990;4(2):221–226. doi: 10.1111/j.1365-2915.1990.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Manoochehri A, Ghiasseddin M, Shahgudian ER. Anopheles dthali Patton, 1905, a new secondary vector in southern Iran. Ann Trop Med Parasitol. 1972;66(4):537–538. doi: 10.1080/00034983.1972.11686857. [DOI] [PubMed] [Google Scholar]
- Mehravaran A, Oshaghi MA, Vatandoost H, Abai MR, Ebrahimzadeh A, Roodi AM, Grouhi A. First report on Anopheles fluviatilis U in southeastern Iran. Acta Trop. 2011;117(2):76–81. doi: 10.1016/j.actatropica.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Moosa-Kazemi SH, Vatandoost H, Nikookar H, Fathian M. Culicinae (Diptera: Culicidae) mosquitoes in Chabahar County, Sistan and Baluchistan Province, southeastern Iran. Iran J Arthropod-Borne Dis. 2009;3:29–35. [PMC free article] [PubMed] [Google Scholar]
- Mousakazemi SH. Fauna and Ecology of culicidae mosquitoes in Zarrin-Shahr and Mobarakeh areas, Isfahan Province, [MSPH dissertation] School of Public Health, Tehran University of Medical Sciences; Tehran, Iran: 2000. (Persian) [Google Scholar]
- Mousakazemi SH, Zaim M, Zahraii-Ramazani AL. Fauna and Ecology of Culicidae Mosquitoes of Zarrin-Shahr and Mobarakeh, Isfahan Province, center of Iran. Medicine and Health in the tropics congress; Marseille, France. 2005. [Google Scholar]
- Naficy K, Saidi S. Serological survey on viral antibodies in Iran. Trop Geogr Med. 1970;2:183–88. [PubMed] [Google Scholar]
- Oshaghi MA, Yaghobi-Ershadi MR, Shemshad Kh, Pedram M, Amani H. The Anopheles superpictus complex: introduction of a new malaria vector complex in Iran. Bull Soc Pathol Exot. 2008;101:429–434. [PubMed] [Google Scholar]
- Knight KL, Malek AA. A morphological and biological study of Culex pipiens in the Cairo area of Egypt. Sot Fouad Ent Bull. 1951;35:175–185. [Google Scholar]
- Koosha M. Genetic structure and species composition Culex pipiens complex in Guilan and Hormozgan Provinces, Iran. [MSPH dissertation] School of Public Health, Tehran University of Medical Sciences; Tehran, Iran: 2011. (In Persian) [Google Scholar]
- Reinert JF. Revised list of abbreviation for genera and subgenera of Culicidae (Diptera) and the notes on generic and subgeneric changes. J Am Mosq Cont Assoc. 2001;17:51–55. [PubMed] [Google Scholar]
- Rioux J. Autogeny and Anautogeny in the Culex pipiens L. complex. WHO. 1965;125:81–85. [Google Scholar]
- Saebi ME. Morphological study on Anopheline larvae and their distribution in Iran. [PhD dissertation] School of Public Health, Tehran University of Medical Sciences; Tehran, Iran: 1987. (In Persian) [Google Scholar]
- Saidi S, Tesh R, Javadian E, Nadim A. The prevalence of human infection of West Nile in Iran. Iranian J Publ Health. 1976;5:8–14. [Google Scholar]
- Sedaghat MM, Linton YM, Oshaghi MA, Vatandoost H, Harbach RE. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterisation and recognition of a new species. Bull Entomol Res. 2003;93:527–535. doi: 10.1079/ber2003272. [DOI] [PubMed] [Google Scholar]
- Shahgudian ER. Notes on Anopheles marteri Senevet and Prunnelle, 1927. Proceedings of the Royal Entomological Society of London Series A General Entomology. 1956;31:71–75. [Google Scholar]
- Shahgudian ER. A key to Anophelines of Iran. Acta Med Iran. 1960;3:38–48. [PubMed] [Google Scholar]
- Tempelis CH. Host-feeding patterns of mosquito with a review of advances in analysis of blood meals by serology. J Med Entomol. 1975;11(6):635–653. doi: 10.1093/jmedent/11.6.635. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Oshaghi MA, Abaie MR, Shahi M, Yaghoobi F, Baghaii M, Hanafi-Bojd AA, Zamani G, Townson H. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan Province, southern Iran. Acta Trop. 2006;97:196–205. doi: 10.1016/j.actatropica.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Emami SN, Oshaghi MA, Abai MR. Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan Province, south-east Islamic Republic of Iran. Eastern Mediterr Health J. 2011;17(5):439–445. [PubMed] [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. Indication of Pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pacific J Trop Med. 2012. pp. 722–726. [DOI] [PubMed]
- Vinogradova EB. Ecophysiological and morphological variations in mosquitoes of the Culex pipiens complex (Diptera: Culicidae) Acta Soc Zool Bohem. 2003;67:41–50. [Google Scholar]
- Vinogradova EB. Culex pipiens pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics and Control. Pensoft Publisher; Sofia: 2000. [Google Scholar]
- World Health Organization. Practical entomology in malaria Eradication. Part I, Field and laboratory techniques. WHO/PA/62. 1963;63:8–11. [Google Scholar]
- World Health Organization. Manual of Practical Entomology in Malaria. Part II: Methods and Techniques. WHO; Geneva: 1975. [Google Scholar]
- World Health Organization. Entomological field techniques for malaria control. Part I and II Lerner and tutor guide. WHO; Geneva: 1992. [Google Scholar]
- World Health Organization. Global Malaria Control and Elimination. WHO; Geneva: 2008. [Google Scholar]
- Zaim M, Cranston PS. Checklist and keys to the Culicinae of Iran (Diptera: Culicidae) Mosq Syst. 1986;18:233–245. [Google Scholar]
- Zaim M, Subbaroa SK, Manouchehri AV, Cochran AH. Role of Anopheles culicifacies s.l. and Anopheles pulcherrimus in malaria transmission in Ghasreghand, Baluchistan, Iran. J Am Mosq Control Assoc. 1993;9:23–26. [PubMed] [Google Scholar]
- Zaim M, Manouchehri AV, Yaghoobi-Ershadi MR. Mosquito fauna of Iran 2-Culex. Iran J Publ Health. 1985;14:1–12. [Google Scholar]
- Zaim M. The distribution and larval habitat characteristics of Iranian Culicinae. J Am Mosq Cont Assoc. 1987;3:568–573. [PubMed] [Google Scholar]