Abstract
The radiation-induced bystander effect is the phenomenon which non-irradiated cells exhibit effects along with their different levels as a result of signals received from nearby irradiated cells. Responses of non-irradiated cells may include changes in process of translation, gene expression, cell proliferation, apoptosis and cells death. These changes are confirmed by results of some In-Vivo studies. Most well-known important factors affecting radiation-induced bystander effect include free radicals, immune system factors, expression changes of some genes involved in inflammation pathway and epigenetic factors.
Keywords: Bystander effect, Immune system, Free radicals, Mitochondria, Epigenetic
Introduction
Traditionally it was believed in radiobiology that only direct radiation exposure to genetic material of nucleus causes damage to cells. This model predicts that radiation-induced mutations are created in irradiated area during a short time after irradiation. This dogma was challenged in 1992 with an experiment performed by nagasawa. This experiment revealed that irradiation of 1% cells with alpha particles lead to chromatid exchange in more than 30% of cells. This is called as bystander effect which demonstrates the relationship between irradiated and non-irradiated cells [1]. Results of studies on survivors of Chernobyl explosion as well as radiotherapy of patients with cancer revealed that adding blood serum of these individuals to the same non-irradiated cell culture causes chromosomal damage [2]. These effects are persistent and would remain even in whom had a radiation exposure twenty years before [3]. It is proposed that irradiated people blood has clastogenic factors. Bystander effect is more obvious in cells with gap junction. Therefore, intercellular relationships between cells is an necessary requirement to transfer signals of radiation-induced bystander effect [4].
Distant Bystander effect is proved to be existed outside of radiation field according to in-Vivo studies. Local irradiation to a small area of body causes chromosomal damages and changes in the cell and molecule levels of distant tissues. After a local irradiation, the chromosomal breaks, P53 activity, DNA repair enzymes, mitotic death and apoptosis of distant tissues from radiation target would impressively increased [5]. These signs are a threat for carcinogenesis due to radiation induced bystander effect. Mancuso and their teammates found that irradiated mice with high sensitivity increase induced medulloblastoma cancer associated with chromosomal damages and abnormalities apparently. Their study proved carcinogenesis of the bystander effect directly [6].
It is supposed that local radiation to an area, like what is seen in radiotherapy, could cause systemic damages and even lead to carcinogenesis incidence beyond therapy field. An example of secondary cancer which is attributed to this phenomenon is high incidence of lung cancer among people who have had radiation therapy to treat prostate cancer [7, 8]. Mechanisms involved in establishing the bystander effect or the radiation effect of outside therapy field include immune system, Free radicals, oxidative stress, changes in gene expression of inflammation pathway and epigenetic modulators.
Mechanisms involved in the bystander effect
Immune systeme
The immune system consists of various types of molecules which protect body against infection and cellular damages. Immune system applies its effects through molecules called cytokines. Cytokines could change the secretion of some molecules as well as cell proliferation by affecting expression or membrane proteins inhibition. Therefore cytokines have effect on regulation of immune response, inflammation and proliferation of blood cells. The most important immune system factors involved in radiation-induced bystander effect are lymphocytes and macrophages [9]. Ionizing radiation by stimulating these cells elevates the level of most cytokines such as IL-1, IL-2, IL-6, IL-8, TNFα and TGFβ in non-irradiated cells. Partial irradiation of the lung demonstrated the increases of these cytokines in the shielded lung area [10]. Elevation of these cytokines plays a key role in second malignancy after radiotherapy [11]. Most of these cytokines are involved in proliferation and differentiation of stem cells [12]. Tumor Necrosis Factor Alpha (TNFα) which is increased evidently after acute exposure [13] leads to induce necrosis and cell death in tumor cells, however, this is not happened to normal cells. TNFα along with other stated cytokines are placed in the inflammation pathway causes nitric oxide production. Activated macrophages by increasing cytokines production lead to increased chromosomal damages, change in DNA bases, mutagenesis and apoptosis in non-irradiated cells. Increasing production level of cytokines via macrophages stimulates NO production which leads to oxidative stress. Superoxide anions are known as an important mediator for damages of clastogenic factors [14].
Free radicals
Free radicals have a too short life time which causes their inability to reach other cells after being produced in the cells by the collision of radiation with water molecules. Therefore, free radicals are not considered as a factor in the damage of non-irradiated cells. Free radicals in the presence of oxygen can be converted to long-lived peroxides. Studies using electron spin shows that these peroxides can have over twenty hours half-life [15]. Although half-life of most peroxides makes them be lesser reactive than free radicals, but this helps them to pass longer paths within or outside the cell. Thus peroxides through this can cause damage to cells which are not exposed to radiation. Many In-Vitro experiments have indicated that free radical and peroxides scavengers such as DMSO and vitamin C reduce chromosomal damage such as chromosomal breaks, apoptosis and micronuclei [16, 17]. These results suggest that production of free radicals after irradiation plays a significant role in chromosomal damage of non-irradiated cells, however, even using free radical scavengers cannot completely suppress these damages, thus it reflects the role of other factors in the establishment of the bystander effect [17].
Mitochondrial activity has a serious role in development of the bystander effect through the production of free radicals. Studies represent that chain suppression of mitochondrial cellular respiratory, calcium uptake with mitochondria and also depleting the cells out of mtDNA, would decrease the chromosomal damage significantly [18]. A malfunction in cytochrome c which is located on the inner wall of mitochondria, and involved in the chain of electron transport illustrated similar results [19]. Other studies also confirmed that mitochondria can increase the cellular damage caused by bystander effect through increase in production of superoxide and free radicals, [20, 21].
Another factor affecting the production of free radicals in cells is NADPH Oxidase. NADPH Oxidase is an enzyme which is dependent on cell membrane and can has the ability to generate free radicals continually leads to cellular and chromosomal damage. Suppressing this enzyme after total body radiation has proved to be effective on the reduction of radiation effects on hematopoietic cells [22]. NADPH Oxidase gene expression may be increased by the influence of protein kinase c and p38 [23]. Studies on fibroblasts and epithelial cell lines suggest that this enzyme by production of free radicals has a key role in inducing the bystander effect [24].
Altering gene expression
Genes which are involved in the inducing of the bystander effect are often the same genes involved in inflammatory pathways. The most important types of these genes are mitogen activated protein kinases (MAPKs), nuclear factor of kappa B (NFkB), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Increase of these genes Expression which occurs by various factors leads to inflammation and NO production which consequently elevates oxidative stress [14]. As it is above explained, radiation exposure induce macrophages to produce cytokines such as IL-1, IL-2, IL-8, TGFβ and TNFα. Receptors of these cytokines which are located on the cell surface are stimulated by these factors and alter a cascade of genes expression. Such alterations among tissues which are exposed to direct irradiation are the main factor of the tissues inflammation [25].
Considered cytokines through stimulating the expression of NFkB gene or MAPK genes such as ERK, JUN and P38 genes causes transcription activation of cyclooxygenase-2 and iNOS. Cyclooxygenase-2 is not expressed in all tissues, besides its expression level is too low. Hence, a smallincrease in the expression of this gene is too obvious. Cyclooxygenase-2 is the main factor in producing prostaglandins like PG-E2 and PG-I2 which induces the blood vessels dilatation as well as incidence of inflammation symptoms [26, 27]. iNOS also produces nitric oxide and thereby increases the level of oxidative stress. Over expression of this gene is often associated with an increase in cyclooxygenase-2 [28].
According to above mentioned, it is expected that acute doses of radiation through stimulating macrophages activities, causes the production of cytokines which leads to increase the expression of COX-2 and iNOS genes in non-irradiated cells. In-Vitro Studies have shown that the bystander effect can have a threefold increase of cyclooxygenase-2 in nearby non-irradiated cells [27]. In-vivo study also has shown multi fold increase in the expression of this gene during 72 hours after radiation of lung [29, 30]. (figure 1)
Figure 1.
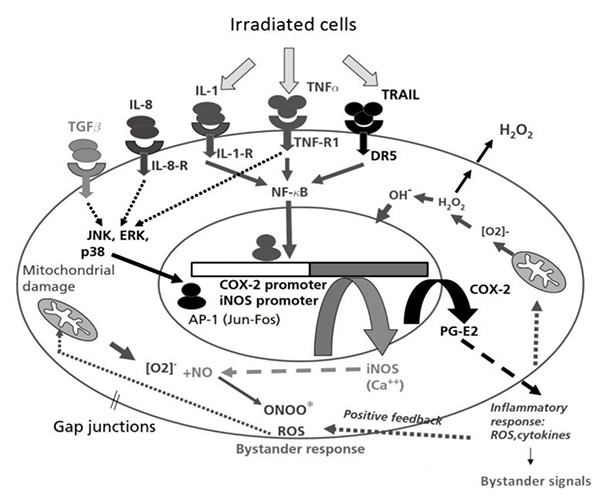
The mechanisms of radiation-induced bystander effect with alteration the expression of involved genes in the inflammation pathway
Epigenetic modulators
Epigenetic term was used by Conrad Waddington for the first time in 1942 to explain the effect of environment on genes which create a new phenotype [31]. Today, epigenetic includes the changes in the genome without any change in DNA sequence. Epigenetic is a system which makes changes in gene expression by mediation of molecules which bind to chromosomes. These combinations include the groups of methyl, acetyl, phosphor, ubiquitinae and molecules such as miRNA, siRNA and piRNA. The methyl group plays the most important role in the changes of genes expression. Adding methyl groups to chromosomes is called methylation which reduces the transcription proteins binding to chromosome and subsequently decreases gene expression. Besides, reduction of methyl groups in the promoter region of genes increases gene expression [32]. Such these changes (Methylation and demethylation) can be transmitted to the future generations [33].
CpG islands are located in the regions of genes promoter and connect to particular molecules (initial proteins) which are familiar with these regions and make the genes to be expressed. In somatic cells, approximately 70% of CpG zones in the human genome are naturally methylated, which contributes the suppression of numerous genes expressions [33]. When chromosome regions are in an open position, CpG islands relatively become demethylated to increase access to gene promoters. By contrast, the connection of this group to cytosine base makes less exposure of DNA strand to expression (hypermethylation), as the result, these changes makes the gene to be turned off [34]. Hypomethylation by increasing of oncogenes activity and hypermethylation by decreasing of tumor suppressor genes both involved in carcinogenesis. Loosing methylation among denucleotides of CPG is the first unnatural sign of cancerous cells which increases DNA breaks, aniopolosis, mutation increase and consequently the phenomenon of genomic instability [34].
In mammals, there are three types of DNA methyltransferase which are mainly responsible for setting and establishing methyl patterns in CPG regions including DNA methyltransferase 1, methyltransferase 3B, and methyltransferase 3A. Among these enzymes, DNA methyltransferase1 is the main enzyme in the methylation patterns after DNA replication [35]. Generally, it can be said that epigenetic alterations are permanent mutagenesis changes which mainly include DNA methylation and histone modifications.
miRNA, siRNA and piRNA are molecules which their bind to genes and turns off genes expression. These molecules can get out from the origin tissue and alter gene expression in distant tissues [36]. Decrease and increase of miRNA along with increase of oncogenes or decrease of tumor suppressor genes expression are contributed factors of carcinogenesis [37]. Hence, non-coding RNAs play role in epigenetic alterations by reduction of genes expression. Other epigenetic alterations include changes in proteins such as histones which bind to DNA to shape it as a specific structure [38].
Epigenetic factors can significantly affected by direct irradiation. It is demonstrated that radiation can affect methylation patterns. Acute exposures to radiation of x or gamma-ray can lead to a global hypomethylation. It is proved that radiation may change the methylation level among directly irradiated tissues. Observed hypomethylation is related to DNA modification after radiation. This phenomenon is associated with induced changes in the expression of DNA methyltransferases especially DNMT3a and DNMT3b methyltransferases which are affected by irradiation. The most important change which may be affected by this phenomenon is hypomethylation which plays a key role in genomic instability and mutagenesis [39 , 40].
Bystander effect may be effective in lots of genetic alterations, including chromosomal abnormalities, sister chromatid exchanges, deletions, transcriptions, mutations and gene expressions. In-Vitro studies suggest that the bystander effect of radiation causes irregularity in the level of chromosome methylation which leads to increase in genomic instability, chromatid and chromosome abnormalities, apoptosis and cell death [5].
Some studies have been continued by 3D phantoms. These studies showed a significant increase in H2AX phosphorylations,, a reduction in DNA methylation levels and a increase in chromosome breaks among non-irradiated cells. There was a significant increase in the amount of H2AX histone phosphorylations among non-irradiated tissues. In the long-term the rate of apoptosis and micronucleus increased, DNA metylation of nuclear decreased then the growth was stopped and a rise in the damage of sensitive cell were observed. The considerable note under these studies is loosing methylation among non-irradiated cells. Reduction of DNA methylation which relates to other epigenetic factors illustrates the effects of epigenetic factors in inducing of radiation-induced bystander effect [41].
It is shown by analyses that a number miRNAs , particularly let-7 family play an important role in the cellular response to oxidative stress conditions. These miRNAs are important in regulation of RAS genes expressions and cellular proliferation. Direct radiation exposure reduces expressions of these miRNAs abviously [42]. Small miRNAs are involved in many of cellular processes such as cell-cell communication. They are small in size, relatively stable, and able to pass through the cells long distances in the body. Thus these molecules are good candidates for inducing of radiation-induced bystander effect.
Radiation exposure leads to over-expression of miR-29, miR-16 and miR-17. MiR-29 reduces the expression of the DNA methyltransferase 3A and MCL1 which is an important factor of methylation and apoptosis. MiR-16 is effective in altering expression of BCL-2. This protein also plays a significant role in the regulation of apoptosis. Over-expression of miR-17 is associated with reduction of E2F1 and RB1. These two genes contribute to regulate the cell cycle especially in the passing of cell cycle from G1 phase to S phase and to induce apoptosis. These results show that microRNAs are an important agent in inducing of the bystander effect. [43].
In-Vivo studies have shown that when only one side of body is irradiated, chromosomal damages in the skin of mice increases. In these studies hypomethylation on skin was only observed on the area under direct radiation but the level of Dnmts in the shield area was decreased. Also cranial irradiation leads to the reduction of methylation in the long interspersed nuclear element 1 (LINE1) and expression reduction in DNA methyltransferase 3A of the spleen. Reduced expression of DNA methyltransferase 3A enzymes was attributed to the micro RNA-194 in this survey which is a significant factor in regulation of this enzyme expression. Further studies have confirmed the increase in expression of this microRNA even several months after irradiation of the spleen [43, 44].
Studies suggest that changes in methylation pattern after irradiation is tissue-dependent and methylation levels may vary in different tissues. Cranial irradiation causes lasting hypomethylation in the spleen while a short time hypomethylation may be observed in the skin [45]. Changes in miRNAs expression are significantly regarding to gender. Assessment of male and female mice spleen after cranial irradiation showed that some miRNAs in females and some other miRNAs in males had higher expressions. Also gonadectomy of mice leads to changes in miRNA expression in both males and females. These results indicate that sex hormones have role in miRNA expression and chromosome methylations after irradiation [46].
The bystander effect can cause epigenetic heritage
As it was noted, epigenetic changes are heritable and can be transmitted to future generations. Radiation can lead to genomic instability in generic cells and affect the oncoming generations. In-Vivo studies have reported that the bystander effects can affect the generic cells to change the methylation pattern in future generations. Cranial irradiation is associated with increase of chromosomal damage in mouse sperms. Furthermore, studying the level of methylation at CCGG sites shows a significant decrease in methylation of the testes and sperm. Evaluation of the thymus, spleen, liver and bone marrow in the next generation of mice which were irradiated by their cranial area depicted that level of methylation and enzymes of DNA methyltransferase is diminished in the bone marrow, thymus and spleen significantly, however, no reduction was observed in the liver. Most of these changes have been observed in the bone marrow respectively [47].
Further studies pointed that the expression of MIR-29a and MIR-29b in the germ cells and the next generations of mice which were irradiated by their cranial area were significantly high. These two expressions regulate the enzymes of DNA methyltransferase 3a and 3b. Therefore, increase of MIR-29a and MIR-29b reduces the production of DNA methyltransferase enzymes which ultimately diminishes the level of methylation [48].
Radioprotective effect of the bystander effect
Bystander effect may be a protective mechanism. Bystander effect may decrease the risk of transformation in the irradiated multicellular organism. The organism may be able to detect damage from irradiation and respond to it by removing of damaged cells. It seems, apoptosis is the best way to remove of damaged cells. Apoptosis allows the elimination of damaged cells without a negative impact on other cells via inflammatory responses. The second way to remove injury is to quick damaged cells into permanent differentiation. Following exposure to low dose radiation, tissues remove all potentially damaged cells from the system to keep away from the risk of carcinogenesis. It seems, the bystander effect induced differentiation play vital role in this process. Obviously cellular senescence is a powerful tumor suppressor mechanism. Based on this theory, irradiated tissue would respond as a single unit. The damaged cells would produce some the bystander signals.
Radiotherapy and the bystander effect
More than half of cancer patients use the radiotherapy for tumor treatment [49]. The risk of second cancers caused by radiation exposure maintain for long years after radiotherapy. Second cancer is one of complications of radiotherapy and it may lead to a decrease in the overall survival after the treatment of primary cancers. The bystander effect is one of the complications of radiation exposure which cause chromosomal damage and may lead to carcinogenesis risk in distant tissues. Studies have also shown that the bystander effect is more conspicuous in fractionated doses [50]. So the bystander effect could have an impact at the incidence of second cancers in the distant tissues in radiotherapy. Several major cancers that could have a link with these effects including cancers of the lung, bronchus, melanoma and sarcoma after radiotherapy for prostate cancer [8]. The high incidence of lung cancer in patients with rectal, cervical and ovarian cancer radiotherapy has been confirmed [51-53]. High incidence of these cancers cannot be due to scatter radiation. That is why it is thought that the bystander effect plays a role in the development of these cancers. According to these results, the cancer risk due to bystander effects in patients with cancer which use of radiotherapy for treatment are be considered.
Result and Conclusion
Radiation-induced bystander effect is an important biological response that leads to damages very similar to direct irradiation in distant non-irradiated cells and tissues. Nowadays several mechanisms which are involved in the bystander effects have been discovered. Chromosome damages induced by free radicals which cause bystander effect are not created by direct irradiation. These free radicals are produced by some cytokines, enzymes and genes expression. Mitochondria can increase production of free radicals in oxidative stress situation. Epigenetic modulators like methylation pattern and miRNAs are important changes which are investigated by the bystander effect. The methylation pattern and miRNAs which regulate gene expression could be involved in oxidative stress and radiation induced carcinogenesis.
Conflict of Interest: None
References
- 1.Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52(22):6394–6. PubMed PMID: 1423287. [PubMed] [Google Scholar]
- 2.Marozik P, Mothersill C, Seymour CB, Mosse I, Melnov S. Bystander effects induced by serum from survivors of the Chernobyl accident. Exp Hematol. 2007;35(4):55–63. doi: 10.1016/j.exphem.2007.01.029. doi: 10.1016/j.exphem.2007.01.029. PubMed PMID: 17379088. [DOI] [PubMed] [Google Scholar]
- 3.Emerit I, Oganesian N, Sarkisian T, Arutyunyan R, Pogosian A, Asrian K, et al. Clastogenic factors in the plasma of Chernobyl accident recovery workers: anticlastogenic effect of Ginkgo biloba extract. Radiat Res. 1995;144(2):198–205. PubMed PMID: 7480646. [PubMed] [Google Scholar]
- 4.Azzam EI, de Toledo SM, Little JB. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003;22(45):7050–7. doi: 10.1038/sj.onc.1206961. doi: 10.1038/sj.onc.1206961. PubMed PMID: 14557810. [DOI] [PubMed] [Google Scholar]
- 5.Koturbash I, Loree J, Kutanzi K, Koganow C, Pogribny I, Kovalchuk O. In Vivo Bystander Effect: Cranial X-Irradiation Leads to Elevated DNA Damage, Altered Cellular Proliferation and Apoptosis, and Increased p53 Levels in Shielded Spleen. Int J Radiat Oncol Biol Phys. 2008;70(2):554–62. doi: 10.1016/j.ijrobp.2007.09.039. doi: 10.1016/j.ijrobp.2007.09.039. PubMed PMID: 18207032. [DOI] [PubMed] [Google Scholar]
- 6.Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc Natl Acad Sci USA. 2008;105(34):12445–50. doi: 10.1073/pnas.0804186105. doi: 10.1073/pnas.0804186105. PubMed PMID: 18711141; PubMed Central PMCID: PMC2517601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate cancer. Cancer. 2006;107(5):991–8. doi: 10.1002/cncr.22083. doi: 10.1002/cncr.22083. PubMed PMID: 16878323. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88(2):398–406. doi: 10.1002/(sici)1097-0142(20000115)88:2<398::aid-cncr22>3.0.co;2-v. PubMed PMID: 10640974. [DOI] [PubMed] [Google Scholar]
- 9.Liu SZ, Jin SZ, Liu XD. Radiation-induced bystander effect in immune response. Biomed Environ Sci. 2004;17(1):40–6. PubMed PMID: 15202863. [PubMed] [Google Scholar]
- 10.Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005;81(12):887–99. doi: 10.1080/09553000600568002. doi: 10.1080/09553000600568002. PubMed PMID: 16524844. [DOI] [PubMed] [Google Scholar]
- 11.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7(5):651–8. doi: 10.7150/ijbs.7.651. PubMed PMID: 21647333; PubMed Central PMCID: PMC3107473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore MA. Cytokine and chemokine networks influencing stem cell proliferation, differentiation, and marrow homing . J Cell Biochem Suppl. 2002;38:29–38. doi: 10.1002/jcb.10105. PubMed PMID: 12046847. [DOI] [PubMed] [Google Scholar]
- 13.Veeraraghavan J, Natarajan M, Aravindan S, Herman TS, Aravindan N. Radiation-triggered tumor necrosis factor (TNF) α-NFκB cross-signaling favors survival advantage in human neuroblastoma cells. J Biol Chem. 2011;286(24):21588–600. doi: 10.1074/jbc.M110.193755. doi: 10.1074/jbc.M110.193755. PubMed PMID: 21527635; PubMed Central PMCID: PMC3122217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, et al. Mechanism of radiation-induced bystander effects: a unifying model. J Pharm Pharmacol. 2008;60(8):943–50. doi: 10.1211/jpp.60.8.0001. doi: 10.1211/jpp.60.8.0001. PubMed PMID: 18644187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyama S, Kodama S, Suzuki K, Matsumoto T, Miyazaki T, Watanabe M. Radiation-induced long-lived radicals which cause mutation and transformation. Mutat Res. 1998;421(1):45–54. doi: 10.1016/s0027-5107(98)00153-5. PubMed PMID: 9748497. [DOI] [PubMed] [Google Scholar]
- 16.Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24(12):2096–103. doi: 10.1038/sj.onc.1208439. doi: 10.1038/sj.onc.1208439. PubMed PMID: 15688009. [DOI] [PubMed] [Google Scholar]
- 17.Konopacka M, Rzeszowska-Wolny J. The bystander effect-induced formation of micronucleated cells is inhibited by antioxidants, but the parallel induction of apoptosis and loss of viability are not affected. Mutat Res. 2006;593:32–8. doi: 10.1016/j.mrfmmm.2005.06.017. doi: 10.1016/j.mrfmmm.2005.06.017. PubMed PMID: 16040062. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Zhao Y, Han W, Zhao G, Zhu L, Wang J, et al. Mitochondria-dependent signalling pathway are involved in the early process of radiation-induced bystander effects. Br J Cancer. 2008;98(11):1839–44. doi: 10.1038/sj.bjc.6604358. doi: 10.1038/sj.bjc.6604358. PubMed PMID: 18475304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang G, Wu L, Chen S, Zhu L, Huang P, Tong L, et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs radiation-induced bystander effect. Br J Cancer. 2009;100(12):1912–6. doi: 10.1038/sj.bjc.6605087. doi: 10.1038/sj.bjc.6605087. PubMed PMID: 19455142; PubMed Central PMCID: PMC2714242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Zhao Y, Zhao G, Han W, Bao L, Yu KN, et al. Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects. Mutat Res. 2009;666:68–73. doi: 10.1016/j.mrfmmm.2009.04.006. doi: 10.1016/j.mrfmmm.2009.04.006. PubMed PMID: 19393669. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and nuclear factor-κB–mediated signaling in radiation-induced bystander effects. Cancer Res. 2008;68(7):2233–40. doi: 10.1158/0008-5472.CAN-07-5278. doi: 10.1158/0008-5472.can-07-5278. PubMed PMID: 18381429; PubMed Central PMCID: PMC3715144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pazhanisamy SK, Li H, Wang Y, Batinic-Haberle I, Zhou D. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis. 2011;26(3):431–5. doi: 10.1093/mutage/ger001. doi: 10.1093/mutage/ger001. PubMed PMID: 21415439; PubMed Central PMCID: PMC3081334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamori T, Inanami O, Nagahata H, Cui Y-D, Kuwabara M. Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett. 2000;467(2):253–8. doi: 10.1016/s0014-5793(00)01167-4. PubMed PMID: 10675549. [DOI] [PubMed] [Google Scholar]
- 24.Little JB, Azzam EI, De Toledo S, Nagasawa H. Bystander effects: intercellular transmission of radiation damage signals. Radiat Prot Dosimetry. 2002;99(1-4):159–62. doi: 10.1093/oxfordjournals.rpd.a006751. PubMed PMID: 12194273. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118(2):503–8. doi: 10.1378/chest.118.2.503. PubMed PMID: 10936147. [DOI] [PubMed] [Google Scholar]
- 26.Streicher JM, Wang Y. The role of COX-2 in heart pathology. Cardiovasc Hematol Agents Med Chem. 2008;6(1):69–79. doi: 10.2174/187152508783329948. PubMed PMID: 18220723. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, et al. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci USA. 2005;102(41):14641–6. doi: 10.1073/pnas.0505473102. doi: 10.1073/pnas.0505473102. PubMed PMID: 16203985; PubMed Central PMCID: PMC1253564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi YJ, Kim HS, Lee J, Chung J, Lee JS, Choi JS, et al. Down-regulation of oxidative stress and COX-2 and iNOS expressions by dimethyl lithospermate in aged rat kidney. Arch Pharm Res. 2014;37(8):1032–8. doi: 10.1007/s12272-014-0332-6. doi: 10.1007/s12272-014-0332-6. PubMed PMID: 24469601. [DOI] [PubMed] [Google Scholar]
- 29.Chai Y, Calaf GM, Zhou H, Ghandhi SA, Elliston CD, Wen G, et al. Radiation induced COX-2 expression and mutagenesis at non-targeted lung tissues of gpt delta transgenic mice. Br J Cancer. 2012;108(1):91–8. doi: 10.1038/bjc.2012.498. doi: 10.1038/bjc.2012.498. PubMed PMID: 23321513; PubMed Central PMCID: PMC3553512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai Y, Lam RK, Calaf GM, Zhou H, Amundson S, Hei TK. Radiation-induced non-targeted response in vivo: role of the TGFβ-TGFBR1-COX-2 signalling pathway. Br J Cancer. 2013;108(5):1106–12. doi: 10.1038/bjc.2013.53. doi: 10.1038/bjc.2013.53. PubMed PMID: 23412109; PubMed Central PMCID: PMC3619070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waddington CH. The epigenotype. Int J Epidemiol. 2012;41(1):10–3. doi: 10.1093/ije/dyr184. doi: 10.1093/ije/dyr184. PubMed PMID: 22186258. [DOI] [PubMed] [Google Scholar]
- 32.Holliday R. Epigenetics: a historical overview. Epigenetics. 2006;1(2):76–80. doi: 10.4161/epi.1.2.2762. PubMed PMID: 17998809. [DOI] [PubMed] [Google Scholar]
- 33.Bird A. On the track of DNA methylation: an interview with Adrian Bird by Jane Gitschier. PLOS Genet. 2009;5(10):e1000667–e. doi: 10.1371/journal.pgen.1000667. doi: 10.1371/journal.pgen.1000667. PubMed PMID: 19834538; PubMed Central PMCID: PMC2753650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22(22):4632–42. doi: 10.1200/JCO.2004.07.151. doi: 10.1200/jco.2004.07.151. PubMed PMID: 15542813. [DOI] [PubMed] [Google Scholar]
- 35.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–402. doi: 10.1093/hmg/9.16.2395. PubMed PMID: 11005794. [DOI] [PubMed] [Google Scholar]
- 36.Zaratiegui M, Irvine DV, Martienssen RA. Noncoding RNAs and gene silencing. Cell. 2007;128(4):763–76. doi: 10.1016/j.cell.2007.02.016. doi: 10.1016/j.cell.2007.02.016. PubMed PMID: 17320512. [DOI] [PubMed] [Google Scholar]
- 37.Naeini MM, Ardekani AM. Noncoding RNAs And Cancer. Avicenna J Med Biotechnol. 2009;1:55–70. PubMed PMID: 23407615; PubMed Central PMCID: PMC3558126. [PMC free article] [PubMed] [Google Scholar]
- 38.Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010;330(6004):612–6. doi: 10.1126/science.1191078. doi: 10.1126/science.1191078. PubMed PMID: 21030644; PubMed Central PMCID: PMC3772643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalinich JF, Catravas GN, Snyder SL. The effect of γ radiation on DNA methylation. Radiat Res. 1989;117(2):185–97. PubMed PMID: 2922465. [PubMed] [Google Scholar]
- 40.Minamoto T, Mai M, Ronai Z. Environmental factors as regulators and effectors of multistep carcinogenesis. Carcinogenesis. 1999;20(4):519–27. doi: 10.1093/carcin/20.4.519. PubMed PMID: 10223177. [DOI] [PubMed] [Google Scholar]
- 41.Sedelnikova OA, Nakamura A, Kovalchuk O, Koturbash I, Mitchell SA, Marino SA, et al. DNA double-strand breaks form in bystander cells after microbeam irradiation of three-dimensional human tissue models. Cancer Res. 2007;67(9):4295–302. doi: 10.1158/0008-5472.CAN-06-4442. doi: 10.1158/0008-5472.can-06-4442. PubMed PMID: 17483342. [DOI] [PubMed] [Google Scholar]
- 42.Saleh AD, Savage JE, Cao L, Soule BP, Ly D, DeGraff W, et al. Cellular stress induced alterations in microRNA let-7a and let-7b expression are dependent on p53. PLoS One. 2011;6(10):e24429. doi: 10.1371/journal.pone.0024429. doi: 10.1371/journal.pone.0024429. PubMed PMID: 22022355; PubMed Central PMCID: PMC3191136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koturbash I, Boyko A, Rodriguez-Juarez R, McDonald RJ, Tryndyak VP, Kovalchuk I, et al. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis. 2007;28(8):1831–8. doi: 10.1093/carcin/bgm053. doi: 10.1093/carcin/bgm053. PubMed PMID: 17347136. [DOI] [PubMed] [Google Scholar]
- 44.Koturbash I, Rugo RE, Hendricks CA, Loree J, Thibault B, Kutanzi K, et al. Irradiation induces DNA damage and modulates epigenetic effectors in distant bystander tissue in vivo. Oncogene. 2006;25(31):4267–75. doi: 10.1038/sj.onc.1209467. doi: 10.1038/sj.onc.1209467. PubMed PMID: 16532033. [DOI] [PubMed] [Google Scholar]
- 45.Ilnytskyy Y, Koturbash I, Kovalchuk O. Radiation-induced bystander effects in vivo are epigenetically regulated in a tissue-specific manner. Environ Mol Mutagen. 2009;50(2):105–13. doi: 10.1002/em.20440. doi: 10.1002/em.20440. PubMed PMID: 19107897. [DOI] [PubMed] [Google Scholar]
- 46.Koturbash I, Zemp FJ, Kutanzi K, Luzhna L, Loree J, Kolb B, et al. Sex-specific microRNAome deregulation in the shielded bystander spleen of cranially exposed mice. Cell Cycle. 2008;7(11):1658–67. doi: 10.4161/cc.7.11.5981. PubMed PMID: 18560276. [DOI] [PubMed] [Google Scholar]
- 47.Tamminga J, Koturbash I, Baker M, Kutanzi K, Kathiria P, Pogribny IP, et al. Paternal cranial irradiation induces distant bystander DNA damage in the germline and leads to epigenetic alterations in the offspring. Cell Cycle. 2008;7(9):1238–45. doi: 10.4161/cc.7.9.5806. PubMed PMID: 18418050. [DOI] [PubMed] [Google Scholar]
- 48.Filkowski JN, Ilnytskyy Y, Tamminga J, Koturbash I, Golubov A, Bagnyukova T, et al. Hypomethylation and genome instability in the germline of exposed parents and their progeny is associated with altered miRNA expression. Carcinogenesis. 2010;31(6):1110–5. doi: 10.1093/carcin/bgp300. doi: 10.1093/carcin/bgp300. PubMed PMID: 19959559. [DOI] [PubMed] [Google Scholar]
- 49.Ron E. Ionizing radiation and cancer risk: evidence from epidemiology. Radiat Res. 1998;150(5s):S30–S41. PubMed PMID: 9806607. [PubMed] [Google Scholar]
- 50.Mothersill C, Seymour CB. Bystander and delayed effects after fractionated radiation exposure. Radiat Res. 2002 ;158(5):626–33. doi: 10.1667/0033-7587(2002)158[0626:badeaf]2.0.co;2. PubMed PMID: 12385640. [DOI] [PubMed] [Google Scholar]
- 51.Dent SF, Klaassen D, Pater JL, Zee B, Whitehead M. Second primary malignancies following the treatment of early stage ovarian cancer: Update of a study by the National Cancer Institute of Canada—Clinical Trials Group (NCIC—CTG) Ann Oncol. 2000;11(1):65–8. doi: 10.1023/a:1008356806417. PubMed PMID: 10690389. [DOI] [PubMed] [Google Scholar]
- 52.Kleinerman RA, Boice JD, Storm HH, Sparen P, Andersen A, Pukkala E, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995;76(3):442–52. doi: 10.1002/1097-0142(19950801)76:3<442::aid-cncr2820760315>3.0.co;2-l. PubMed PMID: 8625126. [DOI] [PubMed] [Google Scholar]
- 53.Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol. 2005;23(25):6126–31. doi: 10.1200/JCO.2005.02.543. doi: 10.1200/jco.2005.02.543. PubMed PMID: 16135478. [DOI] [PubMed] [Google Scholar]


