Abstract
Gas chromatography with mass spectrometric detection combined with head-space needle trap extraction as the pre-concentration technique was applied to identify and quantify volatile organic compounds released or metabolised by human umbilical vein endothelial cells. Amongst the consumed species there were eight aldehydes (2-methyl 2-propenal, 2-methyl propanal, 2-methyl butanal, 3-methyl butanal, n-hexanal, benzaldehyde, n-octanal and n-nonanal) and n-butyl acetate. Further eight compounds (ethyl acetate, ethyl propanoate, ethyl butyrate, 3-heptanone, 2-octanone, 2-nonanone, 2-methyl-5-(methylthio)-furan and toluene) were found to be emitted by the cells under study. Possible metabolic pathways leading to the uptake and release of these compounds by HUVEC are proposed and discussed. The uptake of aldehydes by endothelial cells questions the reliability of species from this chemical class as breath or blood markers of disease processes in human organism. The analysis of volatiles released or emitted by cell lines is shown to have a potential for the identification and assessment of enzymes activities and expression.
Keywords: HUVEC, Volatile organic compounds, GC–MS, Needle trap, Uptake, Release, Aldehydes
Introduction
There is considerable evidence that volatile organic compounds (VOCs) released by human organism mirror normal physiological processes as well as pathological disorders and have, thereby, a great potential for medical diagnosis and therapy [1–4]. Breath analysis holds in this context a distinguished status as it is non-invasive and some breath constituents have already been linked to various disease processes [5–10]. The main obstacle limiting the clinical application of breath tests is the insufficient understanding of the origin and metabolic fate of breath markers. In vitro studies involving pathogenic microorganisms (e.g. bacteria, fungi) or human cells (both normal and cancerogenous) are, within this framework, an invaluable tool capable of revealing the biochemical pathways of breath biomarkers production or metabolism. For instance, over the last few years, a substantial progress was made to pinpoint volatiles emitted or consumed by cancer cells [11–16], bacteria [17, 18], or fungi [19].
In the current study, human umbilical vein endothelial cells (HUVEC) were investigated. These cells are isolated from the vein of the umbilical cord and are commonly used for physiological and pharmacological investigations [20–22]. In breath gas analysis, endothelial cells play a crucial role as they form the interior surface of the vascular system—a trunk line transporting volatile markers from distant parts of the body to lungs. Consequently, the uptake and release of VOCs by these cells can considerably modify the profile of blood and breath VOCs and, thereby, distort the information they provide. Hence, the main goal of this work was to identify and quantify volatile organic compounds being emitted or metabolised by human umbilical vein endothelial cells. Their determination relied on gas chromatography with mass spectrometric detection (GC–MS) and head-space needle trap extraction (HS-NTE) as the pre-concentration method.
Materials and Methods
Chemicals and Standards
Calibration mixtures were prepared from high-purity liquid substances. The majority of them were purchased from Sigma-Aldrich (Austria): 2-methyl 2-propenal (95 %), 2-methyl propanal (99.5 %), 3-methyl butanal (97 %), n-hexanal (98 %), n-octanal (99 %), ethyl butyrate (99 %), toluene (99.8 %) and ethyl acetate (99.9 %). Moreover, n-butyl acetate (99.7 %), benzaldehyde (99 %), n-nonanal (95 %), 2-octanone (99.5 %) and 2-methyl butanal (99 %) were obtained from Fluka (Switzerland), whereas ethyl propanoate (97 %) was provided by SAFC (USA). 3-heptanone (98 %) was purchased from Alfa Aesar (USA), 2-methyl-5-(methylthio) furan (99 %) from Chemos (Germany) and 2-nonanone (98 %) was purchased from Merck Schuchardt (Germany).
Gaseous humid calibration mixtures were prepared using a GasLab calibration mixtures generator (Breitfuss Messtechnik, Germany). The GasLab consists of an integrated zero air generator, a 2-stage dynamic injection module, for evaporating a liquid and diluting it with zero air, and a humidification module enabling the preparation of gas mixtures at pre-defined humidity levels. When using pure liquid substances, GasLab produces a flow of up to 10 L/min of complex trace gas mixtures in dry or humidified zero air containing 10 ppb–100 ppm of each solute. Since in this study the observed levels of compounds of interest were much lower, pure substances were additionally diluted (1:1,000–1:2,000) with distilled water or methanol prior to the evaporation. Effectively, humid gas mixtures (100 % RH at 37 °C) with volume fractions ranging from approximately 0.04 to 150 ppb were used for the purpose of calibration and validation.
Cell Cultivation
For the experiments human umbilical vein endothelial cells (HUVEC) passage 5 (P5) from pooled donors were used (PromoCell, C-12203). After building up a confluent monolayer (cell density 80–90 %) in a 75 cm2 cell culture bottle HUVEC were split 1:3 to cultivation glass bottles coated with 0.2 % gelatin solution (Sigma, G1393). The cultivation/measurement bottles had diameters of 21 cm × 5.5 cm × 11.5 cm (1,000 mL nominal volume, bottom area of approximately 240 cm2) and are shown in Fig. 1. The bottles were closed with a special Teflon plug equipped with a rubber septum enabling the insertion of the needle trap devices into the head-space of the culture and the Teflon tube being the inlet of the zero air. The inner end of the Teflon tube protruded 15–17 cm from the plug into the head-space volume, whereas the outer end was equipped with a sterile filter. The cell culture medium (EBM-2, CC-3156, supplemented with EGM-2 single quotes, CC-4147; both Clonetics) was changed every other day. After building up a confluent monolayer in the glass bottles, cells were washed three times with PBS (PAA, H15-002) and cultured in 30 mL medium. A glass bottle coated with gelatin solution (no cells) and filled with medium was used as background control. HUVEC were incubated for 24–30 h in a humidified atmosphere (37 °C and 5 % CO2) and consequently processed for the GC–MS analyses of the head-space composition. For the 24 to 30 h of cultivation, the bottles were tightly closed to boost the accumulation of species released by the cells and to block the gas exchange with the ambient air. Cell viability counts (trypan blue exclusion method) were performed immediately after the measurements. In total, 7 experiments (each involving 1 cell culture and 1 control) were performed.
Fig. 1.
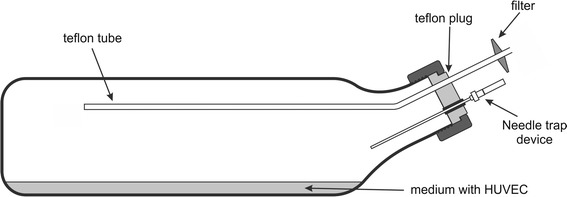
Cultivation/measurement bottle
Head-Space Sampling Procedure and Chromatographic Analysis
Head-space volatile organic compounds were pre-concentrated using three-bed side-hole 23-gauge stainless steel needle trap devices (NTD) (PAS Technology, Germany) [23, 24]. All needles were Silcosteel-treated and their sorbent beds consisted of 1 cm of Tenax TA (80/100 mesh), 1 cm of Carbopack X (60/80 mesh) and 1 cm of Carboxen 1,000 (60/80 mesh). Prior to the first use, all NTDs were pre-conditioned at 290 °C by flushing them with high-purity nitrogen (6.0–99.9999 %) for 4 h. Their re-conditioning was performed before each sampling, however, with shorter flushing times of 10 min. Since NTDs exhibited relatively huge disparities with respect to the extraction efficiency (deviations of up to 70 %, even when originating from the same production lot), the NTDs used during experiments were pre-selected according to the condition that their inter-needle variability should be below 10 %.
The sampling was performed dynamically by inserting the NTD through a rubber septum into the head-space of the bottle and drawing 200 mL of head-space gas at a steady flow rate of 10 mL/min at 37 °C. These conditions were achieved with the help of a membrane pump (Vacuubrand, Germany) and a mass flow controller (RED-Y, Burde Co. GmbH, Austria). Consequently, no transfer line was present between the sampled head-space and a needle trap. To maintain the constant pressure in the bottle during sampling, high-purity zero air was continuously introduced into it via the Teflon tube (see Fig. 1) at a flow equal to the sampling flow. Immediately after an extraction, the NTD was manually introduced into the inlet of the gas chromatograph where the compounds of interest were thermally desorbed at 290 °C in a splitless mode (1 min).
Chromatographic analyses were performed using an Agilent 7890A/5975C GC–MS system (Agilent, USA). During desorption, the split/splitless inlet operated in the splitless mode (1 min), followed by a split mode at ratio 1:20. The analytes were separated using a PoraBond Q column (25 m x 0.32 mm, film thickness 5 μm, styrene–divinylbenzene copolymer phase, Varian, USA) working under a constant flow of helium (1.5 mL/min). The column temperature programme was as follows: 40 °C for 5 min, increase to 260 °C at a rate of 7 °C/min, followed by a constant temperature phase at 260 °C for 6 min. The mass spectrometer worked in a SCAN mode with an m/z range set from 20 to 200. The quadrupole, ion source and transfer line temperatures were kept at 150, 230 and 280 °C, respectively.
The identification of compounds was performed in two steps. First, the peak spectrum was checked against the NIST mass spectral library (NIST/EPA/NIH mass spectral library version 2.0f). Next, the NIST tentative identifications were validated by collating the respective retention times with the list of retention times obtained on the basis of analyses of standard mixtures. Peak integration was based on extracted ion chromatograms. The retention times of the investigated compounds for the applied chromatographic parameters as well as the quantifier ions used for the integration are presented in Table 1.
Table 1.
Retention times R t (min), quantifier ions, LODs (ppb), RSDs (%), coefficients of variation (R 2) and linear ranges (ppb) of compounds under study
| VOC | CAS | R t (min) | Quantifier ion | LOD (ppb) | RSD (%) | R 2 | Linear range (ppb) |
|---|---|---|---|---|---|---|---|
| 2-Propenal, 2-methyl- | 78-85-3 | 18.99 | 70 | 0.03 | 8 | 0.993 | 0.1–12 |
| Propanal, 2-methyl- | 78-84-2 | 19.27 | 72 | 0.3 | 9 | 0.977 | 0.9–150 |
| Ethyl acetate | 141-78-6 | 21.00 | 43 | 0.12 | 5.5 | 0.987 | 0.36–20 |
| Butanal, 3-methyl- | 590-86-3 | 23.36 | 44 | 0.14 | 9 | 0.978 | 0.4–170 |
| Butanal, 2-methyl- | 96-17-3 | 23.42 | Not quantified, RT confirmed | ||||
| Ethyl propanoate | 105-37-3 | 24.64 | 57 | 0.04 | 9 | 0.998 | 0.12–1.5 |
| Toluene | 108-88-3 | 26.21 | 91 | 0.1 | 6 | 0.999 | 0.3–20 |
| n-Hexanal | 66-25-1 | 27.73 | 56 | 0.2 | 9 | 0.994 | 0.6–15 |
| Ethyl butyrate | 105-54-4 | 27.93 | 71 | 0.02 | 9 | 0.996 | 0.06–1 |
| n-Butyl acetate | 123-86-4 | 28.18 | 56 | 0.04 | 10 | 0.997 | 0.12–8 |
| 3-Heptanone | 106-35-4 | 30.51 | 85 | 0.03 | 2.5 | 0.997 | 0.09–7 |
| Benzaldehyde | 100-52-7 | 30.87 | 106 | 0.05 | 12 | 0.998 | 0.15–12 |
| Furan, 2-methyl-5-(methylthio)- | 13678-59-6 | 31.00 | 128 | 0.03 | 7 | 0.988 | 0.09–4 |
| 2-Octanone | 111-13-7 | 33.56 | 58 | 0.05 | 9 | 0.991 | 0.15–5.5 |
| n-Octanal | 124-13-0 | 33.76 | 84 | 0.1 | 10 | 0.993 | 0.3–13 |
| 2-Nonanone | 821-55-6 | 36.19 | 58 | 0.07 | 11 | 0.974 | 0.21–5.7 |
| n-Nonanal | 124-19-6 | 36.41 | 57 | 0.4 | 12 | 0.930 | 1.2–12 |
Compounds are ordered with respect to increasing retention time
Results and Discussion
Validation Parameters
Limits of detection (LODs) were calculated using the mean value of the blank responses and their standard deviations obtained on the basis of 10 blank measurements [25]. The LOD values ranged from 0.02 ppb for ethyl butyrate to 0.3 ppb for 2-methyl propanal, see Table 1. The relative standard deviations (RSDs) were calculated on the basis of five consecutive analyses of humid standard mixtures. The calculated RSDs varied from 2.5 to 12 % and were recognised as adequate for the aim of this study. The system response was found to be linear within the investigated concentration ranges with the coefficients of variation ranging from 0.930 to 0.999, as shown in Table 1.
HUVEC Cultures
The total number of HUVEC and their viabilities after 24–30 h incubation in the closed measurement bottles are shown in Table 2. The total number of cells fell within the range of 3.3 mio and 45.5 mio (mean 15.8 mio), whereas the viability varied from 81.4 to 99.8 % (mean 92.7 %). Consequently, the applied experimental protocol did not affect relevantly the cells’ viability, and it can be assumed that the release and uptake of the head-space VOCs mirror their metabolism.
Table 2.
Total number of cells, number of living cells and viability at the end of the cultivation period
| Culture | Total number of cells (mio) | Number of living cells (mio) | Viability (%) |
|---|---|---|---|
| 1 | 13.6 | 13.2 | 97.4 |
| 2 | 45.5 | 42.8 | 93.9 |
| 3 | 3.3 | 2.7 | 83.7 |
| 4 | 5.3 | 4.3 | 81.4 |
| 5 | 12.8 | 12.1 | 94.8 |
| 6 | 14 | 13.7 | 97.7 |
| 7 | 15.9 | 15.9 | 99.8 |
| Mean | 15.8 | 14.9 | 92.7 |
Uptake of VOCs by HUVEC
A total of nine species were found to be consumed by the HUVEC (Wilcoxon signed-rank test, p < .05). Their incidences and concentration ranges in the head-space of cell cultures and controls are given in Table 3. The predominant chemical class in this group were aldehydes with eight compounds (2-methyl 2-propenal, 2-methyl propanal, 2-methyl butanal, 3-methyl butanal, n-hexanal, benzaldehyde, n-octanal and n-nonanal). Apart from aldehydes there was one ester, n-butyl acetate. In the case of 2-methyl butanal, proper integration and quantification was not possible due to the poor separation from 3-methyl butanal and the absence of unique ion that could be used for these purposes. Aliphatic and saturated aldehydes seemed to be more preferred substrates for HUVEC than unsaturated or aromatic ones. For instance, the levels of n-hexanal and 3-methyl butanal were reduced by approximately 90 % after the 1-day-long incubation, whereas the concentrations of 2-methyl 2-propenal and benzaldehyde dropped only by 40 and 60 %, respectively.
Table 3.
Detection (n d) and quantification (n q) incidences and ranges (means) of VOCs under study in the head-space of medium and cell cultures
| VOC | CAS | Cell cultures | Medium | ||
|---|---|---|---|---|---|
| Incidence n d (n q) | Range (mean) (ppb) | Incidence n d (n q) | Range (mean) (ppb) | ||
| Uptake | |||||
| 2-Propenal, 2-methyl- | 78-85-3 | 7(5) | 0.67–3.1(1.5) | 7(7) | 0.9–4.1(2.4) |
| Propanal, 2-methyl- | 78-84-2 | 7(7) | 1.5–16(5.6) | 7(7) | 16–125 (56) |
| Butanal, 3-methyl- | 590-86-3 | 6(6) | 1.2–17.2(5.3) | 7(7) | 1.7–95.5(44) |
| n-Hexanal | 66-25-1 | 7(6) | 0.7–3(1.5) | 7(7) | 5.8–15.5(9.8) |
| n-Butyl acetate | 123-86-4 | 7(4) | 0.13–0.58(0.38) | 7(7) | 0.15–0.88(0.52) |
| Benzaldehyde | 100-52-7 | 7(7) | 0.17–1.1(0.48) | 7(7) | 0.63–2.53(1.2) |
| n-Octanal | 124-13-0 | 4(0) | <LOQ | 7(7) | 0.32–3.34(0.98) |
| n-Nonanal | 124-19-6 | 1(0) | <LOQ | 7(6) | 1.8–2.2(2.0) |
| Release | |||||
| Ethyl acetate | 141-78-6 | 7(7) | 3.7–16.2(10.1) | 7(7) | 0.5–3.2(1.8) |
| Ethyl propanoate | 105-37-3 | 7(7) | 0.14–0.90(0.49) | 6(0) | <LOQ |
| Toluene | 108-88-3 | 7(7) | 1.8–18.6(7.8) | 7(7) | 1.2–5.9(3.6) |
| Ethyl butyrate | 105-54-4 | 7(6) | 0.07–0.22(0.16) | 1(0) | <LOQ |
| 3-Heptanone | 106-35-4 | 7(7) | 0.3–1.6(1.0) | 7(7) | 0.1–0.79(0.45) |
| Furan, 2-methyl-5-(methylthio)- | 13678-59-6 | 7(7) | 0.11–0.36(0.25) | 0(0) | <LOD |
| 2-Octanone | 111-13-7 | 7(6) | 0.18–0.39(0.28) | 7(1) | 0.16 |
| 2-Nonanone | 821-55-6 | 7(6) | 0.25–0.50(0.37) | 6(0) | <LOQ |
A potential pathway leading to the uptake of aldehydes by HUVEC involves aldehyde dehydrogenases (ALDHs). ALDHs irreversibly oxidise a wide spectrum of endogenous and exogenous aldehydes to their corresponding carboxylic acids [26, 27]. Although ALDHs in endothelial cells are rather poorly expressed, their activity has been evidenced in the literature [28, 29]. Moreover, the observed differences in the uptake of different types of aldehydes are consistent with the reported specificity of human ALDHs towards species from this chemical class [26]. Alternatively, aldehydes can be reduced to alcohols by alcohol dehydrogenases (ADHs). ADHs were found to be abundant in human blood vessels; however, their primary function there seems to be the first-pass extrahepatic ethanol metabolism [30]. Thus, the oxidation rather than reduction appears to be the main reason of the aldehydes’ uptake noted within this study [30].
The decrease of n-butyl acetate can mirror the activity of carboxylesterases (CESs), enzymes ubiquitous in human tissues [31]. This ester could be hydrolysed by CESs into acetic acid and 1-butanol being subsequently converted into n-butanal by ADHs, and next butanoic acid by ALDHs.
The uptake of aldehydes has already been noted in human cells cultures. Filipiak et al. [14, 15] and Sponring et al. [12] reported the consumption of species from this chemical family by lung cancer and normal cells. In our recent paper [16] we evidenced similar phenomenon in cultures of human hepatocellular carcinoma cells (HepG2). ALDHs are particularly expressed in both lung and liver cells [27], moreover, their activity is additionally increased in their cancer counterparts [32, 33]. Both lung and liver cells were also shown to metabolise n-butyl acetate during in vitro studies [12, 14–16].
Emission of VOCs by HUVEC
Eight compounds increased their levels at the presence of HUVEC (see Table 3). Amongst them there were three esters (ethyl acetate, ethyl propanoate and ethyl butyrate), three ketones (3-heptanone, 2-octanone and 2-nonanone), one volatile sulphur compound (2-methyl-5-(methylthio) furan) and one aromatic hydrocarbon (toluene). The highest concentrations were observed for ethyl acetate (mean of 10.1 ppb in cell cultures vs. 1.8 ppb in media) and toluene (7.8 vs. 3.6, respectively). However, the toluene levels increased only by a factor of two, whereas the ethyl acetate ones almost six-fold.
Ketones production by the HUVEC can be attributed to the aforementioned high expression of alcohol dehydrogenases (ADHs) in human vascular endothelium [30]. Although primary alcohols seem to be the most preferred substrates for ADHs, they can also oxidise longer-chain cyclic and secondary alcohols [27, 34–36]. The latter were shown to be rather poor substrates for ADHs [36], nevertheless their conversion into ketones has been documented in the literature [34, 36]. Consequently, 2-octanone could be the product of the 2-octanol oxidation and 2-nonanone possibly stemmed from 2-nonanol. The origin of these secondary alcohols remains unclear. Probably they were present in small amounts in the applied medium. An alternative pathway leading to the formation of ketones in humans employs β-oxidation of branched-chain fatty acids. For example, valproic acid was demonstrated to be metabolised into 3-heptanone [37] and 2-ethylhexanoic acid was reported to be oxidised to 2-heptanone and 4-heptanone [38]. The respective branched-chain fatty acids can in turn be the metabolites of the appropriate branched-chain primary alcohols or/and aldehydes (e.g. 2-propyl pentanol or 2-propyl pentanal in case of 3-heptanone). However, it is not clear if these substrates were present in the applied medium.
Interestingly, all esters found to be released by HUVEC stemmed from ethanol. Indeed, huge amounts of this alcohol (exceeding the dynamic range of the MS detector) were detected in the head-space of both cell cultures and blanks. Consequently, it seems plausible that the esterification reaction involving ethanol and the respective fatty acids could induce the production of the observed esters. Although such a reaction in the absence of a catalyst is very slow and the products relatively unstable, small amounts of esters could form and go into the gas phase. Thus, ethyl acetate was presumably generated by a reaction of ethanol with acetic acid—a product of the oxidation of the former by a tandem of ADHs and ALDHs. The high concentrations of ethyl acetate as compared with the other liberated esters seem to confirm this hypothesis. Analogously, propanoic and butanoic acids—substrates necessary for the production of the remaining esters—could in turn be produced from 1-butanol and 1-propanol (or n-propanal and n-butanal). Apart from 1-butanol, all these potential substrates were found in the head-space of the cell cultures. Consequently, the release of esters seems to be an indirect reflection of ADH and ALDH activities.
The origin of toluene and 2-methyl-5-(methylthio)-furan remains unclear; however, the latter was found to be produced also by human hepatocellular carcinoma cells [16].
The release of ketones was reported also in case of lung and liver cells [13, 15, 16], which is consistent with the ADHs’ expression in different human tissues [27]. In terms of esters, both cancer liver and lung cells were evidenced to emit n-propyl acetate [15, 16].
Conclusions
In the present study, gas chromatography with mass spectrometric detection coupled with head-space needle trap extraction (HS-NTE) as the pre-concentration technique was applied for the identification and quantification of volatiles being released or metabolised by human umbilical vein endothelial cells (HUVEC). Seventeen VOCs were found to change their levels in the presence of HUVEC cells (Wilcoxon signed-rank test, p < .05). Amongst the consumed species, there were eight aldehydes (2-methyl 2-propenal, 2-methyl propanal, 2-methyl butanal, 3-methyl butanal, n-hexanal, benzaldehyde, n-octanal and n-nonanal) and n-butyl acetate. Eight compounds were emitted by the cells under study. This group embraces three esters (ethyl acetate, ethyl propanoate and ethyl butyrate), three ketones (3-heptanone, 2-octanone and 2-nonanone), one volatile sulphur compound (2-methyl-5-(methylthio) furan) and one aromatic hydrocarbon (toluene). The uptake and release of majority of these analytes can be attributed to the expression of enzymes in the endothelial cells, such as ADHs and ALDHs. Thus, the analysis of volatiles released or emitted by cell lines has a potential for the identification and assessment of enzymes activities.
The uptake of aldehydes by HUVEC is particularly interesting as some species from this chemical class (e.g. n-pentanal, n-hexanal, n-heptanal and n-octanal) have been proposed as blood and breath biomarkers of various forms of cancer [28, 39–42] or oxidative stress [43, 44]. The human vascular endothelium exhibits enormous surface of 500–700 m2 [30]. Consequently, it is likely that blood vessels reduce significantly the blood levels of aldehydes in general and disease-related aldehydes in particular. This observation questions aldehydes as reliable markers providing the information on disease processes, or metabolic disorders occurring in distant parts of the body. Moreover, in the context of breath gas analysis, the vascular system cannot be considered as an inert trunk line transporting volatile markers to lungs. Thus, the selection of new breath markers should embrace the studies on their stability in blood and vascular system.
Acknowledgments
P. M. and K. U. gratefully acknowledge support from the Austrian Science Fund (FWF) under Grant No. P24736-B23.
Contributor Information
Paweł Mochalski, Phone: +43-512-504-24636, Email: Pawel.Mochalski@uibk.ac.at.
Anton Amann, Phone: +43-512-504-24636, Email: anton.amann@i-med.ac.at.
References
- 1.Miekisch W, Schubert JK, Noeldge-Schomburg GF. Diagnostic potential of breath analysis—Focus on volatile organic compounds. Clinica Chimica Acta. 2004;347:25–39. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Horvath, I., & de Jongste, J. C. (2010). Exhaled biomarkers (European Respiratory Monograph). European Respiratory Society.
- 3.Amann, A., & Smith, D. (2005). Breath analysis for clinical diagnosis and therapeutic monitoring. Hackensack, NJ: World Scientific. xviii, 536 pp.
- 4.Amann A, Corradi M, Mazzone P, Mutti A. Lung cancer biomarkers in exhaled breath. Expert Review of Molecular Diagnostics. 2011;11:207–217. doi: 10.1586/erm.10.112. [DOI] [PubMed] [Google Scholar]
- 5.Poli D, Carbognani P, Corradi M, Goldoni M, Acampa O, Balbi B, Bianchi L, Rusca M, Mutti A. Exhaled volatile organic compounds in patients with non-small cell lung cancer: Cross sectional and nested short-term follow-up study. Respiratory Research. 2005;6:71. doi: 10.1186/1465-9921-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips M, Cataneo RN, Saunders C, Hope P, Schmitt P, Wai J. Volatile biomarkers in the breath of women with breast cancer. Journal of Breath Research. 2010;4:026003. doi: 10.1088/1752-7155/4/2/026003. [DOI] [PubMed] [Google Scholar]
- 7.Phillips M, Boehmer JP, Cataneo RN, Cheema T, Eisen HJ, Fallon JT, Fisher PE, Gass A, Greenberg J, Kobashigawa J, Mancini D, Rayburn B, Zucker MJ. Heart allograft rejection: detection with breath alkanes in low-levels (the HARDBALL study) Journal of Heart and Lung Transplantation. 2004;23:701–708. doi: 10.1016/j.healun.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Kanoh S, Kobayashi H, Motoyoshi K. Exhaled ethane: An in vivo biomarker of lipid peroxidation in interstitial lung diseases. Chest. 2005;128:2387–2392. doi: 10.1378/chest.128.4.2387. [DOI] [PubMed] [Google Scholar]
- 9.Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor M, Ligor T, Filipiak W, Denz H, Fiegl M, Hilbe W, Weiss W, Lukas P, Jamnig H, Hackl M, Haidenberger A, Buszewski B, Miekisch W, Schubert J, Amann A. Non-invasive detection of lung cancer by analysis of exhaled breath. BMC Cancer. 2009;9:348. doi: 10.1186/1471-2407-9-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amann A, Ligor M, Ligor T, Bajtarevic A, Ager C, Pienz M, Denz H, Fiegl M, Hilbe W, Weiss W, Lukas P, Jamnig H, Hackl M, Haidenberger A, Sponring A, Filipiak W, Miekisch W, Schubert J, Buszewski B. Analysis of exhaled breath for screening of lung cancer patients. Magazine of European Medical Oncology. 2010;3:106–112. doi: 10.1007/s12254-010-0219-2. [DOI] [Google Scholar]
- 11.Sponring A, Filipiak W, Mikoviny T, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Research. 2009;29:419–426. [PubMed] [Google Scholar]
- 12.Sponring A, Filipiak W, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Analysis of volatile organic compounds (VOCs) in the headspace of NCI-H1666 lung cancer cells. Cancer Biomarker. 2010;7:153–161. doi: 10.3233/CBM-2010-0182. [DOI] [PubMed] [Google Scholar]
- 13.Hanai Y, Shimono K, Oka H, Baba Y, Yamazaki K, Beauchamp GK. Analysis of volatile organic compounds released from human lung cancer cells and from the urine of tumor-bearing mice. Cancer Cell International. 2012;12:7. doi: 10.1186/1475-2867-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipiak W, Sponring A, Mikoviny T, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-1 in vitro. Cancer Cell International. 2008;8:17. doi: 10.1186/1475-2867-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filipiak W, Sponring A, Filipiak A, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. TD-GC-MS analysis of volatile metabolites of human lung cancer and normal cells in vitro. Cancer Epidemiology Biomarkers & Prevention. 2010;19:182–195. doi: 10.1158/1055-9965.EPI-09-0162. [DOI] [PubMed] [Google Scholar]
- 16.Mochalski, P., Sponring, A., King, J., Unterkofler, K., Troppmair, J., & Amann, A. (2013) Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell International, 13, 72. [DOI] [PMC free article] [PubMed]
- 17.Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesenhofer H, Nagl M, Troppmair J, Amann A. Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa. BMC Microbiology. 2012;12:113. doi: 10.1186/1471-2180-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filipiak W, Sponring A, Baur MM, Ager C, Filipiak A, Wiesenhofer H, Nagl M, Troppmair J, Amann A. Characterization of volatile metabolites taken up by or released from Streptococcus pneumoniae and Haemophilus influenzae by using GC-MS. Microbiology. 2012;158:3044–3053. doi: 10.1099/mic.0.062687-0. [DOI] [PubMed] [Google Scholar]
- 19.Filipiak, W., Sponring, A., Filipiak, A., Baur, M., Ager, C., Wiesenhofer, H., Margesin, R., Nagl, M., Troppmair, J., & Amann, A. (2013) Volatile organic compounds (VOCs) released by athogenic microorganisms in vitro: Potential breath biomarkers for early-stage diagnosis of disease. In D. Smith & A. Amann (Eds.), Volatile biomarkers: Non-invasive diagnosis in physiology and medicine. Elsevier.
- 20.Tas PW, Stossel C, Roewer N. Inhibition of the histamine-induced Ca2+ influx in primary human endothelial cells (HUVEC) by volatile anaesthetics. European Journal of Anaesthesiology. 2008;25:976–985. doi: 10.1017/S0265021508004778. [DOI] [PubMed] [Google Scholar]
- 21.Digby JE, Martinez F, Jefferson A, Ruparelia N, Chai J, Wamil M, Greaves DR, Choudhury RP. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:669–676. doi: 10.1161/ATVBAHA.111.241836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, Vasiljevic D, Rong S, Wolf AM, Bahlmann FH, Patsch JR, Wolf D, Schratzberger P, Mahata SK, Kirchmair R. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circulation Research. 2010;107:1326–1335. doi: 10.1161/CIRCRESAHA.110.219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mieth M, Kischkel S, Schubert JK, Hein D, Miekisch W. Multibed needle trap devices for on site sampling and preconcentration of volatile breath biomarkers. Analytical Chemistry. 2009;81:5851–5857. doi: 10.1021/ac9009269. [DOI] [PubMed] [Google Scholar]
- 24.Filipiak W, Filipiak A, Ager C, Wiesenhofer H, Amann A. Optimization of sampling parameters for collection and preconcentration of alveolar air by needle traps. Journal of Breath Research. 2012;6:027107. doi: 10.1088/1752-7155/6/2/027107. [DOI] [PubMed] [Google Scholar]
- 25.Huber W. Basic calculations about the limit of detection and its optimal determination. Accreditation and Quality Assurance. 2003;8:213–217. [Google Scholar]
- 26.Klyosov AA. Kinetics and specificity of human liver aldehyde dehydrogenases toward aliphatic, aromatic, and fused polycyclic aldehydes. Biochemistry. 1996;35:4457–4467. doi: 10.1021/bi9521102. [DOI] [PubMed] [Google Scholar]
- 27.Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proceedings of the Nutrition Society. 2004;63:49–63. doi: 10.1079/PNS2003327. [DOI] [PubMed] [Google Scholar]
- 28.Li SY, Gomelsky M, Duan J, Zhang Z, Gomelsky L, Zhang X, Epstein PN, Ren J. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. Journal of Biological Chemistry. 2004;279:11244–11252. doi: 10.1074/jbc.M308011200. [DOI] [PubMed] [Google Scholar]
- 29.Balber AE. Concise review: aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: characteristics, activities, and emerging uses in regenerative medicine. Stem Cells. 2011;29:570–575. doi: 10.1002/stem.613. [DOI] [PubMed] [Google Scholar]
- 30.Allali-Hassani A, Martinez SE, Peralba JM, Vaglenova J, Vidal F, Richart C, Farres J, Pares X. Alcohol dehydrogenase of human and rat blood vessels. Role in ethanol metabolism. FEBS Letters. 1997;405:26–30. doi: 10.1016/S0014-5793(97)00151-8. [DOI] [PubMed] [Google Scholar]
- 31.Imai T. Human carboxylesterase isozymes: catalytic properties and rational drug design. Drug Metabolism and Pharmacokinetics. 2006;21:173–185. doi: 10.2133/dmpk.21.173. [DOI] [PubMed] [Google Scholar]
- 32.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Molecular Cancer Research. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 33.Jelski W, Szmitkowski M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clinica Chimica Acta. 2008;395:1–5. doi: 10.1016/j.cca.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Wagner FW, Pares X, Holmquist B, Vallee BL. Physical and enzymatic properties of a class III isozyme of human liver alcohol dehydrogenase: chi-ADH. Biochemistry. 1984;23:2193–2199. doi: 10.1021/bi00305a014. [DOI] [PubMed] [Google Scholar]
- 35.Kedishvili NY, Bosron WF, Stone CL, Hurley TD, Peggs CF, Thomasson HR, Popov KM, Carr LG, Edenberg HJ, Li TK. Expression and kinetic characterization of recombinant human stomach alcohol dehydrogenase. Active-site amino acid sequence explains substrate specificity compared with liver isozymes. Journal of Biological Chemistry. 1995;270:3625–3630. doi: 10.1074/jbc.270.8.3625. [DOI] [PubMed] [Google Scholar]
- 36.Ditlow CC, Holmquist B, Morelock MM, Vallee BL. Physical and enzymatic properties of a class II alcohol dehydrogenase isozyme of human liver: pi-ADH. Biochemistry. 1984;23:6363–6368. doi: 10.1021/bi00321a012. [DOI] [PubMed] [Google Scholar]
- 37.Erhart S, Amann A, Haberlandt E, Edlinger G, Schmid A, Filipiak W, Schwarz K, Mochalski P, Rostasy K, Karall D, Scholl-Burgi S. 3-Heptanone as a potential new marker for valproic acid therapy. Journal of Breath Research. 2009;3:016004. doi: 10.1088/1752-7155/3/1/016004. [DOI] [PubMed] [Google Scholar]
- 38.Walker V, Mills GA. Urine 4-heptanone: A beta-oxidation product of 2-ethylhexanoic acid from plasticisers. Clinica Chimica Acta. 2001;306:51–61. doi: 10.1016/S0009-8981(01)00390-4. [DOI] [PubMed] [Google Scholar]
- 39.Yazdanpanah M, Luo X, Lau R, Greenberg M, Fisher LJ, Lehotay DC. Cytotoxic aldehydes as possible markers for childhood cancer. Free Radical Biology and Medicine. 1997;23:870–878. doi: 10.1016/S0891-5849(97)00070-1. [DOI] [PubMed] [Google Scholar]
- 40.Phillips M, Gleeson K, Hughes JM, Greenberg J, Cataneo RN, Baker L, McVay WP. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet. 1999;353:1930–1933. doi: 10.1016/S0140-6736(98)07552-7. [DOI] [PubMed] [Google Scholar]
- 41.Kato S, Burke PJ, Koch TH, Bierbaum VM. Formaldehyde in human cancer cells: detection by preconcentration-chemical ionization mass spectrometry. Analytical Chemistry. 2001;73:2992–2997. doi: 10.1021/ac001498q. [DOI] [PubMed] [Google Scholar]
- 42.Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. International Journal of Cancer. 2010;126:2663–2670. doi: 10.1002/ijc.24970. [DOI] [PubMed] [Google Scholar]
- 43.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- 44.Corradi M, Pignatti P, Manini P, Andreoli R, Goldoni M, Poppa M, Moscato G, Balbi B, Mutti A. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. European Respiratory Journal. 2004;24:1011–1017. doi: 10.1183/09031936.04.00002404. [DOI] [PMC free article] [PubMed] [Google Scholar]


