Abstract
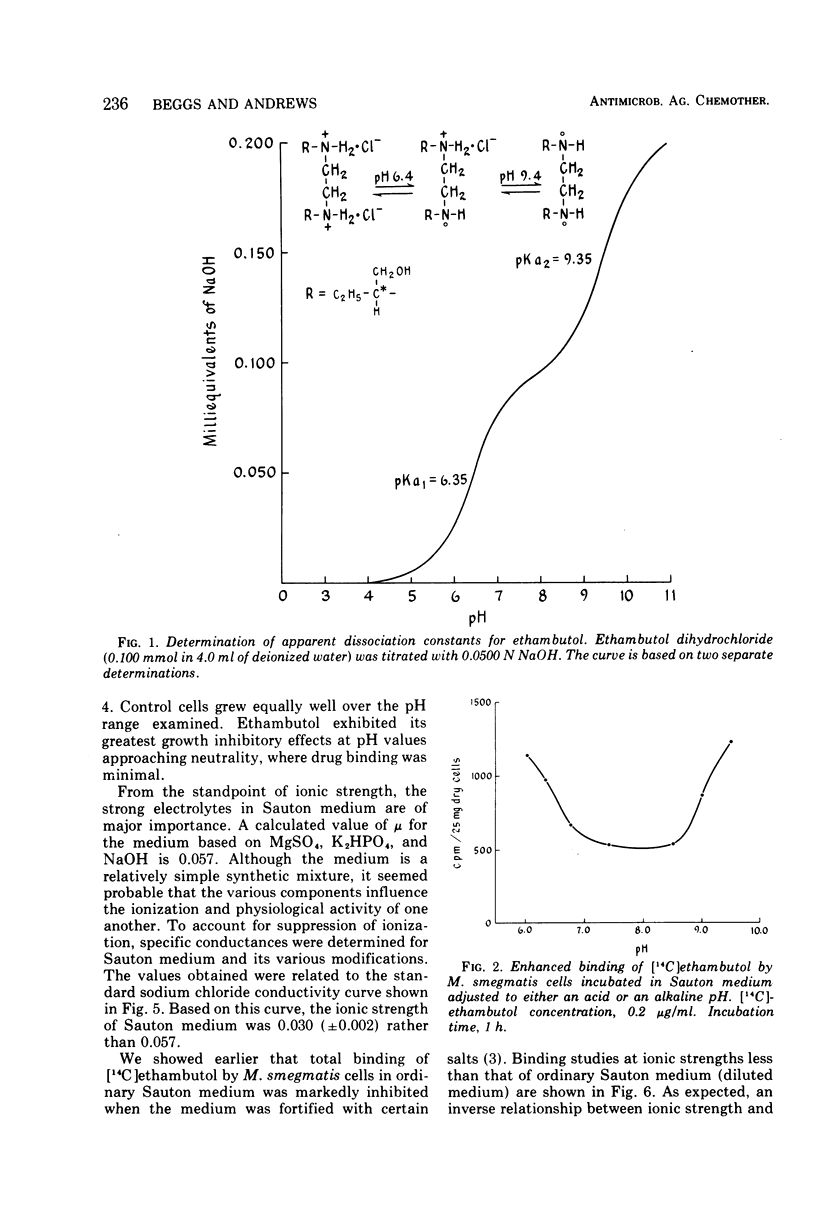
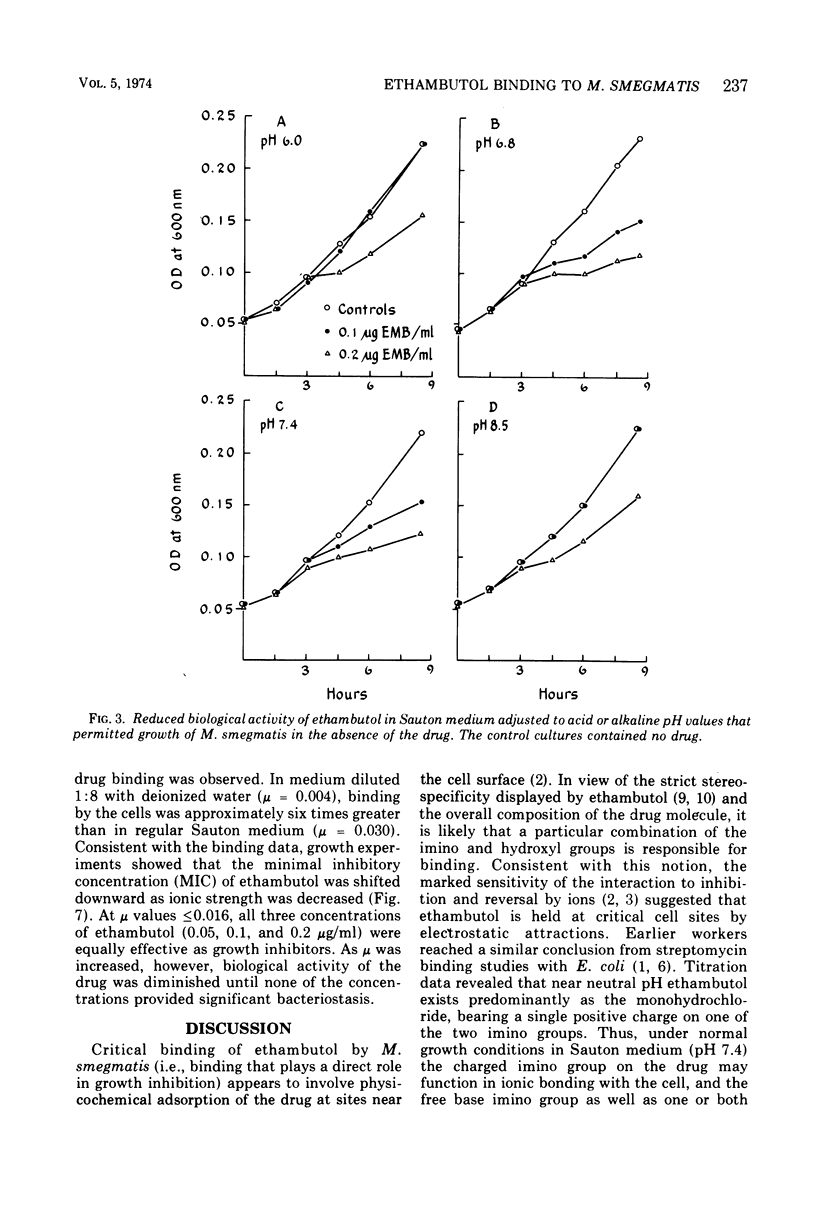
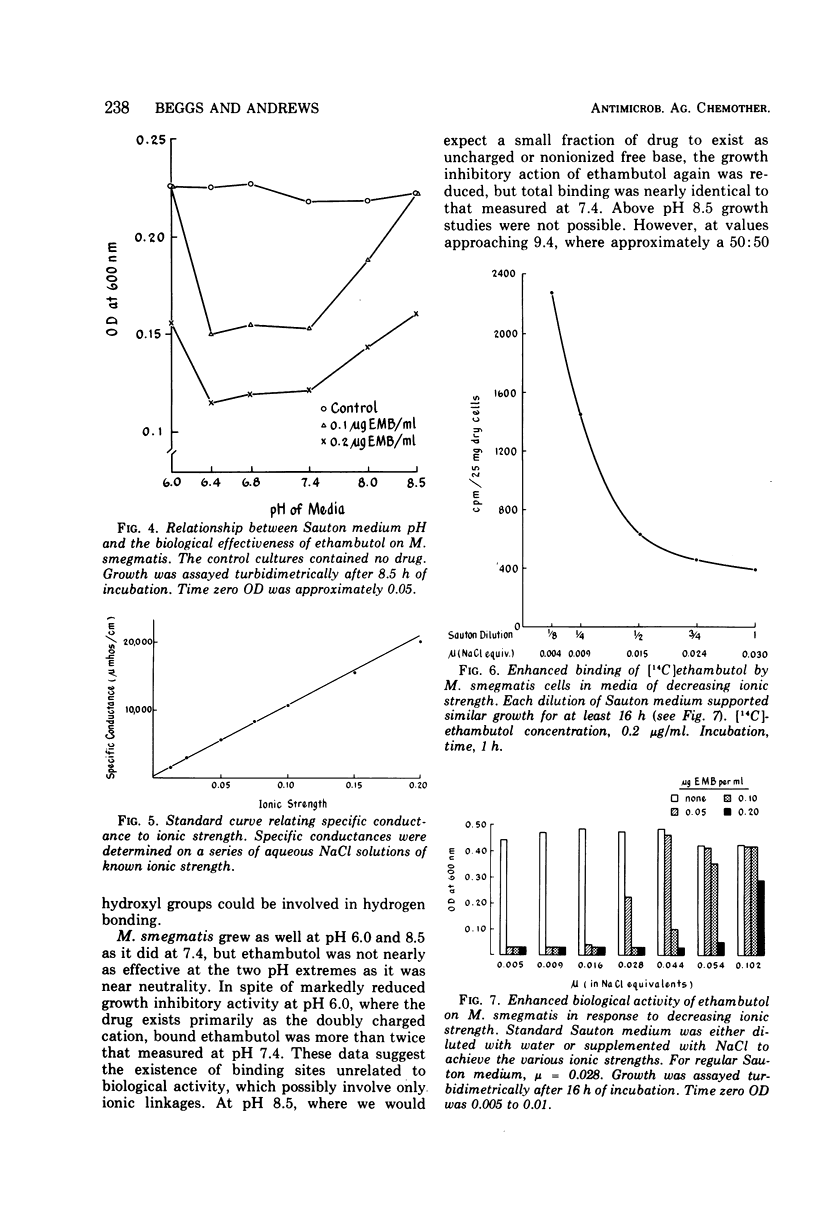
Recent studies showed that critical binding of ethambutol to Mycobacterium smegmatis was both inhibited and reversed by ions. This apparent ion-susceptible characteristic suggested that ethambutol is held at critical sites by electrostatic bonds. Dissociation constant, pH, and ionic strength studies were designed to further characterize ethambutol binding. M. smegmatis was grown in Sauton synthetic liquid medium (pH 7.4) under aerated conditions at 37 C. The pH or ionic strength of the medium was modified to meet the needs of particular experiments. Titration data revealed that ethambutol dihydrochloride has two apparent dissociation constants (pKa1 = 6.35, pKa2 = 9.35). Uptake experiments, in which pH was varied, showed that dihydrochloride and free base ethambutol were bound to a greater extent than the monohydrochloride. However, dihydrochloride and free base binding were not related to biological activity. Ethambutol exerted its maximal growth inhibitory effect at pH values near neutrality, where it exists primarily as the monohydrochloride and showed minimal binding. The increased ethambutol binding observed at pH 7.4 in media of lowered ionic strength was consistent with growth studies showing a reduction in the minimal inhibitory growth concentration in such media. However, nonspecific as well as critical binding was enhanced at low ionic strength. We conclude that binding of ethambutol by M. smegmatis involves a heterogeneous group of drug binding sites, only one of which is directly related to biological activity. Although nothing is known about the ethambutol target site itself, critical binding of the drug seems to require the single positively charged monohydrochloride form. Both hydrogen bonds and ionic linkages are probably involved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D., ARMITAGE A. K. Uptake of streptomycin by Escherichia coli. Nature. 1960 Jan 2;185:23–24. doi: 10.1038/185023a0. [DOI] [PubMed] [Google Scholar]
- Beggs W. H., Andrews F. A. Primary binding of ethambutol by Mycobacterium smegmatis. Am Rev Respir Dis. 1973 Oct;108(4):983–984. doi: 10.1164/arrd.1973.108.4.983. [DOI] [PubMed] [Google Scholar]
- Beggs W. H., Andrews F. A. Uptake of ethambutol by Mycobacterium smegmatis and its relation to growth inhibition. Am Rev Respir Dis. 1973 Sep;108(3):691–693. doi: 10.1164/arrd.1973.108.3.691. [DOI] [PubMed] [Google Scholar]
- Beggs W. H., Jenne J. W. Mechanism for the pyridoxal neutralization of isoniazid action of Mycobacterium tuberculosis. J Bacteriol. 1967 Oct;94(4):793–797. doi: 10.1128/jb.94.4.793-797.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. N., Flaks J. G. Binding of dihydrostreptomycin to Escherichia coli ribosomes: characteristics and equilibrium of the reaction. Antimicrob Agents Chemother. 1972 Oct;2(4):294–307. doi: 10.1128/aac.2.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T., HANCOCK R., DAVIS B. D. THE SEQUENCE OF SOME EFFECTS OF STREPTOMYCIN IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 13;74:476–489. doi: 10.1016/0006-3002(63)91390-8. [DOI] [PubMed] [Google Scholar]
- FORBES M., KUCK N. A., PEETS E. A. Mode of action of ethambutol. J Bacteriol. 1962 Nov;84:1099–1103. doi: 10.1128/jb.84.5.1099-1103.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes M., Peets E. A., Kuck N. A. Effect of ethambutol on Mycobacteria. Ann N Y Acad Sci. 1966 Apr 20;135(2):726–731. doi: 10.1111/j.1749-6632.1966.tb45518.x. [DOI] [PubMed] [Google Scholar]



