Abstract
Background
Acetylcholine (ACh) is known to be a key neurotransmitter in the central and peripheral nervous systems, which is also produced in a variety of non-neuronal tissues and cell. The existence of ACh in maxilla in vivo and potential regulation role for osteogenesis need further study.
Results
Components of the cholinergic system (ACh, esterase, choline acetyltransferase, high-affinity choline uptake, n- and mAChRs) were determined in maxilla of rat in vivo, by means of Real-Time PCR and immunohistochemistry. Results showed RNA for CarAT, carnitine/acylcarnitine translocase member 20 (Slc25a20), VAChT, OCTN2, OCT1, OCT3, organic cation transporter member 4 (Slc22a4), AChE, BChE, nAChR subunits α1, α2, α3, α5, α7, α10, β1, β2, β4, γ and mAChR subunits M1, M2, M3, M4, M5 were detected in rat’s maxilla. RNA of VAChT, AChE, nAChR subunits α2, β1, β4 and mAChR subunits M4 had abundant expression (2-ΔCt > 0.03). Immunohistochemical staining was conducted for ACh, VAChT, nAChRα7 and AChE. ACh was expressed in mesenchymal cells, chondroblast, bone and cartilage matrix and bone marrow cells, The VAChT expression was very extensively while ACh receptor α7 was strongly expressed in newly formed bone matrix of endochondral and bone marrow ossification, AchE was found only in mesenchymal stem cells, cartilage and bone marrow cells.
Conclusions
ACh might exert its effect on the endochondral and bone marrow ossification, and bone matrix mineralization in maxilla.
Electronic supplementary material
The online version of this article (doi:10.1186/0717-6287-47-72) contains supplementary material, which is available to authorized users.
Keywords: Non-neuronal cholinergic system, Maxilla, Real-Time PCR, Immunohistochemistry
Background
Acetylcholine (Ach) acting solely as neurotransmitter has been revised by findings published both early and late in the last century which all demonstrated the existence of Ach in non-neuronal systems [1, 2]. Cholinergic communication and regulation are established from the beginning of life, that is, in primitive uni- and multicellular organisms such as bacteria, algae, protozoa, sponge and primitive plants and fungi [3–5].
Increasing evidence indicates that ACh acts as an paracrine and autocrine signaling molecule which controlling basic cell functions, such as proliferation, dif ferentiation, cell–cell contact, immune functions, secretion, and absorption in non-neuronal cell including epithelial, endothelial, mesothelial, immunocompetent, and smooth muscle cells [2, 6, 7]. Non-neuronal cells possess cholinergic components uptake choline by means of the high affinity choline transporter (CHT1) [8], and then synthesize ACh by choline acetyltransferase (ChAT) [9] from choline and acetyl-coenzyme A (acetyl-CoA).
ACh is translocated into small synaptic vesicles by vesicular ACh transporter (VAChT), and released via exocytosis [10]. Once released, ACh exerts its cellular functions via nicotinic acetylcholine receptors (nAChRs), including 16 subunits (α1, α2, α3, α4, α5, α6, α7, α9, α10, β1, β2, β3, β4, γ, δ and ϵ) and muscarinic acetylcholine receptor (mAChRs), which including 5 subtypes (M1, M2, M3, M4 and M5). Finally, ACh is rapidly degraded into choline and acetate by acetylcholinesterase and butyrylcholinesterase (AChE and BChE) [11].
As described in the non-neuronal cholinergic systems of other cell types and tissues, the presence of all the necessary molecular components of ACh synthesis, release, reception, degradation, and reuptake existed in mouse and human osteoblast-like cells [12]. Osteoblasts express specific acetylcholine receptors and cholinergic components, and ACh plays a potential role in regulating the proliferation and differentiation of osteoblasts [13]. nAChRs include the α2 nAChRs subunit have a key role in regulating skeletal remodeling mainly through an inhibitory tone of bone resorption [14]. However, whether Ach existed in bone in vivo is still undetermined. In this study, we studied the existence of ACh in maxilla in vivo and their potential regulation role for osteogenesis.
Results and discussion
The morphology of maxilla
Essentially, mammalian bones are in the form of 2 different ways: long bones via endochondral ossification; and flat bones via intramembranous ossification. Orofacial bone is mainly formed via intramembranous ossification [15, 16]. These bony types show considerable differences in protein composition [17]. The morphology of maxilla harvested in this study showed in Figure 1A. A complete palatal shelf structure in a normal SD rat is shown in Figure 1B. The bony plates were interposed between two layers of soft tissue, the nasal and oral mucosa. The palatine vessels and nerves formed large bundles and were housed in bilateral concavities in the bone, and a single layer of osteoblasts lined the oral and nasal surfaces of the bone.
Figure 1.
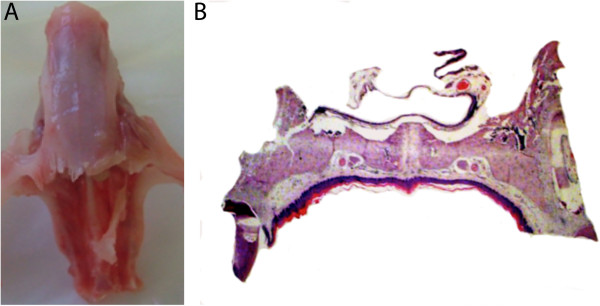
Maxilla from a normal SD rat: A, Maxilla specimen for PCR analysis; B, The palatal shelf structure of maxilla.
Real-Time PCR analysis of the components of non-neuronal cholinergic system in maxilla
We screened RNA for the expression of all non-neuronal cholinergic system components, including esterase, choline acetyltransferase, high-affinity choline uptake, organic cation transporters, carnitine/acylcarnitine translocase, n- and mAChRs. Result showed CHT1, ChAT, OCT2, SLC25a29 and nAChR subunits α4, α6, α9, β3, δ, ϵ were not detected in maxilla. While the RNA of AChE, BChE, CarAT, carnitine/acylcarnitine translocase member 20 (Slc25a20), VAChT, OCTN2, OCT1, OCT3, organic cation transporter member 4 (Slc22a4), nAChR subunits α1, α2, α3, α5, α7, α10, β1,β2, β4, γ and mAChR subunits M1, M2, M3, M4, M5 were detected in rat’s maxilla.
The RNA of VAChT, AChE, nAChR subunits α2, β1, β4 and mAChR subunits M4 had abundant expression (2-ΔCt > 0.03) (Figure 2). And RNA of CarAT, OCT1, nAChR subunits α1, α5, α7, α10, mAChR subunits M2, M3 had less abundant expression(0.005 ≤ 2-ΔCt ≤ 0.03), and the other components had low expression level (2-ΔCt < 0.005).
Figure 2.
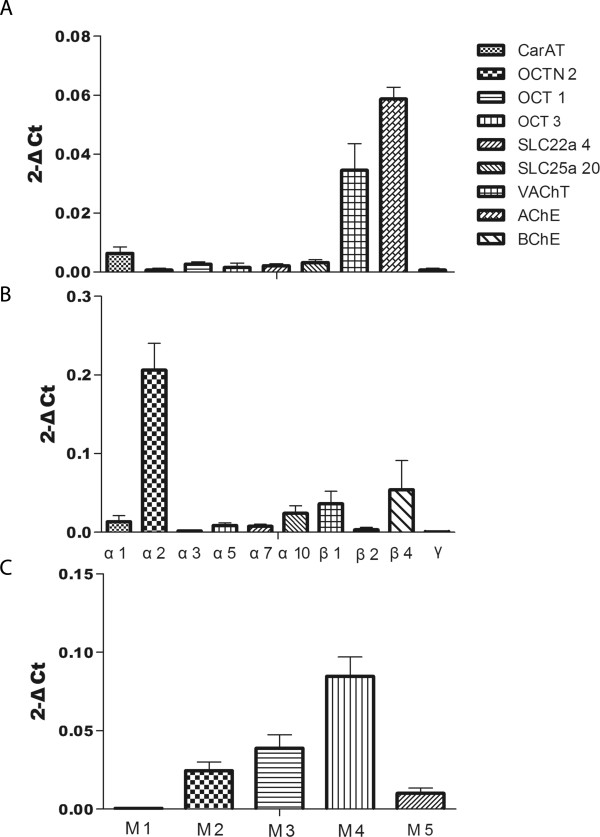
Gene expression of the non-neuronal cholinergic system: A, The expression of synthesis transport and degradation systems, including CarAT, OCTN2, OCT1, OCT3, SLC22a4, SLC25a20, VAChT, AChE, BChE; B, The expression of nAChR subunits, including α1, α2, α3, α5, α7, α10, β1,β2, β4 and γ; C, The expression of mAChR subunits, including M1, M2, M3, M4 and M5.
Immunohistochemistry of ACh, VAChT, nAChRα7 and AChE in maxilla
Traditional methods for the detection of acetylcholine were high performance liquid chromatography (HPLC) and micro dialysis, but location of acetylcholine could not be determined by these methods. Antibodies of choline-protein conjugates introduced by Lolin et al. provided a potentially method to detect ACh [18]. Later Schlereth et al. found conjugated ACh--glutaryl-BSA (bovine serum albumin) the most immunoreactive immunogen by the injection of different ACh- conjugates into AKR and DBA mice followed by ELISA detection for the affinity and specificity of immune serum [19]. The immunogen of anti-acetylcholine polyclonal antibody used for our immunohistochemistry was also acetylcholine-glutaric anhydride- poly lysine.
Figure 3 demonstrated the immunohistochemistry results. As we can see, ACh was expressed in mesenchymal stem cells, chondroblast, bone and cartilage matrix and bone marrow cells (Figure 3A), this indicates that the maxilla also expressed non-neuronal acetylcholine. The immunohistochemistry results of negative control and positive control of ACh antibodies can be found in Additional file 1: Figure S1. The VAChT was expressed very extensively, but less intense (Figure 3B), which showed that ACh was transported to many parts of the maxilla, and played a part in bone metabolism. As ACh receptor, α7 are strongly expressed in newly formed bone matrix of endochondral and bone marrow ossification (Figure 3C), which showed that ACh must be involved in the formation of bone matrix. The AChE, degrading of acetylcholine, had a narrow distribution, only in mesenchymal stem cells, cartilage and bone marrow cells (Figure 3D), which indicats that ACh might contribute to the metabolism in the maxillary bone of normal rats.
Figure 3.
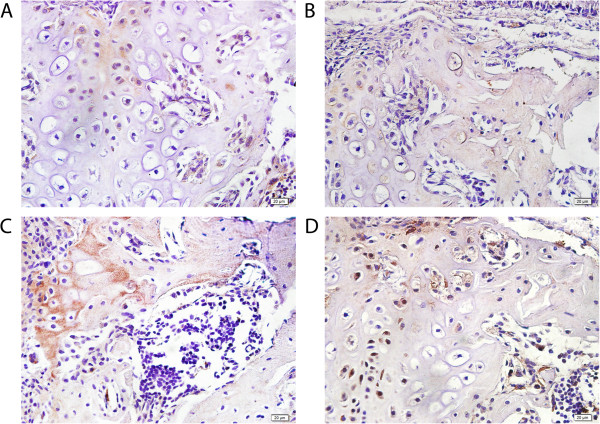
Immunohistochemical results of ACh, VAChT, α7 and AChE in maxilla cells: A, ACh was expressed in mesenchymal stem cells, chondroblast, bone and cartilage matrix and bone marrow cells; B, VAChT was expressed in mesenchymal stem cells, chondroblast and cartilage; C, nAChRα7 was expressed in bone matrix of endochondral and bone marrow ossification, and bone marrow cells; D, AChE was expressed in mesenchymal stem cells, cartilage and bone marrow cells.
ACh was synthesized by practically all living cells and can play an intermediary role in the interactions of non-neuronal cells with the external environment, hormones, growth factors, cytokines and also the neural system [20–23]. Immunohistochemical staining of trabecular bone in the distal femoral metaphysis demonstrated VAChT positive regional in medullary intertrabecular spaces, mainly in the close vicinity of bony struts, one-to-three cell layers away from their surface [14]. The alpha-7 nicotinic acetylcholine receptor (α7-nAChR) was well known as a potent calcium ionophore. Rogers et al. described the expression of α7 during development of the teeth and show that for this nicotinic receptor the distinct spatial and temporal differences in its expression suggested functional pleiotropy in the tooth developmental process [24]. AChE had been detected as nuclear protein in both neuronal and non-neuronal cells [25]. The chondrogenic expression of AChE paralleled the early development of rat lower limbs [26]. AChE fulfilled at least some of the requirements for an osteogenic coordinator.
In maxilla ACh might be produced by carnitine acetyltransferase (CarAT), not choline acetyltransferease (ChAT), which was consistent with some non-neuronal cells such as muscle cells and urothelial epithelial cells [9, 27]. Bajayo et al. [14] demonstrated that VAChT had been expressed in trabecular bone in the distal femoral metaphysic when compared the expression of VAChT mRNA with OCTs’, this indicated that bone transport ACh by VAChT. They also find AChE expressed extremely rich, whereas BChE expressed very little, this illustrated that ACh was degraded mainly by AChE in maxilla.
Sato et al. showed that Nic, an agonist of nAChRs, induced proliferation of osteoblasts [28]. It was demonstrated previously that osteoblasts mainly expressing the muscular type α1, β1, and γ subunits, as well as the neuronal subunits α4, α7, β2, and β4 [14]. But our Real-Time PCR result indicated that many components of nAChRs existed in maxilla, especially the subunits α1, α2, α10, β1 and β4. On the other hand, the ingredients of mAChRs expressed largely, except M1 subunit. Kliemann et al. [29] indicated a significant decrease in relative bone volume, trabecular thickness, trabecular number, bone surface density, and a significant increase in trabecular separation and structure model index of M3R–KO using Micro-CT analysis. All these show that non-neuronal cholinergic system involved in bone formation.
We hypothesized that ACh plays a functional role in bone metabolism. ACh increased the viability, but decreased the proliferation of embryonic stem cells [30]. ACh was expressed in mesenchymal cells, chondroblast, bone and cartilage matrix and bone marrow cells, so we suppose that ACh might participate in the metabolism of the maxilla. ACh had abundant expression in mesenchymal cells, chondroblast and cartilage matrix, this illustrating that ACh might be related with membrane bone and cartilage mineralization. In addition, ACh has been expressed in bone matrix and bone marrow cells, so, we hypothesized that acetylcholine had relevant to bone marrow ossification.
ACh, VAChT, nAChR α7 and AChE are expressed in bone marrow mesenchymal stem cells; ACh might participate in the proliferation, differentiation and death of bone marrow stem cells. ACh, VAChT and nAChR α7 are expressed in bone matrix of endochondral and bone marrow ossification, but AChE is not expressed, further illustrated that the non-neuronal cholinergic system participated in osteogenesis and bone matrix mineralization. These four ingredients were expressed in chondroblast and cartilage cells, indicating that the system were concerned with the cartilage cells formation, and might be involved in cartilage ossification.
Conclusions
The non-neuronal cholinergic system is expressed in the maxilla. ACh mainly transported by VAChT, degraded by AChE, and came into play by parts of nAChR and mAChR subunits, major α1, α2, α10, β1, β4 and M2, M3, M4, M5. The immunohistochemistry of 4 constituents (ACh, VAChT, nAChRα7 and AChE) indicating that non-neuronal cholinergic system participated in the proliferation, differentiation and death of mesenchymal stem cells, cartilage and bone marrow cells, worked in the endochondral and bone marrow ossification, and bone matrix mineralization as well. However, the role of non-neuronal cholinergic system still need be further researched.
Methods
Animals and ethics statement
All of the rats were treated according to the ethical regulations defined by the Ethics Committee of Capital Medical University.
Male Sprague–Dawley rats purchased from Research Institute of Drug Inspection of China (n = 9, 7–8 week-old, mean weight of 302.14 ± 8.42 g). All rat were decapitated under anesthesia on 7th day, and the maxilla was harvested (Figure 1A). Specimens from 6 rats were used for Real-Time PCR analysis and specimens from the other 3 rats were used for immunohistochemistry analysis.
Real-Time PCR analysis of the components of non-neuronal cholinergic system in maxilla
Real-Time PCR analysis was performed with a Roche 480 Sequence Detection System according to manufacturer’s instructions (ROCHE GROUP, Basel, Switzerland) for validation of gene expression of the cholinergic system.
The maxilla from the first to the third molars in rats were harvested free of the overlying soft tissue. Specimens were ground under liquid nitrogen, and then mixed with Trizol solution (Invitrogen, Carlsbad, CA, USA). RNA was treated with DNase I (TAKARA, DALIAN) to eliminate genomic DNA. The yield and purity of RNA was estimated spectrophotometrically using the A260/A280 ratio and agarose gel electrophoresis. RNA was reverse transcribed to cDNA using the superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
The cDNAs were amplified with gene-specific primer pairs (listed in Table 1). Reactions were run on the Roche 480 Real-Time PCR detection system and the results were analyzed using the software supplied with the machine. The running conditions were: incubated at 50°C for 2 minutes and 95°C for 5 minutes, followed by 45 cycles of incubation at 95°C for 15 s and 60°C for 1 minute. The β-actin of rat and 18S gene was used as 2 internal controls. The expression levels of the target genes were correlated to mean of β-actin and 18 s using the 2-ΔCt method.
Table 1.
Sequences and accession numbers for forward and reverse primers used in Real-Time PCR
| Name | Genbank No. | Forward | Reverse |
|---|---|---|---|
| CarAT | NM_001004085 | ATTGTCGCTCTTGTGGACC | TCTGTTTGGCCTTCTCTATGTC |
| OCTN2 | NM_019269 | CGATCCCAGTGAGTTACAAGAC | GAGAAAGTCCGAAGTAGCCC |
| OCT1 | NM_012697 | TGGCCGTAAGCTCTGTCTCT | TCAAGGTATAGCCGGACACC |
| OCT3 | NM_019230 | CAATGGGAAACACCTCTCGT | ATACACCACGGCACTTGTGA |
| SLC22a4 | NM_022270 | CTGGGAGTACAGCAAGGA | GAAGGAGCCACAGAGAAC |
| SLC25a20 | NM_053965 | AACCCATCAGTCCGCTTAAG | TGGTCCCAGAGTACATAGGTG |
| VAChT | X80395 | GCCACATCGTTCACTCTCTTG | CGGTTCATCAAGCAACACATC |
| AChE | S50879 | CTTCTCCCACACCTGTCC | CTGCTTCCTGGTAGAGCC |
| BChE | NM_022942 | ACCTAACCTTGAACACAGAGAAG | TTCCACTCTTGCTCCCTTTC |
| α1 | NM_024485 | GTCACCCACTTTCCCTTCGA | CAGGTCGGGCTGGTCACTT |
| α2 | NM_133420 | CGCTGGTCATCCCACTCAT | GGGAGCGGTGGTGTACATTG |
| α3 | NM_052805 | TCCAGTTTGAGGTGTCCATG | CTTGGTAGTCAGAGGGTTTCC |
| α4 | NM_024354.1 | CGCATCCCCTCTGAACTCAT | CACTGCACCCTTCCGTCATA |
| α5 | NM_017078 | TGGACGCAACCAGCAAACTA | TATGTCCACGAGCCGAATTTC |
| α7 | AY574256 | GCTGGTTCCCTTTTGATG | CTCCGTTGGGGATATAGC |
| α10 | AF196344 | CAGTCTCTCCCCAAAGTG | GAGGTGGGCTTTAGATCC |
| β1 | X74833 | TGGTTGTGGACCGTCTTTTTC | ATGACCGGAGGGTCCTCAAG |
| β2 | L31622 | TTCCTGCTGCTCATCTCCAA | CGCTGGTGACGATGGAGAA |
| β3 | NM_133597.1 | TGGAAACACTCTGCGCTTGA | TCCTGCAGTGGCCGTAAGA |
| β4 | AY574260 | TGCTGGCACTCACGTTCTTC | AAGGTGACCAGCACCATGGT |
| γ | NM_019145 | CACCTACTTCCCCTTCGATTG | ATTCTCTGTGAAAGCCTCGG |
| M1 | NM_080773 | TGGTTTCCTTCGTTCTCTGG | GAGGAACTGGATGTAGCACTG |
| M2 | J03025 | CCACTCCAGAGATGACAACT | GGCTACAACGTTCTGCTTT |
| M3 | M16407 | GGACTGTGGATGTGGAGAG | CGAGGAGTTGGTGTCAGA |
| M4 | NM_031547 | CCCGCCGCACTACTAAGATG | CCTCTTGCCCACCACAAACT |
| M5 | M22926 | CAGCTGCTGCTCACAGACTCA | GGGAAGGAACAGGGCATGAT |
| β-actin | NM_031144 | CTTCAACACCCCAGCCATGT | CAGAGGCATACAGGGACAACA |
| 18S | M11188 | GCGGTTCTATTTTGTTGG | AATGCTTTCGCTCTGGTC |
Hematoxylin and eosin staining of maxilla
The palates from the first to third molars of rats were fixed in 10% formalin solution for 48 h. The blocked tissues were demineralized in 10% (w/v) ethylenediaminetetra-acetic acid (EDTA) for 14 days at 4°C. Routine paraffin embedding procedures were performed. Sections (5 μm thick) were cut and mounted on poly-L-lysine–coated glass slides. Before staining, sections were incubated at 60°C for 1 h and then held in xylene and rehydrated through a series of ethanol solutions. Sections were stained with hematoxylin and eosin to observe the histological morphology. The staining imaging was captured using an OLYMPUS BX61 (Tokyo, Japan) optical microscope.
Immunohistochemistry of Ach, VAChT, nAChR α7 and AChE
Prepared sections were reacted with anti-ACh (Chemicon), anti-VAChT (Abcam), anti-α7 nAChR (Abcam) and anti-AChE (Novus) antibodies. An immunohistochemical staining secondary antibodies kit (Maixin Biotechnology, Inc., Fuzhou China) was used according to the manufacturer’s instructions, and then colored with DAB (Maixin Biotechnology, Inc., Fuzhou China). Sections were counterstained with hematoxylin before they were covered. Immunohistochemical staining imaging was captured using an OLYMPUS BX61 (Tokyo, Japan) optical microscope.
Statistical analysis
Data were expressed as mean ± standard deviation. The data was subjected to ANOVA analysis. P < 0.05 were considered as significant. Statistical analyses were performed using the SPSS 17.0 software.
Electronic supplementary material
Additional file 1: Figure S1: Negative control and positive control of ACh antibodies. (TIFF 3 MB)
Acknowledgments
The authors thank Capital Medical University and Beijing Stomatological Hospital for technical support. This work was funded by the National Natural Science Foundation of China (No. 81070804), Hubei-MOST KLOS&KLOBME (No. 201101), Shandong Province Science and Technique Foundation, China (No. 2014GSF118093).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JG participated in the design of the study, carried out the Realtime PCR and drafted the manuscript. LW carried out the immunohistochemistry. HX participated in the sample preparation and statistical analysis. XC participated in the design of the study, performed the statistical analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Jie Guo, Email: guojiedr@126.com.
Lue Wang, Email: wangluedr@163.com.
Haihua Xu, Email: xuhaihuapro@126.com.
Xiaoxia Che, Email: xiaoxiache77@163.com.
References
- 1.Pochini L, Scalise M, Galluccio M, Indiveri C. Regulation by physiological cations of acetylcholine transport mediated by human OCTN1 (SLC22A4). Implications in the non-neuronal cholinergic system. Life Sci. 2012;91(21–22):1013–1016. doi: 10.1016/j.lfs.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154(8):1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wessler I, Kirkpatrick CJ, Racke K. The cholinergic ‘pitfall’: acetylcholine, a universal cell molecule in biological systems, including humans. Clin Exp Pharmacol Physiol. 1999;26(3):198–205. doi: 10.1046/j.1440-1681.1999.03016.x. [DOI] [PubMed] [Google Scholar]
- 4.Wessler I, Kilbinger H, Bittinger F, Kirkpatrick CJ. The biological role of non-neuronal acetylcholine in plants and humans. Jpn J Pharmacol. 2001;85(1):2–10. doi: 10.1254/jjp.85.2. [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi Y, Kimura R, Kato N, Fujii T, Seki M, Endo T, Kato T, Kawashima K. Evolutional study on acetylcholine expression. Life Sci. 2003;72(15):1745–1756. doi: 10.1016/S0024-3205(02)02478-5. [DOI] [PubMed] [Google Scholar]
- 6.Wessler I, Kirkpatrick CJ, Racke K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther. 1998;77(1):59–79. doi: 10.1016/S0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- 7.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuda T, Haga T. High-affinity choline transporter. Neurochem Res. 2003;28(3–4):483–488. doi: 10.1023/A:1022809003997. [DOI] [PubMed] [Google Scholar]
- 9.Dolezal V, Tucek S. The synthesis and release of acetylcholine in normal and denervated rat diaphragms during incubation in vitro. J Physiol. 1983;334:461–474. doi: 10.1113/jphysiol.1983.sp014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eiden LE. The cholinergic gene locus. J Neurochem. 1998;70(6):2227–2240. doi: 10.1046/j.1471-4159.1998.70062227.x. [DOI] [PubMed] [Google Scholar]
- 11.Kummer W, Lips KS, Pfeil U. The epithelial cholinergic system of the airways. Histochem Cell Biol. 2008;130(2):219–234. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.En-Nosse M, Hartmann S, Trinkaus K, Alt V, Stigler B, Heiss C, Kilian O, Schnettler R, Lips KS. Expression of non-neuronal cholinergic system in osteoblast-like cells and its involvement in osteogenesis. Cell Tissue Res. 2009;338(2):203–215. doi: 10.1007/s00441-009-0871-1. [DOI] [PubMed] [Google Scholar]
- 13.Sato T, Abe T, Chida D, Nakamoto N, Hori N, Kokabu S, Sakata Y, Tomaru Y, Iwata T, Usui M, Aiko K, Yoda T. Functional role of acetylcholine and the expression of cholinergic receptors and components in osteoblasts. FEBS Lett. 2010;584(4):817–824. doi: 10.1016/j.febslet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Bajayo A, Bar A, Denes A, Bachar M, Kram V, Attar-Namdar M, Zallone A, Kovacs KJ, Yirmiya R, Bab I. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci U S A. 2012;109(38):15455–15460. doi: 10.1073/pnas.1206061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y. Skeletal morphogenesis during embryonic development. Crit Rev Eukaryot Gene Expr. 2009;19(3):197–218. doi: 10.1615/CritRevEukarGeneExpr.v19.i3.30. [DOI] [PubMed] [Google Scholar]
- 16.Tuan RS. Biology of developmental and regenerative skeletogenesis. Clin Orthop Relat Res. 2004;427(427 Suppl):S105–S117. doi: 10.1097/01.blo.0000143560.41767.ee. [DOI] [PubMed] [Google Scholar]
- 17.van den Bos T, Speijer D, Bank RA, Bromme D, Everts V. Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: Special emphasis on collagen. Bone. 2008;43(3):459–468. doi: 10.1016/j.bone.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Chagnaud JL, Souan ML, Charrier MC, Geffard M. Monoclonal anti-conjugated acetylcholine antibody and immunohistochemical applications in rat nervous system. J Neurochem. 1989;53(2):383–391. doi: 10.1111/j.1471-4159.1989.tb07346.x. [DOI] [PubMed] [Google Scholar]
- 19.Schlereth T, Birklein F, an Haack K, Schiffmann S, Kilbinger H, Kirkpatrick CJ, Wessler I. In vivo release of non-neuronal acetylcholine from the human skin as measured by dermal microdialysis: effect of botulinum toxin. Br J Pharmacol. 2006;147(2):183–187. doi: 10.1038/sj.bjp.0706451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006;26:219–233. doi: 10.1111/j.1474-8673.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 21.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- 22.Grando SA, Kawashima K, Kirkpatrick CJ, Wessler I. Recent progress in understanding the non-neuronal cholinergic system in humans. Life Sci. 2007;80(24–25):2181–2185. doi: 10.1016/j.lfs.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- 24.Rogers SW, Gahring LC. Nicotinic receptor Alpha7 expression during tooth morphogenesis reveals functional pleiotropy. PLoS One. 2012;7:e36467. doi: 10.1371/journal.pone.0036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos SC, Vala I, Miguel C, Barata JT, Garção P, Agostinho P, Mendes M, Coelho AV, Calado A, Oliveira CR, e Silva JM, Saldanha C. Expression and subcellular localization of a novel nuclear acetylcholinesterase protein. J Biol Chem. 2007;282(35):25597–25603. doi: 10.1074/jbc.M700569200. [DOI] [PubMed] [Google Scholar]
- 26.Umezu Y, Nagata N, Doi Y, Furukawa H, Sagara T, Hayashida T, Ogata H, Fujimoto S. Cytochemical and immunocytochemical demonstration of acetylcholinesterase of the prenatal rat lower limb. Arch Histol Cytol. 1993;56(2):217–224. doi: 10.1679/aohc.56.217. [DOI] [PubMed] [Google Scholar]
- 27.Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol. 2007;51(4):1042–1053. doi: 10.1016/j.eururo.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Sato T, Abe T, Nakamoto N, Tomaru Y, Koshikiya N, Nojima J, Kokabu S, Sakata Y, Kobayashi A, Yoda T. Nicotine induces cell proliferation in association with cyclin D1 up-regulation and inhibits cell differentiation in association with p53 regulation in a murine pre-osteoblastic cell line. Biochem Biophys Res Commun. 2008;377(1):126–130. doi: 10.1016/j.bbrc.2008.09.114. [DOI] [PubMed] [Google Scholar]
- 29.Kliemann K, Kneffel M, Bergen I, Kampschulte M, Langheinrich AC, Durselen L, Ignatius A, Kilian O, Schnettler R, Lips KS. Quantitative analyses of bone composition in acetylcholine receptor M3R and alpha7 knockout mice. Life Sci. 2012;91(21–22):997–1002. doi: 10.1016/j.lfs.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Landgraf D, Barth M, Layer PG, Sperling LE. Acetylcholine as a possible signaling molecule in embryonic stem cells: studies on survival, proliferation and death. Chem Biol Interact. 2010;187(1–3):115–119. doi: 10.1016/j.cbi.2010.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1: Negative control and positive control of ACh antibodies. (TIFF 3 MB)


