Abstract
Background
This study was performed to evaluate the effectiveness of surveillance for screening and treatment of patients with chronic kidney disease undergoing hemodialysis and colonized by Staphylococcus aureus.
Methods
A systematic review and meta-analysis were performed. The literature search involved the following databases: the Cochrane Controlled Trials Register, Embase, LILACS, CINAHL, SciELO, and PubMed/Medline. The descriptors were “Staphylococcus aureus”, “MRSA”, “MSSA”, “treatment”, “decolonization”, “nasal carrier”, “colonization”, “chronic kidney disease”, “dialysis”, and “haemodialysis” or “hemodialysis”. Five randomized controlled trials that exhibited agreement among reviewers as shown by a kappa value of >0.80 were included in the study; methodological quality was evaluated using the STROBE statement. Patients who received various treatments (various treatments group) or topical mupirocin (mupirocin group) were compared with those who received either no treatment or placebo (control group). The outcomes were skin infection at the central venous catheter insertion site and bacteremia.
Results
In total, 2374 patients were included in the analysis, 626 (26.4%) of whom were nasal carriers of S. aureus. The probability of S. aureus infection at the catheter site for hemodialysis was 87% lower in the mupirocin group than in the control group (odds ratio [OR], 0.13; 95% confidence interval [CI], 0.05–0.34; p < 0.001). The risk of bacteremia was 82% lower in the mupirocin group than in the control group (OR, 0.18; 95% CI, 0.08–0.42; p < 0.001). No statistically significant difference in bacteremia was observed between the various treatments group (excluding mupirocin) and the control group (OR, 0.77; 95% CI, 0.51–1.15; p = 0.20).
Conclusions
Twenty-six percent of patients undergoing hemodialysis were nasal carriers of S. aureus. Of all treatments evaluated, topical mupirocin was the most effective therapy for the reduction of S. aureus catheter site infection and bacteremia in patients undergoing chronic hemodialysis.
Electronic supplementary material
The online version of this article (doi:10.1186/1471-2369-15-202) contains supplementary material, which is available to authorized users.
Keywords: Staphylococcus aureus, Colonization, Infection, Hemodialysis, Treatment
Background
Staphylococcus aureus is the most frequently isolated pathogen in hospitals worldwide. Approximately 20% of healthy people are chronic carriers of S. aureus, 30% are intermittent carriers, and 50% are not susceptible to carriage for unknown reasons [1].
Staphylococcus aureus infection has become endemic in health care institutions worldwide. Up to 70% of infections occur in such institutions. The reported half-life of colonization may reach 40 months in individuals who do not receive treatment [2]. An estimated 2 million individuals are carriers of S. aureus based on prevalence data in the Netherlands, while an estimated 53 million are carriers in the United States [3].
In one study, the cloning results of S. aureus isolated from blood were identical to those of S. aureus isolated from nasal specimens in 82.2% of patients undergoing hemodialysis (HD). These findings suggest that in these patients, the organisms isolated from the blood originated from the nasal flora [1].
There are few reports on infection and colonization of S. aureus because few countries conduct epidemiological surveillance of this microorganism. Information on colonization can only be obtained when there is an active search for carriers because colonization is asymptomatic. After patients with chronic kidney disease (CKD) undergoing conservative treatment are colonized with S. aureus during hospitalization, these patients may persist as carriers for a prolonged period of time, even after hospital discharge. They may subsequently reintroduce the bacteria into the hospital during another hospital admission [4].
The greatest risk of transmission occurs when the patient is not identified as a carrier. In a retrospective analysis that evaluated information from hospitalizations during an 8-year period, a German hospital evaluated the risk of acquiring S. aureus by patients who shared the same hospital room with other colonized, but unidentified, patients. The study found a 13% incidence of infection by the same strain of S. aureus among colonized patients [5]. Another study evaluated the prevalence of S. aureus on admission and found that 49% of carrier patients would not have been identified without screening on admission [1].
Infectious complications caused by S. aureus are common, and an increased frequency is being observed. Such complications include bacteremia, endocarditis, osteomyelitis, and metastatic abscesses [6]. The overall mortality rate associated with S. aureus bacteremia ranges from 11.9% to 46.5% [7].
Research performed in our institution demonstrated that application of the topical antibiotic mupirocin at the venous catheter insertion site significantly reduced the risk of colonization and bloodstream infection with S. aureus; moreover, the same result was observed in another study conducted in Australia [8, 9]. A systematic literature review and meta-analysis published in 2011 including 8 randomized controlled trials involving 3396 participants demonstrated that nasal decolonization of patients with S. aureus using mupirocin led to a significant reduction in infections caused by this microorganism [10]. However, the emergence of strains resistant to multiple drugs, including mupirocin, should be considered, such as that demonstrated in a study from Spain [11].
Collection of surveillance cultures has been advocated as a way to control the dissemination of multidrug-resistant pathogens. Early detection of patients colonized with multidrug-resistant microorganisms may permit the effective establishment of measures to control cross-transmission [12].
Eradication of the carrier state of S. aureus includes prevention of both infection and transmission. Several eradication strategies have been evaluated, but these studies differed significantly in their design.
To fill these knowledge gaps, we conducted a systematic review to evaluate the effectiveness of surveillance for screening of S. aureus in patients with CKD and determine the most effective intervention with which to eradicate the transmission of this bacterium among patients with CKD undergoing HD.
Methods
This systematic review and meta-analysis follows the steps proposed by the Cochrane Collaboration [13] and is in accordance with PRISMA guidelines statement for systematic review reporting [14]. The inclusion criterion was S. aureus colonization in patients with CKD undergoing HD as the primary outcome. The exclusion criteria were nonrandomized studies, letters, editorials, and case reports; studies involving patients <18 years of age; evaluation of S. aureus infection treatment outcomes without evaluation of the effect of nasal colonization; and no specification of the therapy administered to the treatment group. The interventions compared in this meta-analysis were surveillance for screening of nasal carriers of S. aureus, prophylactic treatment/decolonization to control cross-transmission, and S. aureus infection (bacteremia and skin infection at the catheter insertion site) between treated and untreated patients undergoing HD.
The following characteristics of each study were extracted: study design; total numbers of patients receiving various treatments (various treatments group), mupirocin (mupirocin group), and placebo or control (control group) with corresponding rates of eradication; colonization by S. aureus; duration of surveillance; number of patients with skin infection at the catheter insertion site; and episodes of bacteremia.
Study identification strategy
Relevant studies published from January 1989 to January 2014 were identified through a search of the following electronic databases: the Cochrane Library (including the Cochrane Controlled Trials Register), Embase, LILACS, SciELO, CINAHL, and Medline/PubMed. The principal descriptors used in the search were “Staphylococcus aureus”, “MRSA”, “MSSA”, “treatment”, “decolonization”, “nasal carrier”, “colonization”,”chronic kidney disease”, “dialysis”, and “haemodialysis” or “hemodialysis”.
Study selection
The studies were read by two independent reviewers (C.G. and M.T.) to ascertain whether they fulfilled the inclusion criteria. The reviewers were not blinded. Each reviewer evaluated the titles and abstracts of all identified studies and obtained complete photocopies of all relevant articles. In cases of doubt or disagreement, a third reviewer (D.A.B.) was solicited to issue an opinion regarding whether the study should or should not be included.
Evaluation of methodological quality
Methodological quality was defined as confidence that the study design and reporting were free of bias. Two independent reviewers used the recommendations of the Cochrane framework and the STROBE statement (STrengthening the Reporting of OBservational studies in Epidemiology). Based on the STROBE recommendations [13], studies included in the meta-analysis were divided into three categories: (A) >80% compliance with the STROBE criteria, (B) 50% to 80% compliance with the STROBE criteria, and (C) <50% compliance with the STROBE criteria (Table 1).
Table 1.
Quality of clinical trials included in the present meta-analysis
| Concealment of allocation | Blinded investigator | Blinded participant | Blinded assessor | Blind data analysis | Intention-to-treat | Lost to follow-up |
|---|---|---|---|---|---|---|
| Adequate: 3 | Yes: 3 | Yes : 4 | Yes : 3 | Yes : 0 | Declared : 3 | Yes : 2 |
| Inadequate: 1 | No : 1 | No: 1 | No: 1 | No: 2 | Not declared: 2 | No: 0 |
| Obscure: 1 | Obscure: 1 | Obscure: 0 | Obscure: 1 | Obscure: 3 | Obscure: 3 |
Data extraction and statistical analysis
The studies were initially stratified according to their design. Based on these results, they were subsequently stratified following the Cochrane methodology. Review Manager 5 software, available from the Cochrane Collaboration, was used for statistical analysis. For dichotomous variables, the odds ratio (OR) with 95% confidence interval (CI) was calculated using random-effects and fixed-effects models. The Mantel–Haenszel chi-squared test and the I2 test were used to calculate heterogeneity [15].
Results
PRISMA flow chart in Figure 1 summarises the search process. Initially, 143 articles were identified in the PubMed/Medline database, 54 in SciELO, 32 in Cochrane, 4 in LILACS, and 10 in Embase. Of the 243 total studies identified, 238 were excluded (36 were articles published and duplicated in different databases, 96 met the exclusion criteria, 84 did not present the principal result, 11 did not evaluate nasal colonization by S. aureus, 7 did not report data on population control, and 4 did not report the duration of follow-up).
Figure 1.
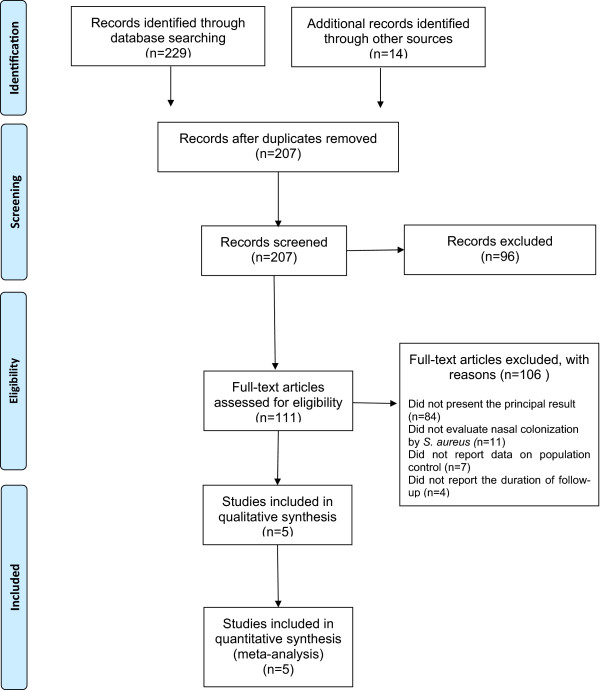
PRISMA flow diagram of systematic review inclusion and exclusion process.
Thus, five studies were evaluated: a prospective double-blind randomized controlled trial [16], three randomized clinical trials [17–19], and one historical cohort [20]. All studies were evaluated and classified as having a low risk of bias and adequate methodological quality by the Cochrane referential [13]. Randomization of the studies included in this review was performed by a computer, and concealment of the allocation was adequate.
These studies involved a total of 2,374 individuals (1,177 patients in the intervention group and 1,197 in the control group). Of these 2374 individuals, 626 (26.4%) were nasal carriers of S. aureus (see Additional file 1).
Based on the STROBE recommendations [13], three studies were placed in category A and two studies were placed in category B. This meta-analysis did not include studies in category C (<50% compliance with the criteria established by STROBE) (Table 1).
Screening ofS. aureus in nasal carriers
One study [20] provided strong evidence of a link between nasal colonization and bloodstream infection caused by S. aureus. In that study, patients who developed infection were transiently recolonized by S. aureus strains identical to the pretreatment colonized strains. The strains were confirmed to be identical by molecular typing and plasma DNA analysis.
Tracking methods that differed in frequency, location, and quantity of samples among the studies were used to identify S. aureus nasal carriers. One study [20] used three nasal samples (one before the intervention, one 3–5 days after the intervention, and one 10 days after the intervention; samples were reevaluated 1, 3, and 12 months after decolonization). Two studies [17, 18] used three to five nasal samples at the beginning and end of treatment. One study [16] measured IgG antibodies for S. aureus type 5 and 8 capsular polysaccharides for 2 years. Finally, one study [19] used nasal samples at the beginning of the study and upon suspicion of infection. Patients with three consecutive negative swabs were considered to be decolonized in most of the studies.
Treatment/decolonization to control cross-transmission
Different interventions were evaluated, including topical antimicrobial agents applied at the catheter insertion site (2% mupirocin calcium ointment [17, 19] and 10% povidone-iodine solution [17, 18]) or in the nasal region (2% mupirocin calcium ointment [20]), bolus injection of cefotaxime through a central venous catheter [CVC] [19], and administration of an S. aureus vaccine containing type 5 and 8 capsular polysaccharides conjugated to nontoxic recombinant proteins [16] (see Additional file 1).
Mupirocin was compared with placebo with respect to eradication of colonization by S. aureus in three studies [17, 18, 20]. In total, 494 patients were evaluated (246 in the mupirocin group and 248 in the control group). Of all 494 patients, 147 (30%) were nasal carriers of S. aureus. The duration of treatment ranged from 140 to 570 days. Eradication of the nasal carrier state was successful in approximately 98% (range, 96–100%) of patients receiving mupirocin after 30 to 90 days of monitoring. One study [19] did not provide information about nasal colonization by S. aureus in the control group, and between-group comparison was therefore not possible. However, eradication of nasal colonization by S. aureus in the mupirocin group was 96% in that study.
The lack of heterogeneity in the results (Q, 0.01; I2, 0.00; p = 0.92) indicates that the probability of S. aureus nasal colonization in patients undergoing HD was 93% lower in mupirocin-treated patients than in untreated or placebo-treated patients [17, 18] (OR, 0.05; 95% CI, 0.01–0.27) (Figure 2).
Figure 2.
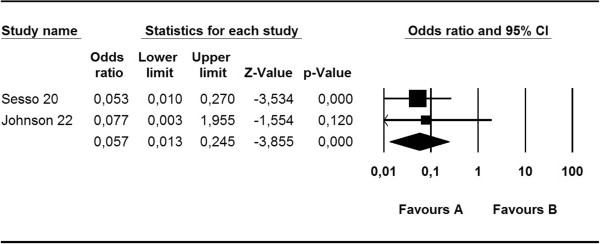
Meta-analysis of mupirocin versus control: eradication of S. aureus nasal colonization in hemodialysis patients.
Other treatments were encountered less frequently. Administration of a povidone-iodine solution eradicated nasal colonizationin 62% of carriers of S. aureus after 30 days of catheter placement [16], bolus catheter injection of cefotaxime eradicated methicillin-resistant S. aureus in 76% (n = 26/34) of patients [19], and administration of a conjugate vaccine containing S. aureus type 5 and 8 capsular polysaccharides offered protection against bacteremia by S. aureus in 90% of patients with antibody levels of ≥80 pg/mL (the estimated minimum level of protection) for up to approximately 40 weeks [16].
The acquisition of mupirocin resistance during treatment was reported in one study. Boelaert et al. [20] identified a strain of high-level mupirocin-resistant S. aureus (minimum inhibitory concentration of >512 yg/mL) after 19 months of mupirocin application among 29 strains isolated from patients colonized with S. aureus. The adverse events attributable to mupirocin use were mild and did not lead to discontinuation of treatment [17, 19, 20]. The incidence of local reactions such as malaise and myalgia was significantly higher in patients who received the vaccine than in patients in the control group, but they were mild or moderate and resolved within 2 days [16].
Table 2 provides details regarding author, year of publication, study population, total number of patients included, number of patients colonized with S. aureus (at entry into the study), number of patients with skin infection at the dialysis catheter insertion site, and bacteremia (see Additional file 1).
Table 2.
Tracking and management of patients with chronic kidney disease colonized with S. aureus undergoing hemodialysis through a central venous catheter
| Author | Year | Design | Patient (n) | NCSA (n/%) | Treatment | Follow up | Eradication | SIIS (n) | Bacteremia (n) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treated group | Control group | Treated group | Control group | Treated group | Control group | Treated group | Control group | Treated group | Control group | Treated group | Control group | ||||
| Hinefield16 | 2002 | DBRCT | 892 | 906 | 197 (22%) | 200 (22%) | Vaccine | Placebo | 17.5 months | 17,2 months | 86% | NT | NT | 37 | 49 |
| Sesso20 | 1998 | RCT | 69 | 67 | 28 (41%) | 27 (40%) | Mupirocin CIS 3x/s | CIS | 5 months | 96% | 5 | 24 | 2 | 11 | |
| Povidine iodine | |||||||||||||||
| Johnson22 | 2002 | RCT | 27 | 23 | 6 (22%) | 6 (26%) | Mupirocin CIS 3x/w | Placebo | 9 months | 76% | 0 | 5 | 1 | 5 | |
| Saxena23 | 2012 | RCT | 39 | 43 | 39 (100%) | 43(100%) | Catheter bolus cefotaxime | Catheter bolus heparin | 12 months | 100% | 7 | 9 | 12 | 22 | |
| Boelaert30 | 1993 | HC | 150 | 15 | 80 (53%) | 0 (0%) | MUpirocin nasal3x\s | No treatment | 18 months | 96% | NT | NT | 4 | 18 | |
Note: n. number of patients; % Percentage; NCSA nasal carrier of S. aureus; SIIS: skin infection at the central venous catheter insertion site; DBRCT: Double blind randomized controlled trial; RCT: randomized clinical trial; HC: historical cohort; NT, not tested; CIS: catheter insertion site; 3x/w: three times per week.
Prevention of skin infection at catheter insertion site and S. aureus bacteremia in patients undergoing HD
Figure 3 shows that the probability of S. aureus skin infection at the CVC insertion site for HD was 87% lower in the mupirocin group than in the control group (OR, 0.13; 95% CI, 0.05–0.34; p = 0.000). There was no significant heterogeneity among the studies (Q, 0.01; I2, 0.00; p = 0.92).
Figure 3.
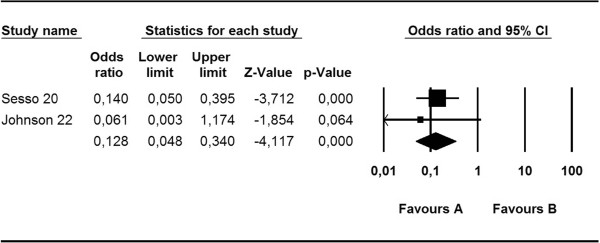
Meta-analysis of mupirocin versus control: S. aureus skin infection at catheter insertion site.
As shown in Figure 4, the risk of bacteremia in patients undergoing HD using a CVC was 82% lower in the mupirocin group than in the control group (OR, 0.18; 95% CI, 0.08–0.42. p < 0.0001). There was no significant heterogeneity among the studies (Q, 0.01; I2, 0.00; p = 0.92).
Figure 4.

Meta-analysis of mupirocin versus control: risk of S. aureus bacteremia.
Finally, no significant difference was observed in S. aureus bacteremia in patients undergoing HD with CVCs between the various treatments group (excluding mupirocin) and the control group (OR, 0.77; 95% CI, 0.51–1.15; p = 0.198) (Figure 5).
Figure 5.
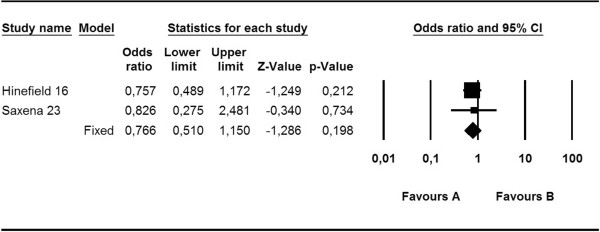
Meta-analysis of diverse treatments versus control: risk of S. aureus bacteremia.
Discussion
Staphylococcus aureus is the leading nosocomial pathogen worldwide [3]. Infection by S. aureus is associated with high morbidity and mortality rates [21]. Effective prevention strategies are essential because of the serious sequelae of this infection. Prevention of S. aureus infection has traditionally been focused on minimizing cross infection. However, it has been repeatedly shown that a high proportion of S. aureus infections originate from the nasal flora [3].
Nasal carriage of S. aureus is a known risk factor for subsequent infection in patients undergoing dialysis with intravascular devices [3]. The rates of S. aureus nasal colonization, skin infection at the exit of the CVC, and bacteremia were studied in the present review, which involved 2374 patients with CKD undergoing HD, 626 (26.4%) of whom were S. aureus nasal carriers.
Tracking methods for S. aureus nasal carrier identification differed in frequency, location, and quantity of samples among the studies in this meta-analysis. Only two studies [17, 18] used the same screening strategy; therefore, it is not possible to establish a strong, evidence-based, uniform recommendation regarding screening strategies.
In this review, mupirocin administration was the most effective treatment for eradication of the S. aureus nasal carrier state in the medium term (30–90 days); the estimated probability of success using mupirocin compared with no treatment was 93% (OR, 12.07; 95% CI, 0.01–0.40). The total estimated decolonization rate using other treatment methods was 76% from 4 to 40 weeks. The efficacy of mupirocin decreased with a prolonged follow-up period (>90 days). Moreover, a longer follow-up period after a short treatment period resulted in an increased risk of recolonization in other parts of the body [5]. Although S. aureus is found in other parts of the body beyond the nasal mucosa, its removal leads to loss of colonization in other locations such as the hands and skin; this indicates that the other body parts are infected via the nasal colonization [7].
Boelaert et al. [20] provided evidence for a link between nasal colonization and bloodstream infection caused by S. aureus. Patients who developed infections were transiently recolonized by S. aureus strains identical to the strains isolated before treatment; the strains were confirmed to be identical by molecular typing and analysis of DNA from plasma. The related infectious strains in the mupirocin group (n = 16 patients) differed from the original nasal strain. In contrast, four of the six infectious strains in the placebo group (n = 18 patients) were similar to the original nasal strain.
Despite improvements in HD techniques and CVC care, infectious complications remain the leading cause of death in patients undergoing HD [6]. Infection of the CVC site is primarily responsible for death in more than half of these patients [22].
A study involving 156 patients with CKD [6] demonstrated that the risk of bloodstream infection was 1.3 times higher in patients with positive skin cultures at the insertion site of the CVC for HD (relative risk, 1.29; 95% CI, 0.83–1.98; p < 0.001) and that the risk of bacteremia was 30% higher in patients with a positive culture at the site of HD catheter insertion (relative risk, 1.29; 95% CI, 0.83–1.98; p < 0.001).
In this review, mupirocin applied to the catheter exit site in S. aureus carriers was the most effective therapy and reduced the likelihood of skin infection at the catheter exit site by 87% in patients with CKD undergoing HD compared with no treatment or placebo (OR, 00.13; 95% CI, 0.05–0.34; p = 0.000). The risk of bacteremia in these patients was 82% lower in the mupirocin group than in the control group (OR, 00.18; 95% CI, 0.08–0.42; p < 0.0001).
Boelaert et al. [20] reported that the incidence of S. aureus bacteremia was four times lower after nasal decolonization with mupirocin in patients with end-stage renal disease than in the untreated group (0.024 vs. 0.097 per patient-year, p = 0.008). Johnson et al. [18] conducted a clinical trial involving patients undergoing HD and found a significantly lower incidence of bacteremia-related bloodstream infection (7% vs. 35%, p < 0.01) and longer survival duration (108 vs. 31 days, p < 0.05) in patients treated with dermal mupirocin at the CVC exit site three times a week than in untreated patients. Sesso et al. [17] performed a prospective randomized study and found that the proportion of patients with S. aureus skin infection at the CVC insertion site was lower in the mupirocin group than in the untreated group (4.3% vs. 23.9%, p = 0.001). In their study, S. aureus bacteremia was observed in 17 patients: 2 in the mupirocin group (0.71 episodes per 1,000 patient-days) and 15 in the control group (8.92 episodes per 1,000 patient-days; p < 0.001).
An issue of concern when mupirocin is used for long periods is the emergence of mupirocin resistance. However, resistance has not been reported when treatment is limited to mupirocin prophylaxis or healthy patients [3].
Three studies in the present meta-analysis evaluated resistance to mupirocin, and only one showed resistance associated with prolonged therapy. Notably, however, the long-term effect on the development of resistance is unknown because no studies have reported follow-up results of more than two Moreover, the techniques used varied among studies, and mupirocin resistance was not evaluated in most of them.
Recent studies of monitoring techniques, however, have reported that mupirocin-resistant S. aureus infections related to the use of CVCs for HD have led to a reduction in drug efficacy in the long term as well as emergence of resistance and recolonization by S. aureus. These are major causes of morbidity and mortality among these patients [10].
Alternatively, long-term studies have offered other treatment options to achieve S. aureus elimination, such as the proposed use of a conjugate vaccine that confers only partial immunity against S. aureus bacteremia for approximately 40 weeks in patients undergoing HD. After this time period, the protective effect decreases as the antibody levels began to decline [16].
One study showed that long-term cefotaxime blockade of CVCs in patients with concurrent end-stage renal disease and nasal colonization by S. aureus undergoing HD led to a significant overall reduction in the incidence of bacteremia (1.47 vs. 3.44 episodes per 1000 catheter-days, p < 0.001) and associated mortality (10.2% vs. 2.9%, p < 0.05) compared with heparin-only blockade of CVCs. This intervention is more effective against Gram-positive cocci (p = 0.032), including methicillin-sensitive S. aureus (p < 0.05). However, cefotaxime blockade provided no protection against methicillin-resistant S. aureus bacteremia and showed antimicrobial resistance associated with prolonged use [19].
There are some limitations in this meta-analysis. The type and duration of HD access were not provided in most studies. Thus, the differences in these potential risk factors for S. aureus catheter-site infection and bacteremia between cases and controls could not be evaluated. Additionally, the studies did not analyze S. aureus strains by typing or DNA analysis to confirm the association between nasal colonization and infection. Finally, no studies showed the results of a prolonged follow-up; therefore, it was not possible to investigate mupirocin resistance. The number of existing high-quality studies is too small to compare the effects of interventions other than the use of mupirocin. Local protocols and patient education interventions for the prevention of infection were not analyzed in the present study.
Notably, a major obstacle faced by these patients is delayed diagnosis using conventional microbiological culture methods. New screening methods such as real-time polymerase chain reaction allow for the detection of nasal colonization in <2 hours. Identification of nasal carriers is possible before infectious complications arise in this group of patients. Combining these screening methods with a short wait for effective treatment would allow for effective treatment of this group of high-risk patients.
Conclusions
Colonization by S. aureus is common in patients undergoing chronic HD and occurred in 26.4% of such patients in this review. It is thus an important risk factor for the development of infections in these patients.
In this meta-analysis, regular clearance of S. aureus from the nose or use of a screening test to guide antibiotic therapy is recommended because the benefits of mupirocin ointment are relatively short-lived.
Mupirocin ointment effectively reduces the risk of infection at the HD venous catheter insertion site as well as the risk of catheter-related bacteremia. There are reports of resistance to mupirocin, and this should be considered in future studies with prolonged follow-up periods.
No other intervention has proven effective in the long term. Given the large number of patients undergoing HD and the importance of infectious complications in these patients, eradication of the carrier state of S. aureus seems justified. To date, topical mupirocin has exhibited the most proven beneficial effects. However, new alternatives should be further investigated in future randomized clinical trials to guide decisions regarding strategies for prevention of infections in patients undergoing chronic HD.
Electronic supplementary material
Additional file 1: PRISMA checklist. (DOC 64 KB)
Acknowledgement
The authors acknowledge the São Paulo State Research Foundation (FAPESP) for its financial support of this study.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CG was the principal researcher and participated in all steps of the study: development of the project, data collection, revision of the database, data analysis, and drafting and revision of the article. MT participated in development of the project, data analysis and article revision. AB participated in revision of the database and revision of the article. RS helped to compose and revise the article. DAB coordinated and conceived of the study and participated in the project design, supervision of data collection, data analysis, and drafting and revision of the article. All of the authors read and approved the final version of the article.
Contributor Information
Cibele Grothe, Email: cibelegrothe@hotmail.com.
Mônica Taminato, Email: mo_tami@yahoo.com.br.
Angélica Belasco, Email: abelasco@unifesp.br.
Ricardo Sesso, Email: rsesso@unifesp.br.
Dulce Barbosa, Email: dulce.barbosa@unifesp.br.
References
- 1.Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Stud Group. 2001;344(1):11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, Leeuwen WV, Belkum AV, Vebrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–756. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Cheng VCC, Li IWS, Wu AKL, Tang BSF, Ng KHL, To KKW, Tse H, Que TL, Ho PL, Yuen KY. Effects of antibiotics on de bacterial load of meticillin-resistant Staphylococcus aureus colonisation in anterior nares. J Hosp Infect. 2008;70:27–34. doi: 10.1016/j.jhin.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Roberts S, West T, Morris A. Duration of methicillin-resistant Staphylococcus aureus colonization in hospitalised in patients. New Zeland Med J. 2004;117:1195. [PubMed] [Google Scholar]
- 5.Schito GC. The importance of the development of antibiotic resistance in Staphylococcus aureus. Clin Microbiol Infect. 2006;12(Suppl. 1):3–8. doi: 10.1111/j.1469-0691.2006.01343.x. [DOI] [PubMed] [Google Scholar]
- 6.Grothe C, Belasco A, Bettencourt A, Diccini S, Vianna L, Pignatari A, Barbosa D. Lethality of endocarditis due to S. Aureus among patients on hemodialysis. Nephrol Nurs J. 2009;36(6):613–632. [PubMed] [Google Scholar]
- 7.Yoon HJ, Choi JY, Kim CO, Kim JM, Song YG. A comparison of clinical features and mortality among methicillin- resistant and methicillin-sensitive strains of Staphylococcus aureus Endocarditis. Yonsei Med J. 2005;46(4):496–502. doi: 10.3349/ymj.2005.46.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kluytmans JA, Manders MJ, van Bommel E, Verbrugh H. Elimination of nasal carriage of Staphylococcus aureus in hemo- dialysis patients. Infect Control Hosp Epidemiol. 1996;17:793–797. doi: 10.2307/30141172. [DOI] [PubMed] [Google Scholar]
- 9.Taminato M, Fram DS, Grothe C, BelascoI AGS, Barbosa DA. Prophylactic use of Mupirocin in Hemodialysis Central Venous Catheters: A Systematic Review and Meta-Analysis Paul. Acta Paul enferm. vol.25 no.1 2012. [Google Scholar]
- 10.Moore C, Dhaliwal J, Tong A, Eden S, Wigston C, Willey B, McGeer A. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) acquisition in roommate contacts of patients colonized or infected with MRSA in an acute-care hospital. Infect Control Hosp Epidemiol. 2008;29:600–606. doi: 10.1086/588567. [DOI] [PubMed] [Google Scholar]
- 11.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MH, Verbrugh HA. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 12.European Antimicrobial Resistance Surveillance System . EARSS Annual Report 2005: Ongoing Surveillance of S. Pneumoniae, S. Aureus, E. Coli, E. Faecium, E. Faecalis. Bilthoven: The Netherlands: Rijksinstituut voor Volksgezondheid en Milieu; 2006. [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook For Systematic Reviews Of Intervention. Chichester (UK): John Wiley & Sons; 2005. [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting itms for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Rev Man Analyses (RevMan) [computer software] Version 1.0 for Windows, in Review Manager 5.1 Oxford England: The Cochrane Collaboration. 2005. [Links]
- 16.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, Fuller S, Johnson J, Fireman B, Alcorn H, Naso R. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 17.Sesso R, Barbosa D, Leme IL, Sader H, Canziani ME, Manfredi S, Draibe S, Pignatari AC. Staphylococcus aureus prophylaxis in hemodialysis patients using central venous catheter: effect of mupirocin ointment. J Am Soc Nephrol. 1998;9(6):1085–1092. doi: 10.1681/ASN.V961085. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DW, MacGinley R, Kay TD, Hawley CM, Campbell SB, Isbel NM, Hollett P. A randomized controlled trial of topical exit site mupirocin application in patients with tunnelled, cuffed haemodialysis catheters. Nephrol Dial Transplant. 2002;17:1802–1807. doi: 10.1093/ndt/17.10.1802. [DOI] [PubMed] [Google Scholar]
- 19.Saxena AK, Panhotra BR, Al-hafiz AA, Sundaram DS, Abu-Oyun B, Mulhim KA. Cafotaxime-heparin lock prophylaxis against hemodialysis catheter-related sepsis among Staphylococcus aureus nasal carriers. Saudi journal of kidney diseases and transplantation: an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2012;23(4):743–754. doi: 10.4103/1319-2442.98154. [DOI] [PubMed] [Google Scholar]
- 20.Boelaert JR, Van Landuyt HW, Godard CA, Landuyt HWV, Godard CA, Daneels RF, Schurgers ML, Matthys EG, De Baere YA, Gheyle DW, Gordts BZ, Herwaldt LA. Nasal mupirocin ointment decreases the incidence of Staphylococcus aureus bacteremias in hemodialysis patients. Nephrol Dial Transplant. 1993;8:235–239. doi: 10.1093/ndt/8.supp1.43. [DOI] [PubMed] [Google Scholar]
- 21.Fram DS, Taminato M, Ferreira D, Neves L, Belasco AGS, Barbosa DA. Prevenção de infecções de corrente sanguínea relacionada a cateter em pacientes em hemodiálise. Acta Paul Enferm. 2009;22:564–568. doi: 10.1590/S0103-21002009000800024. [DOI] [Google Scholar]
- 22.Grothe C, Belasco AG, Bittencourt AR, Vianna LA, Sesso R, Barbosa DA. Incidence of bloodstream infection among patients on hemodialysis by central venous catheter. Rev Lat Am Enfermagem. 2010;18(1):73–80. doi: 10.1590/S0104-11692010000100012. [DOI] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2369/15/202/prepub
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: PRISMA checklist. (DOC 64 KB)


