Abstract
Background
Nitrosative and oxidative stress play a key role in obesity and diabetes-related mitochondrial dysfunction. The objective was to investigate the effect of curcumin treatment on state 3 and 4 oxygen consumption, nitric oxide (NO) synthesis, ATPase activity and lipid oxidation in mitochondria isolated from liver and kidneys of diabetic db/db mice.
Results
Hyperglycaemia increased oxygen consumption and decreased NO synthesis in liver mitochondria isolated from diabetic mice relative to the control mice. In kidney mitochondria, hyperglycaemia increased state 3 oxygen consumption and thiobarbituric acid-reactive substances (TBARS) levels in diabetic mice relative to control mice. Interestingly, treating db/db mice with curcumin improved or restored these parameters to normal levels; also curcumin increased liver mitochondrial ATPase activity in db/db mice relative to untreated db/db mice.
Conclusions
These findings suggest that hyperglycaemia modifies oxygen consumption rate, NO synthesis and increases TBARS levels in mitochondria from the liver and kidneys of diabetic mice, whereas curcumin may have a protective role against these alterations.
Keywords: Diabetes, Mitochondria, Curcumin, ATPase activity, Nitric oxide synthesis, Lipid oxidation
Background
Obesity and diabetes are serious and growing public health problems that result in reduced life expectancy and increased morbidity due to disease-specific vascular complications. Moreover, there is considerable evidence linking obesity and diabetes with oxidative stress and mitochondrial dysfunction in human and animal models. For instance, serum thiobarbituric acid-reactive substances (TBARS) were elevated, and serum total thiols and superoxide dismutase (SOD) activity were decreased in patients with metabolic syndrome compared with healthy subjects [1]. Likely, systemic oxidative stress was increased in obese children with and without metabolic syndrome [2], and reactive oxygen species (ROS) and malondialdehyde (MDA) were higher in diabetic subjects compared with control subjects [3]. With respect to adenosine-5′-triphosphate (ATP) synthesis, abdominal obesity was associated with reduced mitochondrial ATP and ROS production rates in skeletal muscle of men [4]. Moreover, diabetic patients had reduced ATP synthesis and elevated lipid contents in liver [5], as well as reduced ATP synthesis [6, 7]; and reduced expression of the α-subunit of ATP synthase [8] in skeletal muscle as compared to control subjects.
Mitochondria isolated from the livers of mice that were fed a high-fat diet (HFD) exhibit decreased state 3 respiration, decreased uncoupled respiration, and alterations in cytochrome c oxidase activity and mitochondrial membrane potential [9]. In the HFD-fed mice, there was accumulation of 3-nitrotyrosine and increased sensitivity to nitric oxide (NO)-dependent respiratory inhibition compared with non-obese controls.
To reduce oxidative stress caused by obesity and diabetes, dietary supplementation of antioxidants has been proposed. Different studies have shown that curcumin has antioxidant and anti-hyperglycaemic properties in diabetic and obese animal models [10–13]. It was reported that the activity of erythrocyte antioxidant enzymes superoxide dismutase and catalase were significantly higher (2- and 1.5-fold, respectively), and glutathione peroxidase activity was significantly lower (0.7 fold) in diabetic db/db mice than in nondiabetic db/+ mice. In the db/db mice, these enzyme activities were restored to basal values after curcumin treatment. Curcumin also significantly decreased MDA levels from 479.7 to 393.3 nmol/g haemoglobin in erythrocytes of these mice [12]. Furthermore, in PC12 cells that were exposed to 4-hydroxynonenal, curcumin treatment prevented changes in glutathione levels as well as mitochondrial oxidative damage and respiration and decreased the accumulation of carbonylated proteins and apoptosis [14].
We recently showed that curcumin treatment increased oxygen consumption and significantly decreased lipid and protein oxidation levels in liver mitochondria isolated from HFD-induced obese mice compared with those in the untreated obese mice [15]. In kidney mitochondria, curcumin treatment significantly increased oxygen consumption and decreased lipid and protein peroxidation levels in HFD-induced obese mice when compared with those in untreated obese mice. Curcumin treatment neither had any effect on body weight gain or on mitochondrial NO synthesis.
Therefore, we testing hypotheses of that dietary supplementation with curcumin improve indices of lipid oxidation, oxygen consumption rate, nitric oxide (NO) synthesis and ATPase activity in the liver and kidneys of diabetic db/db mice.
Results
Curcumin decreases body weight in db/db mice
To examine the effect of curcumin on the gain of body weight, the mice were weighted at the beginning of the curcumin treatment and no significant differences were observed between the untreated diabetic and curcumin-treated diabetic mice (35.3 ± 0.9 and 35.6 ± 0.5 g, respectively), both groups of diabetic mice were significantly heavier than wild type mice (Figure 1). At the end of the treatment, curcumin treatment significantly decreased the gain of body weight of the diabetic mice (52 ± 0.9 g) compared with diabetic mice that were not treated (60 ± 1.6 g). The diabetic mice, whether treated with curcumin or untreated, were significantly heavier than the wild type mice (29 ± 0.8 g).
Figure 1.
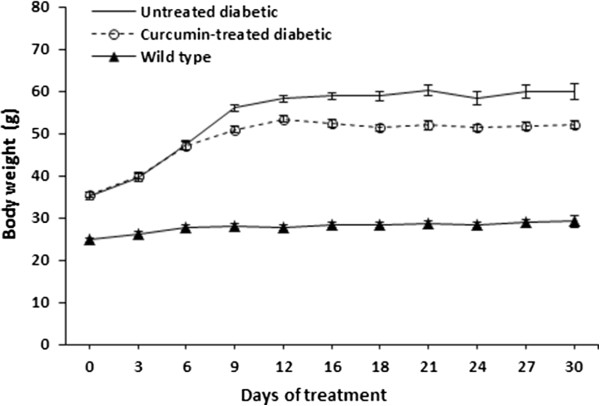
Effect of hyperglycaemia and curcumin on the gain of body weight. Mice were weighted every three days for one month. Wild type mice (n = 4); Untreated-diabetic mice (n = 4); Treated-diabetic mice (n = 9). Data are given as the means ± standard error of the mean (SEM).
Curcumin decreases blood glucose levels in db/db mice
In untreated diabetic mice, blood glucose levels were higher than in the wild type mice (488.2 ± 73 vs. 152 ± 11 mg/ml, p < 0.01). Curcumin treatment decreased blood glucose levels in diabetic mice (196.3 ± 13 mg/ml) as compared to untreated diabetic mice (p < 0.01), but these levels were higher than in wild type mice (p < 0.05). With respect to blood cholesterol levels, these were higher in untreated diabetic mice than in the wild type mice (207 ± 35 vs. 156.5 ± 4.3 mg/ml, p < 0.05); whereas curcumin treatment decreased blood cholesterol levels in diabetic mice (161.4 ± 4 mg/ml) as compared to untreated diabetic mice (p < 0.05). There were no significant differences on blood levels triglycerides among wild-type, untreated diabetic and curcumin-treated diabetic mice (227.7 ± 40.3, 297 ± 59 and 344.8 ± 27 mg/ml, respectively).
Curcumin couples oxygen consumption
In liver mitochondria, state 3 respiration was similar between the diabetic untreated and curcumin-treated mice (40 ± 4.1 and 40 ± 5.2 nmoles O2/mg prot · min, respectively). There was a 66% increase in state 3 mitochondrial oxygen consumption in diabetic untreated and curcumin-treated mouse liver mitochondria compared to mitochondria derived from wt livers (24 ± 5.3 nmoles O2/mg prot · min) (Figure 2A). In mitochondria that were isolated from the diabetic untreated mouse livers, state 4 oxygen consumption was increased from 3.8 ± 0.9 to 9.4 ± 2.4 nmoles O2/mg prot · min, compared with mitochondria from the wt mouse livers, whereas state 4 respiration was 5 ± 0.9 nmoles O2/mg prot · min in mitochondria from the curcumin-treated mouse livers (Figure 2B); no differences were observed between diabetic curcumin-treated and wt mice. Furthermore, in curcumin-treated diabetic mice, state 4 oxygen consumption was decreased by 47% as compared with untreated diabetic mice.
Figure 2.

Effect of hyperglycaemia and curcumin on mitochondrial respiration rate in state 3 (A) and state 4 (B). Black bars, wild type mice (WTM, n = 4); empty bars, untreated-diabetic mice (UDM, n = 4); gray bars, treated-diabetic mice (TDM, n = 9). Data are given as the means ± standard error of the mean (SEM). *p < 0.05 vs. WTM; **p < 0.05 vs. UDM.
The kidney was chosen because is one of the most frequently and severely affected organs. In addition, kidney is an insulin-independent organ in which glucose uptake is facilitated by the SGLT2 transporter, a protein that is abundant in the kidney compared to the liver [16]. As expected, in kidney mitochondria isolated from untreated diabetic mice, the state 3 oxygen consumption rate increased 3-fold compared with wt mice (75 ± 19.4 vs. 24 ± 3.5 nmoles O2/mg prot · min). In kidney mitochondria, curcumin treatment of the diabetic mice decreased state 3 oxygen consumption rates (25 ± 7.4 nmoles O2/mg prot · min) to levels that were similar to those obtained from the wild type mice (Figure 2A). In kidney mitochondria from untreated diabetic mice, state 4 oxygen consumption was two-fold higher compared with wt mice (11.5 ± 1.4 and 6.1 ± 1.6 nmoles O2/mg prot · min, respectively); whereas in kidney mitochondria isolated from diabetic mice, curcumin treatment restored state 4 oxygen consumption (5 ± 1.9 nmoles O2/mg prot · min) to levels similar to mitochondria isolated from wt mouse kidneys (Figure 2B).
Together, these results clearly demonstrate that the curcumin treatment decreased state 3 and 4 oxygen consumption in kidney mitochondria and only state 4 in liver.
Curcumin increases ATPase activity in liver mitochondria
Curcumin treatment decreased oxygen consumption in the liver and kidney; therefore we evaluated the effect of this polyphenol on ATPase activity. ATPase activity is an indirect measurement of the capacity that the mitochondria have to synthesis ATP. In liver mitochondria from untreated diabetic mice, ATPase activity was non-significantly reduced from 2.8 ± 0.24 to 2.05 ± 0.65 nmoles Pi/mg prot · min compared with liver mitochondria from wt mice, whereas curcumin treatment non-significantly increased liver mitochondria ATPase activity by 152% (4.2 ± 0.41 nmoles Pi/mg prot · min) compared with wt mice (Figure 3). Curcumin-treated diabetic mice had significantly higher liver mitochondria ATPase activity than untreated diabetic mice. Kidney mitochondrial ATPase activity was not significantly affected by hyperglycaemia or curcumin; thus, ATPase activity was similar between curcumin-treated diabetic, untreated diabetic and wild type mice (5.8 ± 0.17, 5.3 ± 0.23 and 6.0 ± 0.2 nmoles Pi/mg prot·min) (Figure 3).
Figure 3.
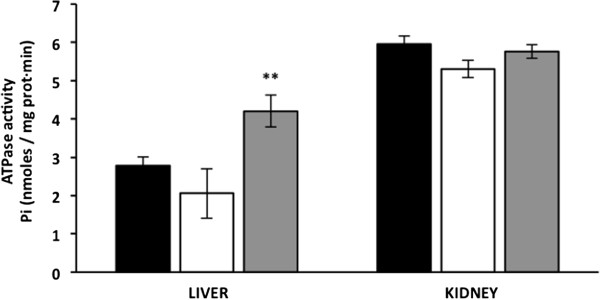
Effect of hyperglycaemia and curcumin on mitochondrial ATPase activity. Black bars, wild type mice (WTM, n = 4); empty bars, untreated-diabetic mice (UDM, n = 4); gray bars, treated-diabetic mice (TDM, n = 9). Data are given as the means ± standard error of the mean (SEM). **p < 0.05 vs. UDM.
Curcumin restores nitric oxide synthesis in liver mitochondria
Mitochondrial NO regulates synthesis of ATP [17, 18] and is altered in diabetes [19]. Therefore, we determined the effect of curcumin on the synthesis of mitochondrial NO in db/db mice. NO synthesis was decreased in liver mitochondria from untreated diabetic mice compared with wt mice mitochondria (0.63 ± 0.1 vs. 2.17 ± 0.37 nmoles/mg prot · min, respectively) (Figure 4); however, in the treated diabetic mice, NO synthesis in liver mitochondria was only reduced by 21% (1.71 ± 0.31 nmoles/mg prot · min) compared with wt mice and was higher than in the untreated diabetic mice.
Figure 4.

Effect of hyperglycaemia and curcumin on mitochondrial nitric oxide synthesis. Black bars, wild type mice (WTM, n = 4); empty bars, untreated-diabetic mice (UDM, n = 4); gray bars, treated-diabetic mice (TDM, n = 9). Data are given as the means ± standard error of the mean (SEM). *p < 0.05 vs. WTM; **p < 0.05 vs. UDM.
Kidney mitochondrial NO synthesis was non-significantly affected by diabetes or curcumin; thus, the values were 0.5 ± 0.1, 0.33 ± 0.05 and 0.28 ± 0.03 nmoles/mg prot · min in untreated diabetic, treated diabetic and wt mice, respectively (Figure 4).
Curcumin decreases lipid peroxidation in liver and kidney mitochondria
Figure 5 shows that in liver mitochondria isolated from the untreated diabetic mice, TBARS values were non-significantly higher than the wt mice (1.6 ± 0.35 and 1.27 ± 0.15 nmoles/mg protein, respectively), whereas curcumin treatment significantly decreased TBARS values (0.86 ± 0.1 nmoles/mg protein) in mitochondria from the diabetic mice compared with the wt and untreated-diabetic mice.
Figure 5.
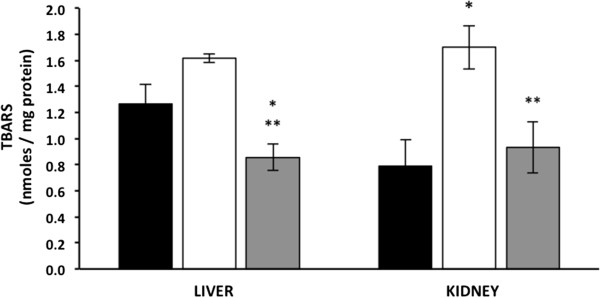
Effect of hyperglycaemia and curcumin on TBARS levels. Black bars, wild type mice (WTM, n = 4); empty bars, untreated-diabetic mice (UDM, n = 4); gray bars, treated-diabetic mice (TDM, n = 9). Data are given as the means ± standard error of the mean (SEM). *p < 0.05 vs. WTM; **p < 0.05 vs. UDM.
In kidney mitochondria, TBARS levels significantly increased in the untreated diabetic mice compared with the wt mice (1.7 ± 0.16 vs. 0.80 ± 0.2 nmoles/mg protein, respectively); however, in the curcumin-treated diabetic mice, TBARS levels were non-significantly higher than (0.93 ± 0.2 nmoles/mg protein) in the wt mice, and were significantly lower than in the untreated diabetic mice.
Discussion
The db/db mouse model develop hyperphagic obesity and nonketotic diabetes similar to non-insulin-dependent diabetes mellitus in humans. Diabetic features in db/db mice follow an age-dependent progression, with early insulin resistance followed by an insulin secretory defect resulting in profound hyperglycemia. Thus, db/db mouse model has provided a useful resource to understand and treat the type 2 diabetic condition. For these reasons, in the present study, diabetic db/db mice were using as diabetes model.
The present results demonstrate that curcumin treatment significantly decreased body weight in db/db mice. It was reported that curcumin decreases body weight by reducing body fat and increasing lean mass in both high-fat-diet-induced obese and ob/ob mice [20].
Using mitochondria isolated from untreated db/db mice, we demonstrated that hyperglycaemia increases mitochondrial oxygen consumption in the liver and kidneys, compared with non-diabetic wild type mice (Figure 2). This finding contrasts with previous observation of mitochondrial dysfunction in liver from these same mice, which was characterized by a reduced respiratory capacity [21]. However, another study, db/db hearts exhibited reduced cardiac function and increased myocardial oxygen consumption; whereas mitochondrial ROS generation and lipid and protein peroxidation products were increased [22]. Thus, heart mitochondria from db/db mice exhibited fatty acid–induced mitochondrial uncoupling and increased expression of fatty acid oxidation genes and electron transfer flavoproteins, whereas the content of the F1 α-subunit of ATP synthase was reduced.
In kidney mitochondria from diabetic mice, curcumin treatment decreased state 3 and 4 oxygen consumption to levels that were similar to that in mitochondria from wild type mice (Figure 2). With respect to liver mitochondria, curcumin treatment only decreased the state 4 oxygen consumption to levels that were similar to that in mitochondria from wild type mice. It is important to consider that glucose uptake in the kidney mainly uses Glut1 transporter and that aerobic oxidation of glucose is the predominant pathway in the kidney; consequently, more redox equivalents are produced in the kidney [23].
We observed that ATPase activity was non-significantly lower in mitochondria from the liver and kidneys of the untreated diabetic mice, whereas treatment of these animals with curcumin significantly increased ATPase activity in the liver but not in the kidneys (Figure 3). Previous studies support that hyperglycaemia decreases ATP synthesis by causing changes in oxygen consumption both in humans and in rodents. For instance, obese and diabetic subjects have a low amount of mitochondrial and reduced capacity for mitochondrial ATP production in skeletal muscle [24–28]. Furthermore, hyperglycaemia decreased functions such as state 3 respiration, electron transport chain activity, ATP synthase activity, mitochondrial membrane potential, ROS production and oxidative damage in the heart of db/db mice [29, 30]. Thus, there is sufficient evidence that obesity and diabetes induce deficiencies in ATP synthesis [4–8, 24–28]. Therefore, the beneficial effect of curcumin treatment on mitochondrial functions suggests that this polyphenol could improve ATP production in the diabetic mouse liver (Figure 3), curcumin also decreases TBARS levels, suggesting that curcumin could reduce the free radicals production or remove these prooxidant molecules. Together, the present results suggest that curcumin increases the ratio of ATP production/oxygen consumption. However, in the present study, we did determine ATPase activity instead of ATP synthesis; for this reason, it is desirable to determine whether curcumin can increase ATP synthesis in vivo.
It has been reported that NO regulates oxygen consumption and consequently ATP production [17, 18]. Our results show that hyperglycaemia decreased NO synthesis in liver and weakly increased this synthesis in kidneys, whereas curcumin restored NO synthesis in both tissues (Figure 4). It is probable that curcumin restores NO synthesis and ATPase activity in liver by preventing the oxidation of the enzymes nitric oxide synthase (NOS) and ATPase or by inducing the expression of these enzymes. Thus, curcumin treatment of db/db mice restored NO synthesis and ATPase activity to wild type levels and also decreased oxygen consumption and lipid oxidation. Therefore, our data suggest that curcumin could be a good alternative to restore the altered mitochondrial NO production that is induced by hyperglycaemia in humans.
Because curcumin treatment restored oxygen consumption, ATPase activity and NO production, we evaluated whether treatment with this polyphenol reduced lipid peroxidation. We observed that diabetic mice had increased mitochondrial TBARS levels that were ameliorated by curcumin treatment both in the liver and in the kidneys (Figure 5). The liver has been demonstrated to have a higher oxidative metabolic rate than the kidneys and to also have increased levels of TBARS [31], which correlated with our results (Figure 5).
Taken together our results suggest that curcumin improves the rate of oxygen consumption as well as ATPase activity probably by scavenging free radicals and preventing protein and lipid oxidation. Given that central role for mitochondria is fuel utilisation and energy production, dysfunctional mitochondria, as are found in obesity and diabetes, can impact whole-body metabolic homeostasis. Mitochondrial uncoupling may be elevated in obesity due to increased FFA concentrations, which may result in the development of diabetes mellitus and/or other metabolic diseases [32, 33]. However, although the association between impaired mitochondrial function and diabetes mellitus is strong, a causal pathogenic relationship remains uncertain. For these reasons, the results presented in this study are important to suggest that hyperglycaemia induces mitochondrial dysfunction, whereas curcumin treatment reverts the lipid oxidation and mitochondrial dysfunction in the liver and kidney of db/db mice that is caused by hyperglycaemia. Our data are consistent with those reported previously in which administration of Resveratrol to db/db mice normalised mitochondrial function and biogenesis [34]. Moreover, curcumin protected brain mitochondria by direct detoxification as well as prevention of 3-nitrotyrosine formation in a mouse model of Parkinson’s disease [35, 36].
Conclusions
Our findings suggest that in db/db mice, dietary supplementation with curcumin diminished mitochondrial dysfunction, likely by decreasing lipid peroxidation, which results in an increase of ATPase activity, restoration of oxygen consumption and NO synthesis in mitochondria isolated from the liver and kidneys of db/db mice. Together, these findings suggest that curcumin could be a good alternative to prevent or decrease nitrosative and oxidative stress as well as mitochondrial dysfunction during obesity and diabetes. Therefore, more research is necessary to elucidate whether curcumin may be effective for prevention and/or treatment of oxidative stress and mitochondrial dysfunction in obese and diabetes humans.
Methods
Animal use and care
Female and male heterozygote non-diabetic db/+ mice (BKS.Cg-m +/+ Leprdb/OlaHsd; Black, Lean) were purchased from Harlan Laboratories (Mexico, DF) and were back-crossed to start our colony. All mice had access to water and food (Harlan Laboratories; Cat. No. T.2018S.15) ad libitum. All animal procedures were performed in accordance with current Mexican legislation (NOM-062-ZOO-1999) and the National Institutes of Health (NIH, Bethesda, MD) Guide for the Care and Use of Laboratory Animals.
Mouse genotypes and curcumin treatment
The mouse genotyping was performed as previously reported with some modifications [37–39]. Briefly, 1 mm of ear tissue was digested in 100 μl lysis buffer [100 mM Tris HCl (pH 8.5), 5 mM EDTA, 0.2% SDS, 200 mM NaCl, and 4 μg proteinase K/ml] overnight at 65°C with agitation, followed by incubation at 95°C for 10 min. Then 100 μl 7.5 M ammonium acetate (pH 2) was added to the lysate prior to centrifugation at 12,000 g for 15 min. The supernatant was recovered and mixed with 300 μl isopropanol followed by centrifugation at 12,000 g for 15 min. The precipitated DNA was washed with 500 μl ethanol, followed by centrifugation at 12,000 g for 15 min. Finally, the DNA was dissolved in 25 μl of water, and 1 μl of the DNA was used as the template for a 12.5 μl PCR reaction with the PCR master kit following the manufacturer’s instructions (ROCHE, Mannheim, Germany). Each primers was used at a concentration of 0.2 μM (forward, 5′-ATGACCACTACAGATGAACCCAGTCTAC-3′; reverse, 5′-CATTCAAACCATAGTTTAGGTTTGTCT-3′), as was previously reported [37–39]. The PCR conditions were as follows: an initial denaturation of 3 min at 95°C followed by 5 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 30 sec; then 30 cycles of 92°C for 15 sec, 50°C for 1 min, and 72°C for 30 sec. Ten microliters of the PCR reaction was digested with the restriction enzyme AccI according to the manufacturer’s instructions (New England Biolabs, Inc.), followed by electrophoresis on a 15% polyacrylamide gel. Digestion with AccI yielded 85- and 24-bp fragments in the wild type (+/+) mice, 85-, 58-, 27-, and 24-bp fragments in heterozygote (db/+) mice, and 58-, 27-, and 24-bp fragments in the diabetic (db/db) mice.
Fourteen-week-old male mice were selected and grouped by genotype. Then 60 mg/kg curcumin was orally administered daily in the morning to 9 db/db mice for 4 weeks; 4 db/db and wt mice were used for sham controls, they received vehicle. Curcumin was dissolved in saline solution and administered using a stainless steel oral cannula. Curcumin was purchased from Sigma-Aldrich (70% purity; Cat. No. 1386. Saint Louis, MO, USA). At the end of the treatment, mice were sacrificed by cervical dislocation; then blood levels of glucose, triglycerides and cholesterol were determined using a commercial kit (Accutrend® GCT, Roche).
Isolation of mitochondria
Liver and kidney mitochondria were prepared by differential centrifugation as we previously described [15].
Oximetry assays
Oxygen consumption was measured at 25°C with an Oxytherm oxygen monitor (Hansatech Instruments Ltd; Norfolk, England) using 0.5 mg/ml mitochondrial protein in 1 ml of oximetry media as we previously described [15]. Briefly, for liver mitochondria, the oximetry media contained 120 mM KCl, 20 mM MOPS, 0.5 mM EGTA, 5 mM NaH2PO4 · H2O, and 10 mM NaCl, pH 7.4. For kidney mitochondria, the oximetry media contained 120 mM KCl, 2 mM EGTA, 10 mM HEPES, 5 mM KH2PO4 · H2O, and 1 mM MgSO4, pH 7.4. In the presence of 15 mM succinate and 2.2 mM rotenone, oxidative phosphorylation (oxygen consumption in state 3) was stimulated with 0.2 mM and 0.1 mM adenosine diphosphate (ADP) for liver and kidney mitochondria, respectively; then oxygen consumption in state 4 was measured when ADP was consumed.
Measurement of NO production
NO production was determined spectrophotometrically by recording the reduction of oxyhaemoglobin (HbO2) by NO to methaemoglobin at 401 nm as we previously described [15].
Determination of ATPase activity
ATPase activity was determined spectrophotometrically by recording the release of Pi from ATP [18]. Briefly, 8 μg of mitochondrial protein in 100 μl of reaction buffer (20 mM Tris-base, 5 mM MgCl2 and 0.1 mM ATP-Tris) were incubated at 25°C for 10 min, followed by addition of 10 μl of 30% trichloroacetic acid. Then 90 μl of water and 10 μl of 10% sodium dodecyl sulphate (SDS) were added, followed by addition of 25 μl of ammonium molybdate solution [11.1 ml of concentrated sulfuric acid and 5 g of molibdato ((NH4)6Mo7O24 · H2O) in 100 ml of water]. Next, 25 μl of ELON solution [0.1 g of 4-(methylamino)phenol hemisulfate salt and 0.3 g of sodium metabisulfite (Na2S2O5) in 10 ml of water] were added, followed by incubation at 25°C for 15 min. The absorbance was recorded at 660 nm and the amount of Pi was determined by a concentration standard curve, using sodium phosphate as standard.
Measurement of lipid peroxidation
Reactive aldehyde levels were quantified with the thiobarbituric acid-reactive substances (TBARS) assay as we previously described [15].
Statistical analysis
Data were analysed with ANOVA followed by Duncan’s multiple range tests. Data are represented as the means ± standard error of the mean (SEM). Values were considered statistically significant if p < 0.05.
Acknowledgements
This work was financially supported by Mexican grants from CONACYT and the University of Guanajuato to JRE; MGSU was a CONACYT scholar.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors have contributed equally to this work. All authors read and approved the final manuscript.
Contributor Information
María G Soto-Urquieta, Email: sugo9883@hotmail.com.
Sergio López-Briones, Email: lobris@yahoo.com.
Victoriano Pérez-Vázquez, Email: vicpe@yahoo.com.
Alfredo Saavedra-Molina, Email: fredisaav2002@yahoo.com.mx.
Gloria A González-Hernández, Email: gonzang@ugto.mx.
Joel Ramírez-Emiliano, Email: joelre@ugto.mx.
References
- 1.Yokota T, Kinugawa S, Yamato M, Hirabayashi K, Suga T, Takada S, Harada K, Morita N, Oyama-Manabe N, Kikuchi Y, Okita K, Tsutsui H. Systemic oxidative stress is associated with lower aerobic capacity and impaired skeletal muscle energy metabolism in patients with metabolic syndrome. Diabetes Care. 2013;36:1341–1346. doi: 10.2337/dc12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faienza MF, Francavilla R, Goffredo R, Ventura A, Marzano F, Panzarino G, Marinelli G, Cavallo L, Di BG. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr. 2012;78:158–164. doi: 10.1159/000342642. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez LJ, Galioto A, Pineo A, Ferlisi A, Ciaccio M, Putignano E, Belvedere M, Costanza G, Barbagallo M. Age, homocysteine, and oxidative stress: relation to hypertension and type 2 diabetes mellitus. J Am Coll Nutr. 2010;29:1–6. doi: 10.1080/07315724.2010.10719810. [DOI] [PubMed] [Google Scholar]
- 4.Chanseaume E, Barquissau V, Salles J, Aucouturier J, Patrac V, Giraudet C, Gryson C, Duché P, Boirie Y, Chardigny JM, Morio B. Muscle mitochondrial oxidative phosphorylation activity, but not content, is altered with abdominal obesity in sedentary men: synergism with changes in insulin sensitivity. J Clin Endocrinol Metab. 2010;95:2948–2956. doi: 10.1210/jc.2009-1938. [DOI] [PubMed] [Google Scholar]
- 5.Schmid AI, Szendroedi J, Chmelik M, Krssák M, Moser E, Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011;34:448–453. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajpeyi S, Pasarica M, Moro C, Conley K, Jubrias S, Sereda O, Burk DH, Zhang Z, Gupta A, Kjems L, Smith SR. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1160–1168. doi: 10.1210/jc.2010-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irving BA, Short KR, Nair KS, Stump CS. Nine days of intensive exercise training improves mitochondrial function but not insulin action in adult offspring of mothers with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:E1137–E1141. doi: 10.1210/jc.2010-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon D, Zorzano A, Nolan JJ. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010;33:645–651. doi: 10.2337/dc09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci. 2005;46:2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256–261. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Seo KI, Choi MS, Jung UJ, Kim HJ, Yeo J, Jeon SM, Lee MK. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 13.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139:919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 14.Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol. 2008;226:161–168. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Morúa A, Soto-Urquieta MG, Franco-Robles E, Zúñiga-Trujillo I, Campos-Cervantes A, Pérez-Vázquez V, Ramírez-Emiliano J. Curcumin decreases oxidative stress in mitochondria isolated from liver and kidneys of high-fat diet-induced obese mice. J Asian Nat Prod Res. 2013;15:905–915. doi: 10.1080/10286020.2013.802687. [DOI] [PubMed] [Google Scholar]
- 16.Perez Lopez G, Gonzalez Albarran O, Cano Megias M. Sodium-glucose cotransporter type 2 inhibitors (SGLT2): from familial renal glucosuria to the treatment of type 2 diabetes mellitus. Nefrologia. 2010;30:618–625. doi: 10.3265/Nefrologia.pre2010.Sep.10494. [DOI] [PubMed] [Google Scholar]
- 17.Sarti P, Lendaro E, Ippoliti R, Bellelli A, Benedetti PA, Brunori M. Modulation of mitochondrial respiration by nitric oxide: investigation by single cell fluorescence microscopy. FASEB J. 1999;13:191–197. doi: 10.1096/fasebj.13.1.191. [DOI] [PubMed] [Google Scholar]
- 18.Saavedra-Molina A, Ramirez-Emiliano J, Clemente-Guerrero M, Pérez-Vázquez V, Aguilera-Aguirre L, González-Hernández JC. Mitochondrial nitric oxide inhibits ATP synthesis. Effect of free calcium in rat heart. Amino Acids. 2003;24:95–102. doi: 10.1007/s00726-002-0331-7. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky SV, Gao S, Li H, Goligorsky MS. Hyperglycemic switch from mitochondrial nitric oxide to superoxide production in endothelial cells. Am J Physiol Heart Circ Physiol. 2002;283:H2130–H2139. doi: 10.1152/ajpheart.00196.2002. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmstrom MH, Iglesias-Gutierrez E, Zierath JR, Garcia-Roves PM. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab. 2012;302:E731–E739. doi: 10.1152/ajpendo.00159.2011. [DOI] [PubMed] [Google Scholar]
- 22.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 23.Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 24.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toledo FGS, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–2147. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 26.Hey-Mogensen M, Hojlund K, Vind BF, Wang L, Dela F, Beck-Nielsen H, Fernström M, Sahlin K. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia. 2010;53:1976–1985. doi: 10.1007/s00125-010-1813-x. [DOI] [PubMed] [Google Scholar]
- 27.Karakelides H, Irving BA, Short KR, O’Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59:89–97. doi: 10.2337/db09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab. 2010;298:E49–E58. doi: 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabkowski ER, Baseler WA, Williamson CL, Powell M, Razunguzwa TT, Frisbee JC, Hollander JM. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes. Am J Physiol Heart Circ Physiol. 2010;299:H529–H540. doi: 10.1152/ajpheart.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-{kappa}B-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85:473–483. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulbert A, Turner N, Hinde J, Else P, Guderley H. How might you compare mitochondria from different tissues and different species? J Comp Physiol B. 2006;176:93–105. doi: 10.1007/s00360-005-0025-z. [DOI] [PubMed] [Google Scholar]
- 32.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 33.Di Paola M, Lorusso M. Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Biochim Biophys Acta. 2006;1757:1330–1337. doi: 10.1016/j.bbabio.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mythri RB, Jagatha B, Pradhan N, Andersen J, Bharath MM. Mitochondrial complex I inhibition in Parkinson’s disease: how can curcumin protect mitochondria? Antioxid Redox Signal. 2007;9:399–408. doi: 10.1089/ars.2006.1479. [DOI] [PubMed] [Google Scholar]
- 36.Mythri RB, Harish G, Dubey SK, Misra K, Bharath MM. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson’s disease. Mol Cell Biochem. 2011;347:135–143. doi: 10.1007/s11010-010-0621-4. [DOI] [PubMed] [Google Scholar]
- 37.Horvat S, Bunger L. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay for the mouse leptin receptor (Leprdb) mutation. Lab Anim. 1999;33:380–384. doi: 10.1258/002367799780487850. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita H, Shao J, Ishizuka T, Klepcyk PJ, Muhlenkamp P, Qiao L, Hoggard N, Friedman JE. Leptin administration prevents spontaneous gestational diabetes in heterozygous Leprdb/+ Mice: Effects on placental leptin and fetal growth. Endocrinology. 2001;142:2888–2897. doi: 10.1210/endo.142.7.8227. [DOI] [PubMed] [Google Scholar]
- 39.Valerio A, Ghisi V, Dossena M, Tonello C, Giordano A, Frontini A, Ferrario M, Pizzi M, Spano P, Carruba MO, Nisoli E. Leptin increases axonal growth cone size in developing mouse cortical neurons by convergent signals inactivating glycogen synthase kinase-3beta. J Biol Chem. 2006;281:12950–12958. doi: 10.1074/jbc.M508691200. [DOI] [PubMed] [Google Scholar]


