Abstract
Chitinases are necessary for fungal cell wall remodeling and cell replication. Methylxanthines have been shown to competitively inhibit family 18 chitinases in vitro. We sought to determine the effects of methylxanthines on fungal chitinases. Fungi demonstrated variable chitinase activity and incubation with methylxanthines (0.5–10 mM) resulted in a dose-dependent decrease in this activity. All fungi tested, except for Candida spp., demonstrated growth inhibition in the presence of methylxanthines at a concentration of 10 mM. India ink staining demonstrated impaired budding and decreased cell size for methylxanthine-treated Cryptococcus neoformans. C. neoformans and Aspergillus fumigatus treated with pentoxifylline also exhibited abnormal cell morphology. In addition, pentoxifylline-treated C. neoformans exhibited increased susceptibility to calcofluor and a leaky melanin phenotype consistent with defective cell wall function. Our data suggest that a variety of fungi express chitinases and that methylxanthines have antifungal properties related to their inhibition of fungal chitinases. Our results highlight the potential utility of targeting chitinases in the development of novel antifungal therapies.
Keywords: Chitinase, Chitin, Anti-fungal, Methylxanthines, Cryptococcus, Aspergillus
Introduction
Chitin, a polymer of β(1,4)-linked N-acetylglucosamine (GlcNAc), is a highly conserved and is essential for structural integrity of the fungal cell wall [1]. The deacetyl derivative of chitin, chitosan, also plays a role in the virulence of some fungi like C. neoformans by linking the capsule to the cell wall [1, 2].
Chitinases are chitin-degrading enzymes produced by viruses, bacteria, fungi, insects, plants, and animals. Fungal chitinases are essential for cell replication and division, and contribute to cell nutrition, morphogenesis, and parasitism [3]. Fungal chitinases appear to be particularly important for cell wall remodeling during periods of growth, replication, and repair [3]. In humans and other mammals, chitinases are thought to play a protective role against chitin containing pathogens [4, 5], but have also been implicated in the pathogenesis of asthma [6, 7].
The methylxanthines, including pentoxifylline, aminophylline, and caffeine, are used to treat a variety of human diseases like asthma, chronic obstructive pulmonary disease, peripheral vascular disease, liver cirrhosis [8], and apnea of prematurity. Methylxanthines are thought to act primarily as phosphodiesterase inhibitors [9], but also act as adenosine receptor antagonists [10] and induce histone deacetylase activity [11]. A recent drug library screening by Rao et al. [12] demonstrated that methylxanthines competitively inhibited the family 18 chitinases in fungi and humans. Likewise, inhibition of Saccharomyces cerevisiae chitinase 1 with a theophylline derivative has been described [13]. In these studies, we sought to determine whether methylxanthines inhibit fungal chitinases and to understand the effects of chitinase inhibition on fungal growth and morphology.
Methods
Organisms and Methylxanthines
The following fungi were used in these studies: C. neoformans (CN), American Type Culture Collection (ATCC) strain 20467; the unencapsulated C. neoformans strain CAP67, C. albicans, ATCC strain 20049; C. parapsilosis strain GA1 (a kind gift of Dr. Joshua Noshanchuk) (13); Alternaria alternata ATCC 20084 and Aspergillus fumigatus ATCC 293 (a kind gift of Dr. Marta Feldmesser). Pentoxifylline (PTX), aminophylline, and caffeine were purchased from Sigma (St. Louis, MO).
Protein Extracts
Protein extracts were prepared as previously described [14]. Briefly, cultures of CN 24067 and C. albicans were grown in YPD (Difco) at 30°C for 3 days. Crude protein extracts were prepared from 1.7 × 109 organisms. Homogenization was performed using a Mini-bead Beater (BioSpec Products) in PBS containing protease inhibitors (Roche, Manheim, Germany). Alternaria alternata was grown in Sabouraud’s dextrose broth for 5 days at 30°C while Aspergillus fumigatus under the same conditions in defined media [15]. Fungi were collected by vacuum filtration and pulverized manually with a pestle in protease inhibitor solution. Supernatants were collected and protein concentration determined by bicinchoninic acid assay (Pierce) [14].
Chitinase Activity
Chitinase bioactivity was measured as previously described [16]. Briefly, 50 ll of protein extracts were incubated with 4-Methylumbelliferyl β-d-N,N′-diacetylchitobioside hydrate (Sigma) in citrate phosphate buffer (pH 5.2). Reactions were stopped with 0.3 M glycine/NaOH buffer (pH 10.6) and fluorescence measured (Perkin Elmer; excitation 355 nm; emission 460 nm). Chitinase activity was extrapolated from a standard curve generated using chitinase from Streptomyces griseus (Sigma). In some experiments, methylxanthines at varying concentrations were incubated with protein extracts at 37°C for 15 min prior to addition of the substrate.
Growth Assays
Turbidity Measurements
For CN 24067, CAP67, and Candida spp., 5 × 104 organisms per well were inoculated on a 96-well plate in 200 μl of either chemically defined media (15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine, and 3.0 μM vitamin B1) or Sabouraud’s dextrose broth with varying concentrations of methylxanthines (0.001–10 mM). The concentrations were determined empirically, based on small pilot studies. Plates were incubated with continuous shaking in an Easy Bioscreen Reader (Oy Growth Curves, Piscataway, NJ) at 37°C or 30°C for CN and 30°C for Candida spp. Absorbance at 600nm was measured every 30 min for 4–5 days. For Aspergillus and Alternaria, organisms were grown for 6–10 days on agar plates. Conidia were harvested in PBS containing 0.025% Tween-20 and diluted to 1.0 × 105 per ml of RPMI 1640 with l-glutamine and without bicarbonate, pH 7.0. PTX was added to conidial suspensions in a flat bottom microtitration plate. Wells containing media only were used to determine background absorbance. Absorbance was measured at 630nm at time 0 and again at 48 h of incubation at 37°C.
Colony-Forming Unit Enumerations
C. albicans, C. parapsilosis, and CN were inoculated into Sabouraud’s dextrose broth or defined media containing different concentrations of methylxanthines and placed in a rotary shaker. CN were grown at 37°C and Candida spp. at 30°C for 3–7 days and an aliquot removed at various times. Organisms were counted with a hemacytometer, appropriately diluted, and plated on Sabouraud’s dextrose agar.
XTT Assay
CN 24067, CAP67, and C. albicans (1 × 104 organisms) were cultured in Sabouraud’s dextrose broth with or without PTX (10 mM). Organisms were cultivated at 37°C (CN) and 30°C (C. albicans) with shaking. After 72 h, the XTT assay was performed as described [17]. For A. fumigatus, 1 × 104 spores were cultured in RPMI (in a 96-well plate) at 37°C for 24 h with varying concentrations of PTX and aminophylline. An aliquot (100 μl) of each culture was combined with XTT salt (1 mg/ml in PBS) and menadione solution (Sigma). Absorbance (492 nm) was measured at 5 h.
Fungal Cell Morphology
CN 24067 were cultured in Sabouraud’s dextrose broth at 37°C for 3 days with or without PTX (10 mM). Non-treated organisms were diluted to produce similar numbers of organisms on microscopy compared with treated cells. Cells were stained with India ink. Forty non-budding organisms of both treated and untreated groups were studied at 100× magnification. Cell diameter and cell capsule thickness were measured using Image J software (NIH, Bethesda, MD). To estimate the budding patterns of CN, 20 random fields at 40× magnification were studied. The percentage of budding cells and presence of one or more buds were also determined.
Fluorescent Microscopy
CN 24067 and A. fumigatus cell structures were visualized with stains specific to the cell wall, capsule, and chitosan as described [18]. CN 24067 and A. fumigatus were cultured in Sabouraud’s dextrose broth at 37°C for 3 days with or without PTX (10 mM). Organisms were washed twice in PBS and suspended in 500 μl of 1% bovine serum albumin in PBS (PBS-BSA) for 30 min at 37°C. Cells were then suspended in 100 μl each of 5 μl/ml solution of Alexa-Fluor 594 conjugated wheat germ agglutinin WGA (Invitrogen, Carlsbad, CA), 25 μM calcofluor white (Sigma), and 20 lg/ml mAb 18B7, and incubated for 30 min at 37°C. The cells were washed in PBS and then suspended in 10 lg/ml fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (Fc specific) antibody. Cells were washed and suspended in PBS. Cell suspensions were mounted on glass slides and analyzed with an Axiovert 200 M inverted microscope (Carl Zeiss Micro Imaging, NY). Confocal images of calcofluor fluorescence were recorded using a multi-channel mode. Z-stack images and measurements were corrected utilizing Axio Vision 4.4 software in deconvolution mode (Carl Zeiss MicroImaging, NY).
Cell Wall Integrity
Sabouraud’s dextrose agar plates containing 1 mg/ml calcofluor white (Sigma) were made. CN 24067 were cultured in Sabouraud’s dextrose broth at 37°C for 3 days with or without PTX (10 mM). Plates were inoculated with 104, 103, 102, and 101 treated and untreated organisms and incubated at 30°C for 4 days. Colony morphology was evaluated as a qualitative assessment of susceptibility to killing by calcofluor in the presence of methylxanthine treatment [19].
Melanization
To determine the effect of PTX on CN 24067 melanization, the organism was grown at 30°C for 10 days in varying concentrations of drug in defined media supplemented with 1 mM l-Dopamine media [20]. In one set of experiments, PTX in the culture media was replenished daily using the same concentration as baseline; while in a second set of experiments, PTX was added only at T0. After 10 days, 1 ml of each culture was aliquoted, spun at 16 G for 45 min, and observed for melanization and growth inhibition.
Results
Chitinase Activity
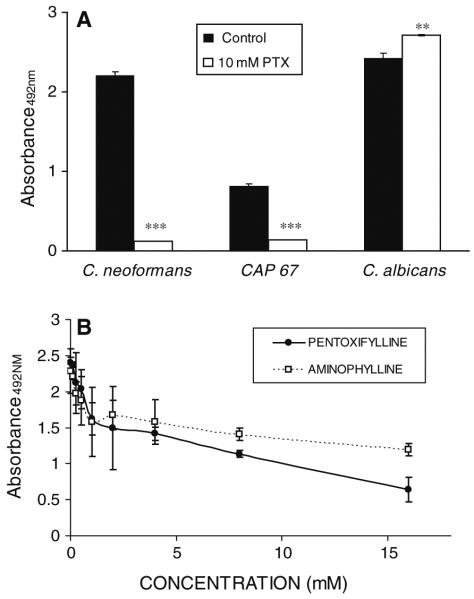
Chitinase activity was detected in the protein extracts of all fungi tested; however, there were differences in basal activity between fungi with A. fumigatus having the highest activity and C. albicans the lowest (Fig. 1a). Co-incubation with PTX produced a dose-dependent decrease in chitinase activity in all fungal extracts tested. Incubation with other methylxanthines (e.g., aminophylline, caffeine) also produced a dose-dependent decrease in the chitinase activity of CN protein extracts (Fig. 1b).
Fig. 1.

Effects of PTX on fungal chitinase activity. a Average chitinase activity in untreated fungal extracts (Control) and fungal extracts treated with (PTX). b Average chitinase activity of CN 24067 treated with aminophylline and caffeine compared to untreated organisms (Control). Activity is expressed milli Units/mg of protein relative to a Streptomyces griseus chitinase standard (Sigma). Bars represent one standard deviation. *P < 0.05 and **P %lt; 0.001 for comparison with untreated fungi (Control)
Fungal Growth Measurements
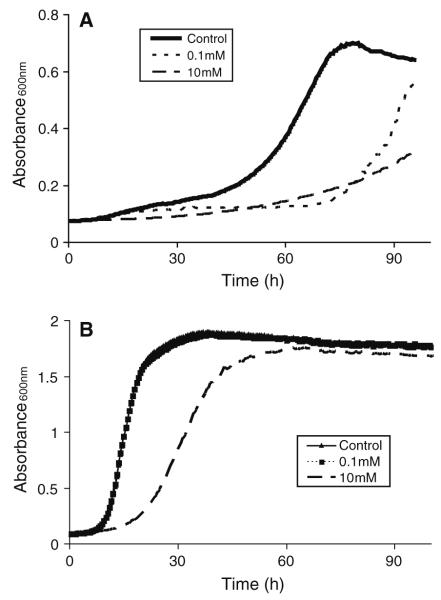
At a concentration of 10 mM, all methylxanthines (PTX, aminophylline, and caffeine) inhibited CN 24067 growth at 37°C in defined media as measured by turbidity (Fig. 2a). Lower concentrations of methylxanthines resulted in a 20–40-h delay in log-phase growth. Growth inhibition was less prominent for CN grown at either 30°C or in Sabouraud’s dextrose broth. Under these conditions, PTX (10 mM) treatment resulted in a delay in log-phase growth (Fig. 2b). In Sabouraud’s media, PTX (10 mM) delayed log-phase growth of CAP67, similar to CN 24067 (not shown). All concentrations of PTX (0.5–10 mM) inhibited A. alternata growth as reflected by an approximately 50% decrease in absorbance readings at 48 h. A similar 50% decrease in absorbance readings was also observed for A. Fumigatus, but only with high doses of PTX (10 mM). For these experiments, average absorbance readings for controls and treated cells were 0.26 ± 0.03 and 0.13 ± 0.01, respectively (P = 0.03). In contrast, no growth inhibition was observed for either C. albicans or C. parapsilosis with any of the methylxanthines (not shown).
Fig. 2.
Effects of PTX on CN 24067 growth. Growth curves were generated from automated turbidity measurements of CN 24067 treated with different concentrations of PTX. Growth was measured in plates incubated with continuous shaking in an Easy Bioscreen Reader (Oy Growth Curves, Piscataway, NJ). Fungi were grown 37°C in defined media (a) at 37°C and Sabouraud’s dextrose broth (b)
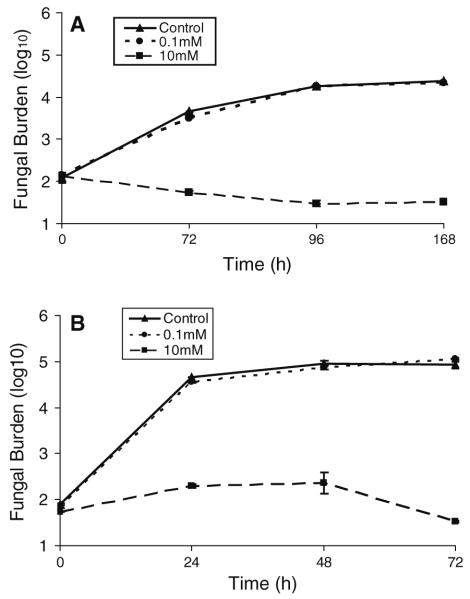
Colony count experiments were performed to confirm that the absorbance readings for all yeasts accurately reflected fungal growth. CN 24067 growth was inhibited by high-dose PTX (10 mM) at all time points, 24, 48, and 72 h (Fig. 3a). CN 24067 grown in defined media had a similar delay in growth for both treated and untreated organisms, and high-dose PTX (10 mM) again inhibited growth completely (Fig. 3b). C. albicans and C. parapsilosis demonstrated no growth inhibition with high- or low-dose PTX (10, 0.1 mM, not shown). Manual colony counts confirmed our findings from automated turbidity measurements that this was a drug class effect with consistent inhibition of growth from pentoxifylline, aminophylline, and caffeine.
Fig. 3.
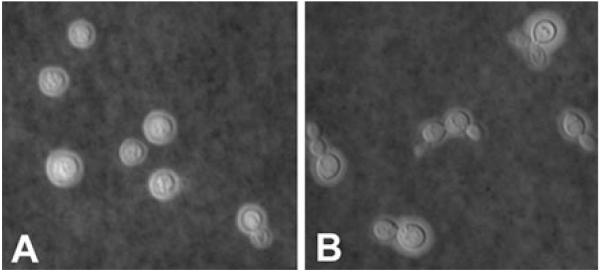
Effects of PTX on CN budding. India ink staining of untreated (Controls, a) and PTX treated CN 24067 (b). PTX treated CN demonstrates increased number of budding cells and increased number of buds per cell. Original magnification, (a and b) ×200
XTT Assay
Absorbance readings and colony-forming unit determinations may not accurately reflect organism numbers due to changes in the physical characteristics of the yeast and changes in the budding pattern. Thus, we chose to measure the metabolic activity of yeasts as a reflection of cell density XTT assay. CN 24067 treated with 10 mM PTX demonstrated a 95% drop in XTT signal compared to untreated organisms (P < 0.001) (Fig. 4a). Likewise, PTX-treated CAP67 showed an 83% decrease in signal activity compared to controls (P < 0.001). In contrast, PTX treatment of C. albicans resulted in a 17% increase in activity (P = 0.01). A. fumigatus treated with either PTX or aminophylline demonstrated a dose-dependent decrease in XTT signal (Fig. 4b).
Fig. 4.
Effects of PTX on CN and C. albicans viability. a Fungal metabolic activity was measured by XTT assay (absorbance 498nm) for untreated fungi (Control) and fungi treated with (PTX). b XTT readings following treatment of A. fumigatus with varying concentrations of PTX or aminophyl line. Average absorbance measurements of 2 different wells are shown. Bars represent one standard deviation, **P < 0.01, ***P < 0.001 for comparison with controls
Fungal Cell Morphology
CN 24067 treated with 10 mM PTX were significantly smaller than untreated organisms with cell diameters of 4.0 ± 0.5 μm and 4.8 ± 0.6 μm, respectively (P < 0.01). PTX had no effect on capsule size (not shown). Approximately 13% of untreated CN were budding, whereas 73% of PTX-treated CN were budding (P < 0.0001) (Fig. 5). None of the untreated CN had more than a single attached bud, while 40% of PTX-treated organisms had multiple buds.
Fluorescent Microscopy
To better appreciate the effects of methylxanthines on fungal structure, CN 24067 were stained using calcofluor white, which labels chitin in the cell wall and wheat germ agglutinin, which labels chitosan [18]. Untreated CN 24067 were spherical with an uninterrupted cell wall and well-healed bud scars. However, CN 24067 grown in 10 mM aminophylline or PTX for 72 h were irregularly shaped and exhibited less-defined bud scarring (Fig. 6a, b). PTX treatment resulted in a change in the distribution of WGA reactivity. Untreated CN demonstrated a diffuse distribution of reactivity over the cell wall surface, whereas PTX-treated cells showed an irregular pattern of WGA staining (Fig. 6c, d). Overall, treated organisms demonstrated a greater degree of cell wall irregularity with incomplete bud detachment and poorly healed bud scars. No differences in capsular staining were noted. Likewise, A. fumigatus treated with 10 mM PTX demonstrated thinner, more delicate hyphae with less extensive branching. Calcofluor staining of treated A. fumigatus was less intense and more irregular compared to untreated organisms (Fig. 6e, f).
Fig. 6.
Effects of PTX on CN and A. fumigatus morphology. Top panels: Calcofluor staining (blue) demonstrates irregular cell wall shape for PTX (10 mM) treated CN 24067 (a) com pared with untreated cells (b). Center panels: WGA staining for chitosan demonstrates decreased and irregular reactivity at the surface of the cell wall for PTX (10 mM) treated CN 24067 (c) compared with controls (d) (original magnification 9365). Bottom panels Calcofluor staining of A. fumigatus reveals thinner hyphae with less extensive branching for PTX (10 mM treatment) organisms (e) compared with controls (f) (original magnification ×200)
Cell Wall Integrity
CN 24067 treated with 10 mM but not 1 mM PTX exhibited poor growth on calcofluor supplemented plates when compared to untreated organisms (not shown).
Melanization
CN 24067 treated with 10 mM PTX formed dark, but significantly smaller pellets when compared to non-treated CN. Of note, the supernatant of treated cultures was significantly darker consistent with leakage of melanin from the cell wall into the supernatant (Fig. 7). Similar results were obtained with daily addition of PTX or a one-time addition of PTX.
Fig. 7.
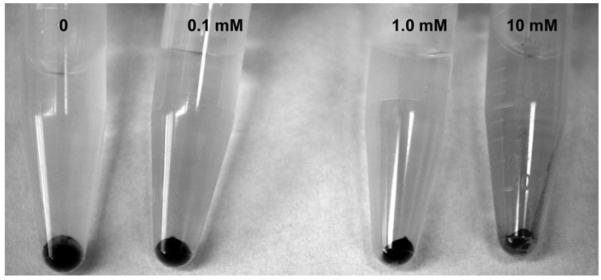
Effects of PTX on melanization of C. neoformans. Pellets and supernatants from CN24067 cultures grown in varying concentrations of PTX for 10 days are shown. Organisms treated with 10 mM of PTX produced a significantly smaller pellet with dark supernatant consistent with release of melanin into the supernatant
Discussion
We document that a variety of genetically diverse fungi including, C. neoformans, A. fumigatus, A. alternata, and C. albicans, constitutively express chitinase activity. Interestingly, there was significant variation in chitinase activity among fungi that could be related to differences in growth rate, mechanisms of growth and replication, the presence of chitin and its precursors in the growth media, or the specificity of the assay for different chitinase types.
We also found that methylxanthines (aminophylline, pentoxifylline, and caffeine) inhibit chitinase activity in a dose-related fashion in these fungi. Fungi with higher baseline chitinase activity like A. fumigatus, C. neoformans, and A. alternata showed an 80–90% inhibition of activity in the presence of methylxanthine. Our results are consistent with those of Rao, who demonstrated competitive binding of methylxanthines (theophylline, pentoxifylline, and caffeine) to Aspergillus chitinases [12]. Candida spp., which had minimal baseline chitinase activity, showed no growth inhibition with PTX treatment. These differences in chitinase inhibition may reflect structural differences among the fungal chitinases and requires additional study. Methylxanthine mediated inhibition of chitinase activity correlated with inhibition of fungal growth as documented by turbidity assays, colony counts, and XTT assays.
Our findings highlight the importance of chitinases in fungal growth and cell remodeling and are consistent with prior studies. In Saccharomyces cerevisiae, the potent chitinase inhibitor demethylallosamidin alters cell septation [21] and disruption of the chitinase gene leads to a defect in cell separation without altering the amount of chitin present [22]. Likewise, deletions in the chitinase genes of C. albicans lead to irregular cell separation [23, 24]. Interestingly, Baker et al. [25] recently demonstrated that genetic deletion of CN chitinases had no effect on asexual growth, but did inhibit sexual reproduction. Furthermore, reduction of chitinase activity in A. fumigatus did not affect morphogenesis [26]. These discrepant results may be explained by the expression of multiple chitinases by fungi that have different roles [27].
To determine the basis of methylxanthine-induced growth inhibition, we studied the effects of these drugs on fungal morphology. CN treated with high-dose PTX demonstrated several findings suggestive of impaired chitin remodeling, including lack of daughter cell detachment, small cell size, and irregular cell shape. Likewise, A. fumigatus treated with methylxanthines exhibited thinner hyphae and less extensive branching. For CN, methylxanthine-induced changes in morphology correlated with functional defects in the cell wall as manifested by increased susceptibility to killing and irregular colonies on calcofluor plates.
One potential explanation for the particular susceptibility of C. neoformans to methylxanthines relates to the polysaccharide capsule [28, 29] and the role chitosan (a chitin derivative) plays in attaching the capsule to the cell wall. Staining for chitosan in treated organisms showed a loss of the punctuate pattern. In addition, we observed leakage of melanin in the supernatant that has also been observed in chitosan-deficient mutants [1]. Nonetheless, we were unable to detect gross differences in the intensity of chitosan staining in PTX-treated organisms. Furthermore, methylxanthine treatment inhibited growth of an acapsular strain of CN, indicating that cell wall-capsule linkage is not the primary target of methylxanthine activity.
While our results suggest the feasibility of chitinase inhibition as an approach to antifungal therapy, there are several caveats regarding the treatment of fungal infections with methylxanthines. Firstly, the concentrations of methylxanthines needed to inhibit fungal growth were relatively high (e.g., 10 mM). We note that these concentrations of methylxanthines are higher than the micromolar concentrations of theophylline derivatives that inhibit S. cervesiae chitinase, and may reflect the particular methylxanthine and conditions under which fungal growth was measured [13]. Methylxanthines like theophylline and aminophylline have a narrow therapeutic index, which limits higher dosing. The pharmacokinetics of methylxanthines may be altered in fungal infection and lead to increased toxicity [30]. Also, methylxanthines may alter the host inflammatory response and inhibit human chitinases. Methylxanthines are known to inhibit TNF-α release [31 33], which has been implicated in the host response to a variety of fungal infections [34 38].
Cumulatively, our data indicate that fungal chitinases may provide a new strategy for antifungal therapy. Furthermore, methylxanthines may serve as a basis for the formulation of new compounds with anti-chitinase activity. In targeting fungal chitinases, an important consideration is any effect on the host inflammatory response [39] and including host chitinase inhibition.
Fig. 3.
Effects of PTX on CN 24067 growth as reflected in colony formation. CN 24067 was cultured in media with or without PTX (Control). At different times an aliquot was removed and incubated on Sabouraud’s agar and resultant colonies counted. a represents growth in defined media, while b represents growth in Sabouraud’s dextrose broth. Colony numbers represent an average of 3 plates, and bars represent one standard deviation
Acknowledgments
We would like to thank Dr. Andre Nicola for his assistance with microscopy studies. Arturo Casadevall is supported by NIH grants AI033142, AI033774, AI052733, and HL059842.
References
- 1.Baker LG, Specht CA, Donlin MJ, Lodge JK. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot Cell. 2007;6(5):855–67. doi: 10.1128/EC.00399-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks IR, Specht CA, Donlin MJ, Gerik KJ, Levitz SM, Lodge JK. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2005;4(11):1902–12. doi: 10.1128/EC.4.11.1902-1912.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duo Chuan L. Review of fungal chitinases. Mycopathologia. 2006;161(6):345–60. doi: 10.1007/s11046-006-0024-y. [DOI] [PubMed] [Google Scholar]
- 4.Boot RG, Blommaart EF, Swart E, Ghauharali-van der Vlugt K, Bijl N, Moe C, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276(9):6770–8. doi: 10.1074/jbc.M009886200. [DOI] [PubMed] [Google Scholar]
- 5.van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, Blommaart EF, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005;17(11):1505–12. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 6.Elias JA, Homer RJ, Hamid Q, Lee CG. Chitinases and chitinase-like proteins in T(H)2 inflammation and asthma. J Allergy Clin Immunol. 2005;116(3):497–500. doi: 10.1016/j.jaci.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357(20):2016–27. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 8.Windmeier C, Gressner AM. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol. 1997;29(2):181–96. doi: 10.1016/s0306-3623(96)00314-x. [DOI] [PubMed] [Google Scholar]
- 9.Meskini N, Nemoz G, Okyayuz-Baklouti I, Lagarde M, Prigent AF. Phosphodiesterase inhibitory profile of some related xanthine derivatives pharmacologically active on the peripheral microcirculation. Biochem Pharmacol. 1994;47(5):781–8. doi: 10.1016/0006-2952(94)90477-4. [DOI] [PubMed] [Google Scholar]
- 10.Fredholm BB, Persson CG. Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol. 1982;81(4):673–6. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Lim S, Caramori G, Cosio B, Chung KF, Adcock IM, et al. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci USA. 2002;99(13):8921–6. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao FV, Andersen OA, Vora KA, Demartino JA, van Aalten DM. Methylxanthine drugs are chitinase inhibitors: investigation of inhibition and binding modes. Chem Biol. 2005;12(9):973–80. doi: 10.1016/j.chembiol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Hurtado-Guerrero R, van Aalten DM. Structure of Saccharomyces cerevisiae chitinase 1 and screening-based discovery of potent inhibitors. Chem Biol. 2007;14(5):589–99. doi: 10.1016/j.chembiol.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski L, Niang R, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107(5):E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 15.Barratt RW, Johnson GB, Ogata WN. Wild type and mutant stocks of Aspergillus nidulans. Genetics. 1965;52(1):233–46. doi: 10.1093/genetics/52.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicencio AG, Narain S, Du Z, Zeng WY, Ritch J, Casadevall A, et al. Pulmonary cryptococcosis induces chitinase in the rat. Respir Res. 2008;9:40. doi: 10.1186/1465-9921-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman DL, Zeng W, Rivera J, Nakouzzi A, Casadevall A. Human serum contains a protease that protects against cytotoxic activity of Bacillus anthracis lethal toxin in vitro. Clin Vaccine Immunol. 2008;15(6):970–3. doi: 10.1128/CVI.00064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonseca FL, Nimrichter L, Cordero RJ, Frases S, De Siqueira JR, Goldman DL, et al. A role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot Cell. 2009 doi: 10.1128/EC.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs BB, Tegos GP, Hamblin MR, Mylonakis E. Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimic rob Agents Chemother. 2007;51(8):2929–36. doi: 10.1128/AAC.00121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformansmelanin and virulence: mechanism of action. Infect Immun. 1995;63(8):3131–6. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuda S, Nishimoto Y, Ohi M, Watanabe M, Takayama S, Isogai A, Yamada Y. Effects of demethylallosamidin, a potent yeast chitinase inhibitor, on the cell division of yeast. Agricultural Biological Chemistry. 1990;54(5):1333–5. [Google Scholar]
- 22.Kuranda MJ, Robbins PW. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem. 1991;266(29):19758–67. [PubMed] [Google Scholar]
- 23.McCreath KJ, Specht CA, Robbins PW. Molecular cloning and characterization of chitinase genes from Candida albicans. Proc Natl Acad Sci USA. 1995;92(7):2544–8. doi: 10.1073/pnas.92.7.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkler A, Walther A, Specht CA, Wendland J. Candida albicans CHT3 encodes the functional homolog of the Cts1 chitinase of Saccharomyces cerevisiae. Fungal Genet Biol. 2005;42(11):935–47. doi: 10.1016/j.fgb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Baker LG, Specht CA, Lodge JK. Chitinases are essential for sexual development but not vegetative growth in Cryptococcus neoformans. Eukaryot Cell. 2009;8(11):1692–705. doi: 10.1128/EC.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcazar-Fuoli L, Clavaud C, Lamarre C, Aimanianda V, Seidl-Seiboth V, Mellado E, et al. Functional analysis of the fungal/plant class chitinase family in Aspergillus fumigatus. Fungal Genet Biol. 48(4):418–29. doi: 10.1016/j.fgb.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Taib M, Pinney JW, Westhead DR, McDowall KJ, Adams DJ. Differential expression and extent of fungal/plant and fungal/bacterial chitinases of Aspergillus fumigatus. Arch Microbiol. 2005;184(1):78–81. doi: 10.1007/s00203-005-0028-x. [DOI] [PubMed] [Google Scholar]
- 28.McFadden D, Zaragoza O, Casadevall A. The capsular dynamics of Cryptococcus neoformans. Trends Microbiol. 2006;14(11):497–505. doi: 10.1016/j.tim.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller K, Louie A, Baltch AL, Smith RP, Davis PJ, Gordon MA. Pharmacokinetics of pentoxifylline and its metabolites in healthy mice and in mice infected with Candida albicans. Antimicrob Agents Chemother. 1998;42(9):2405–9. doi: 10.1128/aac.42.9.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louie A, Baltch AL, Franke MA, Smith RP, Gordon MA. Comparative capacity of four antifungal agents to stimulate murine macrophages to produce tumour necrosis factor alpha: an effect that is attenuated by pentoxifylline, liposomal vesicles, and dexamethasone. J Antimicrob Chemother. 1994;34(6):975–87. doi: 10.1093/jac/34.6.975. [DOI] [PubMed] [Google Scholar]
- 32.Fischer W, Schudt C, Wendel A. Protection by phosphodiesterase inhibitors against endotoxin-induced liver injury in galactosamine-sensitized mice. Biochem Pharmacol. 1993;45(12):2399–404. doi: 10.1016/0006-2952(93)90219-m. [DOI] [PubMed] [Google Scholar]
- 33.Jilg S, Barsig J, Leist M, Kusters S, Volk HD, Wendel A. Enhanced release of interleukin-10 and soluble tumor necrosis factor receptors as novel principles of methylxan thine action in murine models of endotoxic shock. J Phar macol Exp Ther. 1996;278(1):421–31. [PubMed] [Google Scholar]
- 34.Bauman SK, Huffnagle GB, Murphy JW. Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect Immun. 2003;71(1):68–74. doi: 10.1128/IAI.71.1.68-74.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hage CA, Wood KL, Winer-Muram HT, Wilson SJ, Sarosi G, Knox KS. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest. 2003;124(6):2395–7. doi: 10.1378/chest.124.6.2395. [DOI] [PubMed] [Google Scholar]
- 36.Wood KL, Hage CA, Knox KS, Kleiman MB, Sannuti A, Day RB, et al. Histoplasmosis after treatment with anti-tumor necrosis factor alpha therapy. Am J Respir Crit Care Med. 2003;167(9):1279–82. doi: 10.1164/rccm.200206-563OC. [DOI] [PubMed] [Google Scholar]
- 37.Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, et al. TNF-{alpha} from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci USA. 108(13):5360–5. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94(15):8093–8. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsirilakis K, Du Z, Rivera J, Vicencio AG, Goldman DL. C. neoformans induces chitinase activity and expression in the mouse. Am J Respir Crit Care Med. 2010;181:A5653. [Google Scholar]