Abstract
IMPORTANCE
That too few youth with special health care needs make the transition to adult-oriented health care successfully may be due, in part, to lack of readiness to transfer care. There is a lack of theoretical models to guide development and implementation of evidence-based guidelines, assessments, and interventions to improve transition readiness.
OBJECTIVE
To further validate the Social-ecological Model of Adolescent and Young Adult Readiness to Transition (SMART) via feedback from stakeholders (patients, parents, and providers) from a medically diverse population in need of life-long follow-up care, survivors of childhood cancer.
DESIGN
Mixed-methods participatory research design.
SETTING
A large Mid-Atlantic children's hospital.
PARTICIPANTS
Adolescent and young adult survivors of childhood cancer (n = 14), parents (n = 18), and pediatric providers (n = 10).
MAIN EXPOSURES
Patients and parents participated in focus groups; providers participated in individual semi-structured interviews.
MAIN OUTCOMES AND MEASURES
Validity of SMART was assessed 3 ways: (1) ratings on importance of SMART components for transition readiness using a 5-point scale (0-4; ratings >2 support validity), (2) nominations of 3 “most important” components, and (3) directed content analysis of focus group/interview transcripts.
RESULTS
Qualitative data supported the validity of SMART, with minor modifications to definitions of components. Quantitative ratings met criteria for validity; stakeholders endorsed all components of SMART as important for transition. No additional SMART variables were suggested by stakeholders and the “most important” components varied by stakeholders, thus supporting the comprehensiveness of SMART and need to involve multiple perspectives.
CONCLUSIONS AND RELEVANCE
SMART represents a comprehensive and empirically validated framework for transition research and program planning, supported by survivors of childhood cancer, parents, and pediatric providers. Future research should validate SMART among other populations with special health care needs.
The dramatic increase in youth with special health care needs surviving to adulthood in recent decades has prompted attention to transition from pediatric to adult-oriented care and how best to facilitate it. To address the need to improve transition care, the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians jointly authored a clinical report in 2011 that provides an algorithm for providers to implement best practices for transition of patients both with and without special health care needs.1 The report and prior consensus statements emphasize that transition is a multifactorial process requiring the engagement of not only the patient, but also the family and health care providers.1-4 While this effort based on expert consensus was extremely timely and critical for advancing transition care, the algorithm and guidelines were developed in the absence of theoretical models and evidence-based assessment tools and interventions and a paucity of longitudinal data on transition planning and posttransfer outcomes. A valid multifactorial model of transition readiness for youth with special health care needs that addresses the role of multiple stakeholders in the process is needed to inform the implementation of the transition planning algorithm and the development of related evidence-based assessment and intervention.5 The present study fills this gap in transition research and care by using a mixed-methods participatory approach with patient, parent, and provider (PPP) stakeholders to further validate a newly developed model of transition readiness: the Social-ecological Model of Adolescent and Young Adult Readiness to Transition5 (SMART) (Figure 1).
Figure 1.
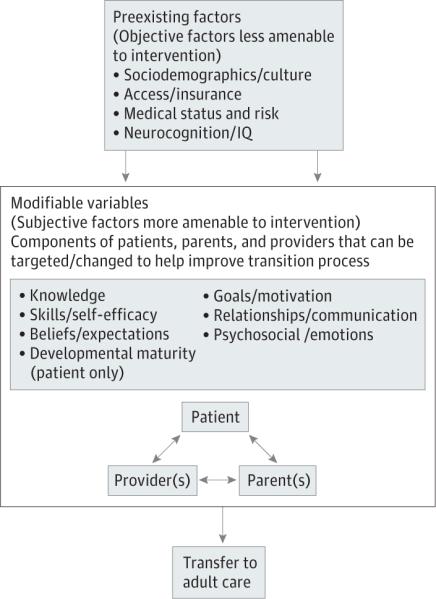
Social-ecological Model of Adolescent and Young Adult Readiness to Transition (SMART) Model
SMART applies a social-ecological framework to transition readiness, emphasizing multiple factors, stakeholders, and systems and their reciprocal relationships in influencing the readiness for and likelihood of success in transfer to adult-oriented care. Transition readiness is defined as indicators that patients and those in their support system (eg, parents and providers) can begin, continue, and finish the transition process from child-centered to adult-oriented health care, through the event of transfer.6,7 SMART purports 4 preexisting objective factors that are not or are less amenable to change and may influence processes among PPP (sociodemographics/culture, access/insurance, medical status/risk, and neurocognitive functioning/IQ), 6 more modifiable subjective factors of PPP (knowledge, beliefs/expectations, goals/motivations, skills/ self-efficacy, relationships/communication, and psychosocial functioning/emotions), and 1 additional factor specific to the patient, developmental maturity. The broader systems issues and disease status (ie, the objective factors) are considered critical for influencing the subjective factors, which are believed to be more modifiable in a clinic setting. While most research on transition focuses on patient disease knowledge and skills, SMART considers additional indicators of transition readiness, attends to multiple stakeholder (ie, PPP) perspectives, and distinguishes between variables more and less amenable to change within the context of clinical settings and multidisciplinary teams. The comprehensive process of developing SMART following the methods of Jaccard and Jacoby8 was the first step of its validation. In particular, SMART was informed by relevant research on transition, disease management, and adjustment to pediatric chronic illness; related theoretical models9-13; and expert clinical opinion on transition readiness from providers in adolescent medicine and oncology (including physicians, nurses, and psychologists).5
Data collection to further support its validity has been conducted in childhood cancer survivorship. While SMART is intended to be generalizable to all adolescents and young adults with compromised health and will be tested in multiple chronic illness populations in future research, childhood cancer is a broad diagnosis with applicability to many different health conditions. Survivors of childhood cancer represent a variety of cancer diagnoses and treatments, subsequent treatment-related medical issues (ie, late effects that affect every organ system such as cardiac, renal, pulmonary, and endocrine) that often emerge in young adulthood, and an increased risk for future cancers.14,15 Thus, survivors of childhood cancers are a heterogeneous population ranging from those experiencing good health but requiring surveillance for future problems to those with significant life-threatening problems and/or disabilities. Guidelines recommend, at minimum, annual lifelong follow-up care,14,16 yet only about 40% of young adult survivors access appropriate medical care related to their prior cancer or treatment,17 highlighting the need for improved transition care for this population.18
Preliminary findings with providers in a pediatric cancer survivorship clinic indicated that SMART variables were related to the providers’ assessments of the level of transition readiness of all 100 patients.5 The findings supported initial validity from the providers’ perspectives, thus warranting data collection with multiple stakeholders to further test the validity of SMART. The current article extends this work by further validating SMART using mixed-methods (quantitative and qualitative) data collection with PPP in the context of pediatric cancer survivorship and provides a framework by which to test SMART in other chronic health conditions.
Methods
The study was approved by the institutional review board at the hospital where the study took place. Patients older than 18 years, parents, and providers provided written informed consent, and patients younger than 18 years provided written assent. A mixed-methods participatory approach with patients (adolescent and young adult survivors of childhood cancer aged 16-28 years), parents, and pediatric providers was used. The validation process was strengthened by using a participatory approach that assures the model is consistent with stake-holder experiences19-21 and mixed methods that allow for complementary types of data to evaluate SMART.20,22-24 Qualitative data collection occurred with patients and parents via focus groups and with pediatric providers via semi-structured interviews, a method that better accommodated their work schedules. Qualitative data were intended to inform modifications to SMART and definitions of its components. The quantitative data—assessment of PPP perceived importance of each SMART component—were used to further validate inclusion of each of the SMART components. Attention was paid to the consistency across reporters and the 2 data collection modalities.23,24
Participants
Patients, parents, and providers participated. Patient participants (n = 14) were required to be (1) at least 5 years from diagnosis of a childhood malignancy, (2) at least 2 years since end of treatment, (3) at least 16 years old, (4) able to speak and read English, and (5) cognitively capable of participating in a focus group and answering questions, as determined by their oncology provider or parent. Parents (n = 18) were eligible if they had a child who met the patient participation criteria and could speak and read English. Sample sizes were based on the number of participants needed (at least 25 across participant groups) to reach redundancy and data saturation during qualitative analysis.25,26 Purposeful sampling27 was used to identify a maximally diverse sample of patients and parents in terms of demographics (age, sex, and race/ethnicity) and cancer-specific variables (type of cancer, treatment modalities received, and severity of late effects). Forty-seven patients and their parents who were potentially eligible were identified by clinical providers on the study team and other pediatric oncology providers. Of those, 30 patients and 29 parents were reached. Of those, 12 patients and 14 of their parents (12 mothers and 2 fathers, including 2 married couples) agreed to participate. An additional 2 patients and 4 parents participated without their parent/child. See the Table for demographics, disease-related information, and type of provider seen for follow-up care. Only 1 patient had transferred all care (oncology and primary care) to adult providers. Reasons for decline were unavailable on days focus groups were offered (n = 9), patient relapsed (n = 2), too busy (n = 2), too far to travel (n = 3), not interested (n = 1), or no reason given (n = 10).
Table.
Demographics and Disease/Treatment Information of Patients and Parentsa
| No. (%) |
||
|---|---|---|
| Patients (n = 14) | Parents (n = 18) | |
| Demographics | ||
| Age, y, mean (SD) [range] | 21.93 (3.45) [16-28] | 52.60 (7.41) [36-66] |
| Race/ethnicity | ||
| White | 8 (57) | 12 (67) |
| Asian | 4 (29) | 4 (22) |
| African American/black | 2 (14) | 2 (11) |
| Female | 8 (57) | 16 (89) |
| Education | ||
| High school | 5 (36) | 1 (6) |
| Some college or completed 2-y degree | 2 (14) | 4 (22) |
| 4-y College degree | 7 (50) | 9 (50) |
| Graduate school | 0 | 4 (22) |
| Marital status | ||
| Single | 9 (64) | 1 (6) |
| Committed relationship | 5 (36) | 2 (11) |
| Married | 0 | 15 (83) |
| Family income, $b | ||
| <25 000 | 1 (7) | 1 (6) |
| 25 000-49 999 | 1 (7) | 0 |
| 50 000-74 999 | 2 (14) | 3 (17) |
| 75 000-99 999 | 1 (7) | 3 (17) |
| ≥100 000 | 6 (43) | 9 (50) |
| Personal income of patient, $ | ||
| No personal income | 6 (43) | ... |
| <25 000 | 1 (7) | ... |
| 25 000-49 999 | 1 (7) | ... |
| 50 000-74 999 | 3 (21) | ... |
| Employment status | ||
| Not working | 7 (50) | 5 (28) |
| Part time | 3 (21) | 5 (28) |
| Full time | 4 (29) | 8 (44) |
| Disease/treatmentc | ||
| Diagnosis | ||
| Leukemia | 3 (21) | 1 (25) |
| Lymphoma | 3 (21) | 1 (25) |
| Brain tumor | 4 (29) | 1 (25) |
| Other solid tumor | 4 (29) | 1 (25) |
| Years since diagnosis, mean (SD) [range] | 14.35 (5.30) [7-23] | 13.83 (5.47) [7-24] |
| Treatment | ||
| Major surgery | 10 (71) | 2 (50) |
| Chemotherapy | 13 (93) | 4 (100) |
| Irradiation | 5 (36) | 3 (75) |
| Bone marrow transplant | 0 | 1 (25) |
| Years not receiving treatment, mean (SD) [range] | 12.50 (5.92) [4-22] | 12.35 (6.06) [3-22] |
| Relapse (yes) | 3 (21) | 1 (25) |
| Second cancer (yes) | 2 (14) | 0 |
| Follow-up care | ||
| Pediatric oncology provider | 13 (93) | 2 (50) |
| Adult oncology provider | 1 (7) | 0 |
| Adult primary care provider | 1 (7) | 1 (25) |
| Pediatric primary care provider | 0 | 0 |
| No follow-up care | 0 | 1 (25) |
Percentages in each category may not add up to 100% because of missing data or participants who selected multiple options.
Parent-reported family income was only reported once for married couples who participated together.
Data in the Parent column for disease/treatment information represents the children of the parents who participated without their children (n = 4). Patient disease characteristics were reported by the parent unless a parent did not participate (n = 2).
For providers, efforts were made to establish a sample unfamiliar with SMART and with variability in terms of years in practice, transition practices (ie, providers ranging from frequently transferring patients to those known to keep patients long term), and medical specialties and interests (eg, nurse practitioner/physician, types of cancers treated, and experience/expertise with adolescents and young adults). Providers (n = 10) with expertise in childhood cancer or transition care who practice at the children's hospital where the study took place (n = 9) or at the affiliated, adjacent adult hospital (n = 1) were invited to participate and all agreed. Six pediatric oncologists, 2 pediatric oncology nurse practitioners, and 2 pediatricians with expertise in transition care participated. Clinical foci of the participants included leukemia and lymphoma (n = 3), neuro-oncology (n = 3), hematopoietic transplant (n = 1), solid tumors (n = 1), and medicine/pediatrics (n = 2). All providers interviewed were non-Hispanic white, and most were female (n = 8). Number of years in practice ranged from 1 to 31 years (median = 15.00 years).
Procedures
Patients and parents and providers were invited to participate in a focus group or interview, respectively, to discuss long-term cancer-related follow-up care. Focus groups and interviews followed parallel procedures using scripts guided by L.A.S. or J.A.D. In addition to a team member taking notes, all sessions were audio-recorded and professionally transcribed.28 To minimize response bias, the focus group leaders called for alternative explanations and possibilities from the participants and performed techniques such as round-robins to assure systematic input of all group members.28 Finally, questionnaires on demographics and ratings of SMART components were completed individually to gather data privately from participants.
Focus Groups
Two patient and 2 parent focus groups were held with 5 to 10 participants each,28 lasting approximately 60 to 90 minutes. Facilitators presented a definition of transition readiness and a visual presentation of SMART to facilitate discussion (Figure 1). Focus group scripts were used to guide inquiry on 3 topics: (1) long-term follow-up care (eg, “How important is [follow-up care, health care appointments] to you/your child)?”; (2) transition readiness (eg, “Do you think you/your child are ready to transition to an adult health care provider?”); and (3) introduction to the SMART model and its individual components (eg, “What are your thoughts on this component of the model?”).
Provider Interviews
The in-person, semi-structured interviews lasted 40 to 60 minutes using a script that paralleled that used in focus groups. Additional questions probing for assessment of transition readiness were asked such as, “How do you advise your patients, if at all, with regards to long-term follow-up care?”
Measures
Demographics and Disease/Treatment Information
Patients and parents provided demographic information on age, sex, race/ethnicity, education level, marital status, income, employment status, and cancer-specific information including diagnosis, date of diagnosis, relapse(s) (if applicable), treatment length, type of treatments, and type of follow-up care they attend (if applicable).
SMART Component Importance
Participants completed a brief questionnaire after the focus groups/interviews that assessed perceived importance of the 11 SMART components (see the eTable in Supplement for list of components) in 2 ways: (1) ratings on a 5-point scale (0 = not at all to 4 = extremely) and (2) nominations of the 3 “most important” components.
Data Analysis
Qualitative Content Coding
Transcripts were uploaded into Atlas.ti software (ATLAS.ti Scientific Software Development GmbH). Using the principles of a directed content analysis, prior definitions of SMART components5 (eTable in Supplement) were used to deductively guide coding, allowing for the application of multiple codes throughout.29 A data auditing system was implemented to ensure rigor of analysis; thus, coding was conducted by a primary coder (L.D.B.), a secondary coder (L.A.S.), and a final reviewer (J.A.D.). Only discussion of survivorship care, transition readiness and planning, and transfer to adult-oriented care were included in coding. Coders wrote field notes as they assignedcodes.20 Theteamdiscussedtheapplicationofcodesand reached a consensus on the final assignment of codes. Inductive methods were then employed to use the qualitative data to enrich and modify definitions of SMART components.
Quantitative Analysis
Descriptive statistics were performed on demographic and disease/treatment data, ratings of SMART component importance, and nominations of “most important” SMART components. Criterion for content validity of each SMART component was defined as an average stakeholder group rating of greater than 2 (on a scale from 0-4).30 To rank the “most important” SMART components and assess potential variability among groups, the percentage of each group that nominated each component as “most important” was calculated. The presence of variability among PPP on nominations of the most important components was examined to assess whether transition readiness is a process that involves multiple, and presumably differing, perspectives—something that is implied by SMART.
Data Integration
Qualitative and quantitative data from PPP regarding SMART components were compared to evaluate the data for consistency and complementarity.23,24 Definitions of components deemed valid (as determined by an average importance rating >2) were modified, as needed, based on qualitative data.
Results
Qualitative Results
The original definitions of SMART components, additions from the current content analysis, and representative quotes are presented in the eTable in Supplement (additions are underlined). The qualitative data provided support for all SMART components and the full model. The PPP endorsed SMART as comprehensive and applicable to their experiences with transition. Very few suggestions were made for changes to the definitions of the objective, pre existing factors. Additions were made to the descriptions of the subjective, modifiable indicators of SMART. There were no recommendations for additional components.
Quantitative Results
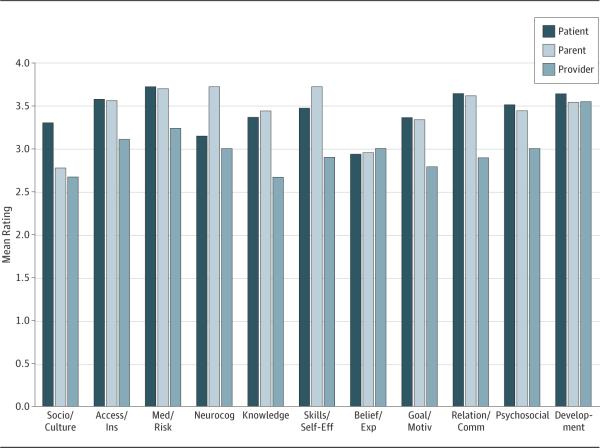
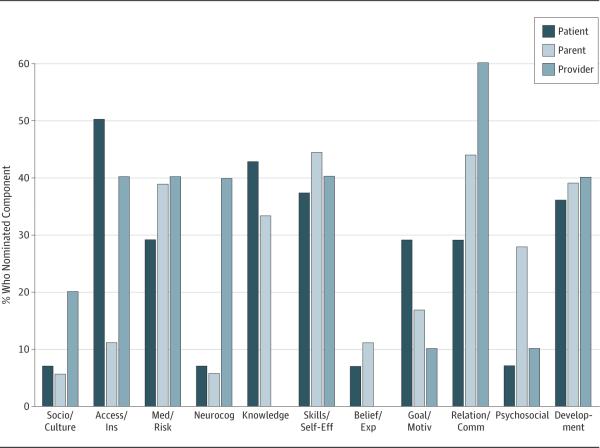
The PPP each reported a mean more than 2 for all SMART components on importance (mean = 2.95-3.60; SD = 0.54-0.93) (Figure 2), meeting criteria for validity. Of patients, 100% rated health, development, and relationships as more than 2 on importance; 100% of parents rated relationships more than 2; and 100% of providers rated developmental maturity more than 2 on importance. The highest-rated components by PPP were medical status/risk (mean = 3.71), skills/self-efficacy and neurocognition/IQ(bothwithmean = 3.72),anddevelopmentalmaturity (mean = 3.55), respectively. The components endorsed by more than 40% of the stakeholder groups as “most important” were access/insurance and knowledge for patients, skills/self-efficacy and relationships/communication for parents, and relationships/communication for providers (Figure 3).
Figure 2.
Ratings of Importance of Social-ecological Model of Adolescent and Young Adult Readiness to Transition (SMART) Components by Stakeholder Group
Figure 3.
Comparison Between Patient, Parent, and Provider Nominations of Social-ecological Model of Adolescent and Young Adult Readiness to Transition (SMART) Components as a “Most Important” Component
Data Integration
High quantitative ratings on importance of SMART components (>2) were consistent with qualitative results endorsing SMART components. The consistency between data collection modalities supported the use of qualitative data to modify and enhance existing definitions of SMART components.
Discussion
To our knowledge, SMART is the only model of transition readiness that has followed multiple steps to establish its validity, including the process of theory development5 and testing it via mixed methods that incorporate the input of stakeholders. Mixed-methods results indicated strong support for SMART and its components. Only minimal additions and some examples were added to component definitions; no information was removed from definitions, and no additional components were suggested. The variability among PPP on nominations of the “most important” SMART components supported the importance of considering multiple stakeholder perspectives. For example, that “relationships” were deemed “most important” by the parents and providers is especially validating of SMART's focus on interactions of multiple stake-holders as an important part of the transition process. It further reinforces the need for transition services to address relationships and needs of parents and providers in addition to patients.19 Furthermore, that no provider endorsed “knowledge” as one of the “most important” components is contrary to the emphasis placed on patient knowledge in the transition literature and related assessment tools, thus further emphasizing the importance of the many SMART constructs.5,31,32 This may indicate that knowledge is an important precursor to transition readiness but may not be sufficient to translate to appropriate skills and motivations that are necessary for transition readiness. Taken together, results support conceptualizing transition readiness as a multi-factorial, dynamic process involving multiple stakeholders and psychosocial variables.
A valid model fills a critical gap in the literature by providing a framework to guide implementation of the transition algorithm, development of evidence-based assessments and interventions, and theoretically informed research on transition. SMART represents targets of intervention identified and corroborated by PPP and should be used to inform development of valid, evidence-based assessment tools and interventions that target all aspects of transition readiness already shown to be important to stakeholders. When implementing the transition algorithm in clinical practice, SMART can help to operationalize the process by identifying specific variables for providers to target in assessment, discussions, and planning.
The findings support the need for multidisciplinary teams to facilitate the transition process for youth with special health care needs. For example, promoting autonomous disease management skills, managing family conflict and traumatic memories related to illness experiences, and changing negative beliefs and expectations about adult health care may require efforts by nurses, psychologists, social workers, and transition coordinators beyond what can be accomplished in a time-limited medical visit with a physician. Addressing these transition-related concerns and barriers with the aid of a multidisciplinary team may ultimately reduce costs by enhancing the likelihood of a successful transfer of care and reducing poor transition outcomes (eg, increased morbidities and use of emergent medical care).33,34
There are several limitations of this study. The majority of participants were middle to high income; thus, future research should elicit perspectives on transition readiness from low-income families to examine if additional or conflicting themes emerge in content analyses. Almost all providers interviewed practiced in the same pediatric setting. Thus, future research may benefit from including adult providers and providers from multiple institutions. Despite these limitations, participants presented diverse perspectives while also supporting the model. Also, the content analysis necessitated an informed coder who may be biased to find more supportive than nonsupportive evidence.29 However, we addressed this limitation by having a 3-fold audit trail of coders and self-report questionnaire data, which supported findings from content analysis. Additionally, results from questionnaires administered after the interview or focus group were relatively reflective of the discussions; future studies may want to administer a more general questionnaire before and after discussion. Finally, the focus on pediatric oncology may limit the generalizability of the findings. However, given the heterogeneity of health status and organ systems affected in childhood cancer survivors, results are expected to generalize to other youth with special health care needs.
In conclusion, SMART is an empirically validated model of transition readiness that lends support to and expands on the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians joint clinical report, consensus statements, and guidelines that were developed without established models of transition readiness.1-4 Advancements in transition care are hindered by the lack of valid measures and interventions that could identify outcomes and best practices. Thus, SMART fills a gap in the burgeoning literature on transition by providing an evidence-based framework to inform research and the development of assessment tools and interventions. Future research is needed to test SMART with other disease populations, further test and confirm the components of SMART via larger samples and quantitative analysis, and understand the criterion level (eg, extent of disease knowledge and management skills, beliefs, emotions) of each component that indicates optimal transition readiness.
Supplementary Material
Acknowledgments
Funding/Support: This work is supported by award R21 CA141332, “Transition Readiness of Adolescent and Young Adult Survivors of Childhood Cancer,” from the National Cancer Institute (Dr Schwartz).
Footnotes
Author Contributions: Dr Schwartz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Schwartz, Tuchman, Barakat, Hobbie, Ginsberg, Kazak, Bevans, Deatrick. Acquisition of data: Schwartz, Brumley, Hobbie, Ginsberg, Kazak, Deatrick.
Analysis and interpretation of data: Schwartz, Brumley, Tuchman, Barakat, Hobbie, Daniel, Kazak, Bevans, Deatrick.
Drafting of the manuscript: Schwartz, Brumley, Ginsberg, Kazak, Deatrick.
Critical revision of the manuscript for important intellectual content: Schwartz, Brumley, Tuchman, Barakat, Hobbie, Daniel, Kazak, Bevans, Deatrick. Statistical analysis: Schwartz, Brumley, Barakat, Bevans.
Obtained funding: Schwartz, Kazak, Deatrick. Administrative, technical, or material support: Schwartz, Brumley, Barakat, Hobbie, Ginsberg, Daniel, Kazak.
Study supervision: Schwartz, Barakat, Ginsberg, Kazak.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank the members of the Writers Seminar of The CHOP/PENN Mentored Psychosocial Research Curriculum, supported by K05 award CA128805 (Dr Kazak), for reading and reviewing prior drafts of this article.
REFERENCES
- 1.Cooley WC, Sagerman PJ, American Academy of Pediatrics. American Academy of Family Physicians. American College of Physicians; Transitions Clinical Report Authoring Group Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182–200. doi: 10.1542/peds.2011-0969. [DOI] [PubMed] [Google Scholar]
- 2.Sable C, Foster E, Uzark K, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young. Council on Cardiovascular Nursing. Council on Clinical Cardiology. Council on Peripheral Vascular Disease Best practices in managing transition to adulthood for adolescents with congenital heart disease: the transition process and medical and psychosocial issues. a scientific statement from the American Heart Association. Circulation. 2011;123(13):1454–1485. doi: 10.1161/CIR.0b013e3182107c56. [DOI] [PubMed] [Google Scholar]
- 3.Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM, Society for Adolescent Medicine Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33(4):309–311. doi: 10.1016/s1054-139x(03)00208-8. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics. American Academy of Family Physicians. American College of Physicians–American Society of Internal Medicine A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6, pt 2):1304–1306. [PubMed] [Google Scholar]
- 5.Schwartz LA, Tuchman LK, Hobbie WL, Ginsberg JP. A social-ecological model of readiness for transition to adult-oriented care for adolescents and young adults with chronic health conditions. Child Care Health Dev. 2011;37(6):883–895. doi: 10.1111/j.1365-2214.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 6.Telfair J, Alexander LR, Loosier PS, Alleman-Velez PL, Simmons J. Providers’ perspectives and beliefs regarding transition to adult care for adolescents with sickle cell disease. J Health Care Poor Underserved. 2004;15(3):443–461. doi: 10.1353/hpu.2004.0049. [DOI] [PubMed] [Google Scholar]
- 7.Betz CL, Telfair J. Health care transitions: an introduction. In: Betz CL, Nehring WM, editors. Promoting Health Care Transitions for Adolescents With Special Health Care Needs and Disabilities. Paul H. Brookes Publishing; Baltimore, MD: 2007. pp. 3–19. [Google Scholar]
- 8.Jaccard J, Jacoby J. Theory Construction and Model-Building Skills. Guilford Press; New York, NY: 2010. [Google Scholar]
- 9.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 10.Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Harvard University Press; Cambridge, MA: 1979. [Google Scholar]
- 11.Kazak AE. Pediatric Psychosocial Preventative Health Model (PPHM): research, practice, and collaboration in pediatric family systems medicine. Fam Syst Health. 2006;24(4):381–395. doi:10.1037/1091-7527.24.4.381. [Google Scholar]
- 12.Kazak AE, Rourke MT, Alderfer MA, Pai A, Reilly AF, Meadows AT. Evidence-based assessment, intervention and psychosocial care in pediatric oncology: a blueprint for comprehensive services across treatment. J Pediatr Psychol. 2007;32(9):1099–1110. doi: 10.1093/jpepsy/jsm031. [DOI] [PubMed] [Google Scholar]
- 13.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 14.Oeffinger KC, Nathan PC, Kremer LC. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Pediatr Clin North Am. 2008;55(1):251–273. xiii. doi: 10.1016/j.pcl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Oeffinger KC, Tonorezos ES. The cancer is over, now what?: understanding risk, changing outcomes. Cancer. 2011;117(10)(suppl):2250–2257. doi: 10.1002/cncr.26051. [DOI] [PubMed] [Google Scholar]
- 16.Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers: version 3.0. Children's Oncology Group; [July 16, 2012]. website. www.survivorshipguidelines.org. [Google Scholar]
- 17.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2(1):61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2008;26(27):4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiss JG, Gibson RW, Walker LR. Health care transition: youth, family, and provider perspectives. Pediatrics. 2005;115(1):112–120. doi: 10.1542/peds.2004-1321. [DOI] [PubMed] [Google Scholar]
- 20.Whittemore R, Chase SK, Mandle CL. Validity in qualitative research. Qual Health Res. 2001;11(4):522–537. doi: 10.1177/104973201129119299. [DOI] [PubMed] [Google Scholar]
- 21.Rich M, Ginsburg KR. The reason and rhyme of qualitative research: why, when, and how to use qualitative methods in the study of adolescent health. J Adolesc Health. 1999;25(6):371–378. doi: 10.1016/s1054-139x(99)00068-3. [DOI] [PubMed] [Google Scholar]
- 22.Onwuegbuzie AJ, Leech NL. Validity and qualitative research: an oxymoron? Qual Quant. 2007;41(2):233–249. doi:10.1007/s11135-006-9000-3. [Google Scholar]
- 23.Breitmayer BJ, Ayres L, Knafl KA. Triangulation in qualitative research: evaluation of completeness and confirmation purposes. Image J Nurs Sch. 1993;25(3):237–243. doi: 10.1111/j.1547-5069.1993.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 24.Teddlie C, Tashakkori A. Foundations of Mixed Methods Research: Integrating Quantitative and Qualitative Approaches in the Social and Behavioral Sciences. Sage; Thousand Oaks, CA: 2009. [Google Scholar]
- 25.Ruff CC, Alexander IM, McKie C. The use of focus group methodology in health disparities research. Nurs Outlook. 2005;53(3):134–140. doi: 10.1016/j.outlook.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Sandelowski M. Sample size in qualitative research. Res Nurs Health. 1995;18(2):179–183. doi: 10.1002/nur.4770180211. [DOI] [PubMed] [Google Scholar]
- 27.Patton M. Purposeful sampling. In: Patton M, editor. Qualitative Research and Evaluation Methods. Sage; Thousand Oaks, CA: 2002. pp. 230–246. [Google Scholar]
- 28.Krueger RA, Casey MA. Focus Groups: Practical Guide for Applied Research. 4th ed. Sage; Thousand Oaks, CA: 2009. [Google Scholar]
- 29.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 30.Polit DF, Beck CT. The content validity index: are you sure you know what's being reported? critique and recommendations. Res Nurs Health. 2006;29(5):489–497. doi: 10.1002/nur.20147. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz LA, Wesley K, Danzi L, et al. 2012 Conference of the Society of Adolescent Health and Medicine. Society of Adolescent Health and Medicine; New Orleans, LA: 2012. A systematic review of transition readiness measures. [Google Scholar]
- 32.Talikoff K, Sheth S, Ferris M, Bickford K, Patel U. Content variation in health care transition tools: can we do better?. Paper presented at: Pediatric American Societies; Denver, CO.. May 2011. [Google Scholar]
- 33.White PH. Access to health care: health insurance considerations for young adults with special health care needs/disabilities. Pediatrics. 2002;110(6)(pt 2):1328–1335. [PubMed] [Google Scholar]
- 34.Williams RG. Fumbling the handoff: managing the transition to adult care for adolescents with chronic conditions. J Adolesc Health. 2009;44(4):307–308. doi: 10.1016/j.jadohealth.2009.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.