Abstract
Background
To ascertain whether use of non-benzodiazepine sedative-hypnotics is associated with risk of falls and compare this to risk of falls associated with use of benzodiazepines.
Methods
Among 4450 community-dwelling men, aged 71 years and older, enrolled in the population-based prospective cohort study, Osteoporotic Fractures in Men (MrOS), use of nonbenzodiazepine sedative-hypnotics and benzodiazepines was assessed by interview and verified from medication containers at the third annual visit of the MrOS study. Falls in the subsequent one-year period were ascertained by tri-annual questionnaires and a computerized dictionary used to categorize type of medication.
Results
In age-adjusted models, non-benzodiazepine sedative hypnotic use was associated with an increased risk of any falls (one or more falls) (RR 1.44, 95% CI 1.15, 1.81) and recurrent falls (2 or more falls) (RR 1.51, 95% CI 1.07, 2.14). Use of benzodiazepines was associated with a similar increase in age-adjusted risk of falling. Depressive symptoms, inability to stand from a chair, and instrumental activities of daily living (IADL) impairment modestly attenuated these associations. The association between non-benzodiazepine sedative-hypnotic use and falls was most pronounced among men without a history of falls in the previous year: in a multivariable model controlling for multiple potential confounders, the RR of any falls was 1.74 (95% CI 1.13, 2.68) in this subgroup.
Conclusions
Use of non-benzodiazepine sedative-hypnotics is associated with an increased risk of falls. Non-pharmacologic approaches to sleep disturbances may represent the safest approach to sleep difficulties in older adults.
Keywords: falls, sedative hypnotics, benzodiazepines, men, zolpidem, adverse drug effects
INTRODUCTION
Nonbenzodiazepine sedative-hypnotics, such as zolpidem, zaleplon, and eszopiclone, are often advocated as safer alternatives to benzodiazepines for the treatment of sleep disturbances due to their short half-life and preservation of normal sleep architecture.[1-5] However, limited data are available about their safety in older patients, in particular regarding postural instability, falls, and fractures.
These so-called “Z-drugs” affect the same receptor as benzodiazepines, suggesting that their risks may be similar. Clinical trials of zolpidem in healthy younger adults have demonstrated central nervous system side effects, including impaired cognitive and motor function, particularly in the first few hours after use.[6-8] In addition, observational data have suggested an association between non-benzodiazepine sedative-hypnotics and risk of fracture.[9,10] However, these studies have relied on administrative data bases and thus had limited or no information on important potential confounders, such as baseline physical function, cognitive function, and comorbidities.
To determine whether use of non-benzodiazepine sedative-hypnotics is associated with an increased risk of falls, and to compare this to the risk of falls observed with benzodiazepine use, we ascertained use of non-benzodiazepine sedative-hypnotics and benzodiazepines in a cohort of 4450 men aged 71 years and older enrolled in the Osteoporotic Fractures in Men study (MrOS) and followed them prospectively for incident falls during one year of follow-up.
METHODS
Participants
From March 2000 through April 2002, 5994 men who were at least 65 years of age were recruited for participation in the baseline examination of the prospective Osteoporotic Fractures in Men (MrOS) study. Men were recruited from population based listings in Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA. Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded.[11,12]
To be included in the present analysis, men must have attended a 3rd clinic examination between March 2007 and March 2009, completed a medication inventory at the 3rd exam, and returned at least two follow-up questionnaires regarding falls in the subsequent one year period. Of the original cohort, 1043 men had died prior to the 3rd exam and 168 had terminated participation in the study; 4682 of the original cohort, (98% of survivors) attended the 3rd exam (baseline for this analysis). 4588 men returned at least two follow-up questionnaires in the subsequent one year period; of these, 4471 completed a medication inventory. 19 men who reported use of both a non-benzodiazepine sedative hypnotic and a benzodiazepine were excluded, as were two men reporting use of ramelteon, a melatonin receptor agonist. In a secondary analysis, we restricted the cohort to those 2722 men participating in an ancillary study evaluating sleep disorders, titled the MrOS Sleep Study, who had attended an earlier exam and completed a measure of subjective sleep quality (average 3.4 ± 0.5 years between ancillary sleep exam and 3rd exam).
The Institutional Review Board (IRB) at each center approved the study protocol and written informed consent was obtained from all subjects.
Medication Use
Participants attending the clinic examination were asked to bring all current (any use within the last 30 days) prescription and nonprescription medications with them to clinic. Interviewers completed a medication history for each participant, including name of medication and frequency of use. All medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).[13] Zolpidem, zaleplon, and eszopiclone were categorized as non-benzodiazepine sedative-hypnotics; benzodiazepines included lorazepam, clonazepam, alprazolam, temazepam, diazepam, triazolam, oxazepam, clorazepate, and chlordiazepoxide. Participants were categorized as users of a medication if they reported any use in the last 30 days.
Ascertainment of Falls
At 4-month intervals, participants were queried by mailed questionnaire about the number of times they had fallen during the interval. Participants who fell in the previous 4 months were asked how many times (1, 2, 3, 4, or ≥5). Participants who did not initially return a tri-annual questionnaire or did not adequately complete the questionnaire were followed up with a telephone call. The present study includes fall reports during the one year period following the subject's Visit 3 examination.
Other Measurements
Participants completed questionnaires and were interviewed at the examination by trained staff, who asked about race/ethnicity, educational achievement, health status, smoking status, alcohol use, medical history, and falls in previous year. Men were asked whether they had difficulty performing any of 5 independent activities of daily living (preparing meals, shopping, heavy housework, walking 2-3 blocks, and climbing up 10 steps). A composite functional impairment score expressed the total number of activities ranging from 0 to 5 that a participant reported difficulty performing.[14] Physical performance measures included chair stand time and walking speed. For the chair stand, participants were asked to stand from a chair without using their arms; the time required to complete five chair stands without using arms was recorded.[15]
Cognitive function was assessed with the Teng Modified Mini-Mental State Examination (mMMSE) (maximum score 100).[16] Depressive symptoms were evaluated using the Geriatric Depression Scale short form (GDS).[17] The total number of selected comorbid conditions reported by participants (stroke, diabetes mellitus, Parkinson's disease, chronic obstructive pulmonary disease, myocardial infarction, angina, and congestive heart failure) was summed for each participant.
Body weight was measured by using a balance beam scale at all sites, except at one site (Portland) which used a digital scale. Height was measured by using a wall-mounted Harpenden stadiometer (Holtain, UK). Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).[18] Among participants in the MrOS Sleep Study, sleep complaints were measured using the Pittsburgh Sleep Quality Index (PSQI), a 19-item self-report questionnaire that assesses sleep quality over a one month time period (range 0-21), with higher scores reflecting poorer sleep quality.[19,20]
Statistical Analysis
Characteristics of the men at the 3rd exam were compared by medication use category (non-user, benzodiazepine user, non-benzodiazepine sedative-hypnotic user) using either analyses of variance or Kruskal-Wallis tests (continuous variables) or chi-squared tests of homogeneity (categorical variables).
For the primary analysis examining the association between medication use and risk of falls, log binomial and Poisson models were used to estimate the relative risk (RR) and 95% confidence interval (CI) of any falls (1 or more falls vs. 0) and recurrent falls (2 or more falls vs. 1 or none) by medication use category, with nonusers as the referent group. Models were initially adjusted for age and then further adjusted for potential confounders one at a time. In addition, covariates known to be risk factors for falls and characteristics related to use of sedativehypnotics were examined for inclusion in a multivariable model, with those factors related to falls, non-benzodiazepine sedative-hypnotic use, or benzodiazepine use at p <0.1 included. Interactions between medication and history of falls were explored. We also conducted secondary analyses in which the analysis was limited to the 2722 men who had data about self-reported sleep quality available from the earlier sleep exam.
All analyses were performed using SAS software (Version 9.1, SAS Institute Inc., Cary, NC).
RESULTS
Of the 4450 men included in this analysis, 94 men reported use of zolpidem (n=81), zaleplon (n=3), or eszopiclone (n=10), and 177 reported use of a benzodiazepine. Baseline characteristics of the cohort overall and by use status appear in Table 1. Sedative-hypnotic users were more likely to report fair or poor health and more IADL impairments than non-users. They were more likely to have difficulty standing from a chair without using their arms and were more likely to have fallen in the previous year. Benzodiazepine users and non-benzodiazepine sedative-hypnotic users were similar in many characteristics, although non-benzodiazepine sedative-hypnotic users were more likely to be college-educated, had higher mean MMSE, and reported more alcohol consumption.
Table 1.
Baseline Characteristics
| Characteristic | Overall | Non-users | Benzodiazepine Users | Non-benzodiazepine sedative hypnotic users | P-value* |
|---|---|---|---|---|---|
| (N=4450) | (N= 4179) | (N= 177) | (N= 94) | ||
| Age, years, mean ± SD | 79.5 ± 5.3 | 79.5 ± 5.3 | 79.1 ± 5.3 | 80.4 ± 5.7 | 0.18 |
| Caucasian, n (%) | 3991 (89.7) | 3744 (89.6) | 162 (91.5) | 85 (90.4) | 0.69 |
| College education or greater, n (%) | 2476 (55.6) | 2314 (55.4) | 92 (52.0) | 70 (74.5) | <0.001 |
| Fair, poor, very poor self-reported health, n (%) | 710 (16.0) | 639 (15.4) | 48 (27.3) | 23 (24.5) | <0.001 |
| Number of IADL impairments (range 0-5), mean ± SD | 0.49 ± 1.0 | 0.47 ± 1.02 | 0.83 ± 1.19 | 0.76 ± 1.26 | <0.001 |
| Walking speed, m/sec, mean ± SD | 1.11 ± 0.2 | 1.12 ± 0.24 | 1.04 ± 0.28 | 1.10 ± 0.25 | <0.001 |
| Inability to stand up without using arms, n (%) | 3860 (10.0) | 347 (9.6) | 23 (16.2) | 16 (19.5) | <0.001 |
| Any fall in previous year, n (%) | 1371 (30.8) | 1249 (29.9) | 79 (44.6) | 43 (45.7) | <0.001 |
| Number of co-morbidities (range 0-7), n (%) | 0.003 | ||||
| None | 2462 (55.4) | 2332 (55.9) | 87 (49.2) | 43 (45.7) | |
| 1 co-morbidity | 1257 (28.3) | 1183 (28.3) | 48 (27.1) | 26 (27.7) | |
| 2+ co-morbidities | 727 (16.4) | 660 (15.8) | 42 (23.7) | 25 (26.6) | |
| MMSE score (range 0-100), mean ± SD | 92.0 ± 7.23 | 91.9 ± 7.3 | 91.7 ± 6.6 | 94.1 ± 5.3 | 0.002 |
| GDS score (range 0-15), mean ± SD | 2.1 ± 2.4 | 2.0 ± 2.3 | 3.5 ± 3.4 | 2.8 ± 2.9 | <0.001 |
| PASE score, mean ± SD | 126.7 ± 70.5 | 128.2 ± 70.5 | 104.4 ± 68.9 | 101.2 ± 58.9 | <0.001 |
| Smoking status, n (%) | 0.59 | ||||
| Never | 1721 (38.9) | 1625 (39.1) | 59 (33.5) | 37 (39.4) | |
| Past | 2616 (59.1) | 2446 (58.9) | 114 (64.8) | 56 (59.6) | |
| Current | 87 (2.0) | 83 (2.0) | 3 (1.7) | 1 (11) | |
| ≥12 alcoholic drinks in past 12 months, n (%) | 2697 (61.2) | 2532 (61.2) | 99 (56.6) | 66 (70.2) | 0.09 |
| BMI, kg/m2, mean ± SD | 27.1 ± 3.9 | 27.1 ± 3.9 | 27.0 ± 4.0 | 27.3 ± 4.6 | 0.81 |
| PSQI score (range 0-21), mean ± SD | 5.5 ± 3.2 | 5.3 ± 3.0 | 7.9 ± 4.3 | 8.9 ± 3.4 | <0.001 |
| Fell at least once in 1st year post V3, n (%) | 1353 (30.4) | 1241 (29.7) | 71 (40.1) | 41 (43.6) | <0.001 |
| Fell at least twice in 1st year post V3, n (%) | 741 (16.7) | 677 (16.2) | 40 (22.6) | 24 (25.5) | 0.005 |
P values are from ANOVA for normally distributed continuous variables and Kruskal-Wallis for skewed continuous variables. P values for categorical data are from a χ2 test for homogeneity.
Abbreviations: IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; PASE, Physical Activity Scale for the Elderly; BMI, Body Mass Index; PSQI, Pittsburgh Sleep Quality Index
Non-benzodiazepine sedative-hypnotic use and falls
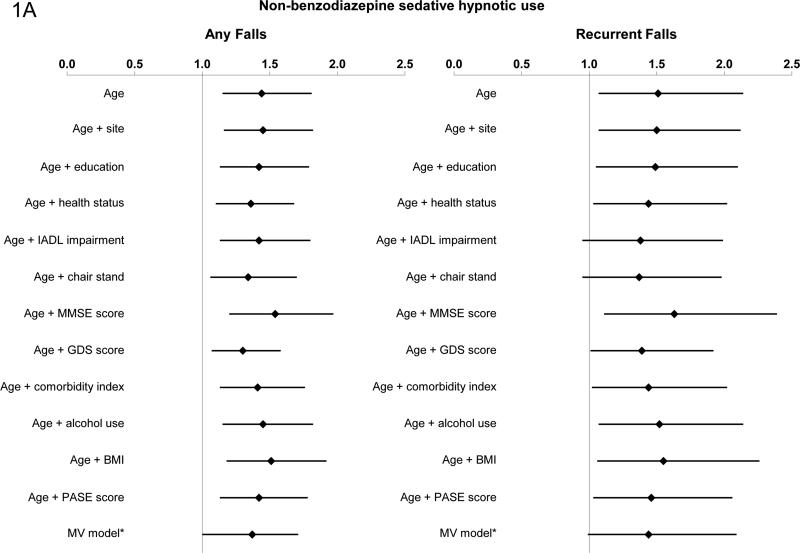
In age-adjusted models, non-benzodiazepine sedative-hypnotic use was associated with an increased risk of any falls (RR 1.44, 95% CI 1.15, 1.81) and recurrent falls (RR 1.5l, 95% CI 1.07, 2.14). The addition of potential confounders one at a time modestly attenuated the associations, with GDS having the greatest impact on the association between nonbenzodiazepine sedative-hypnotic use and risk of any falls (RR 1.30; 95% CI 1.07, 1.58). IADL impairment, inability to stand from a chair, and GDS all attenuated the risk of recurrent falls for non-benzodiazepine sedative-hypnotic users (Figure 1a).
Figure 1a-b. Association between Non-benzodiazepine Sedative-hypnotic use, Benzodiazepine Use and Risk of Falls.
*model adjusted for age, site, GDS score, educational status, BMI, comorbidity index, ability to stand from a chair, PASE score, self-reported health status, IADL impairments, alcohol use, and MMSE.
Abbreviations: IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; BMI, body mass index; PASE, Physical Activity Scale for the Elderly
In multivariable models that simultaneously adjusted for age, site, GDS score, educational status, BMI, comorbidity index, ability to stand from a chair, PASE score, self-reported health status, IADL impairments, alcohol use, and MMSE, use of non-benzodiazepine sedative-hypnotics appeared to be associated with modest increases in the risks of any fall and recurrent falls [(any falls: RR 1.37; 95% CI 1.09, 1.71; p=0.01); (recurrent falls: RR 1.44; 95% CI 0.99, 2.09); p=0.06]. In the subset of participants who attended the Sleep visit (n=2722), results were similar (any falls: RR 1.42; 95% CI 1.08, 1.86); (recurrent falls: RR 1.44; 95% CI .95, 2.16). The addition of the PSQI to the models did not significantly change the results.
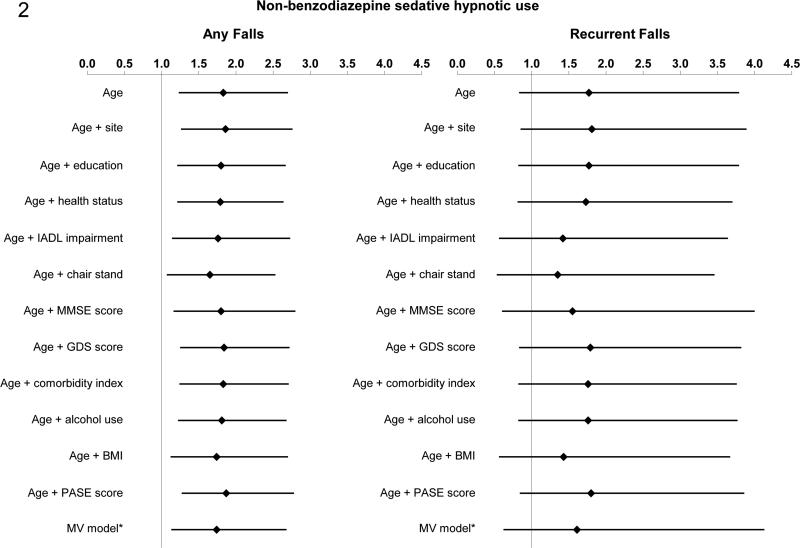
There was a significant interaction between use of non-benzodiazepine sedativehypnotics and history of falls in the age-adjusted model for any falls (p=0.01); for recurrent falls the interaction was not significant (p=0.24), possibly due to limited power. We subsequently stratified on history of falls in the previous year. The risk of falling with non-benzodiazepine sedative hypnotic use appeared to be most pronounced among men without a falling history. For men without a history of previous falls, non-benzodiazepine sedative hypnotic use was associated with an increased risk of any falls in age-adjusted models (RR 1.83, 95% CI 1.23, 2.70) (Figure 2). The point estimate for the age-adjusted relative risk of recurrent falls (RR 1.77, 95% CI 0.83, 3.79) was similar to that for the risk of any falls but did not reach significance, likely due to low power. In the multivariable model, non-benzodiazepine sedative-hypnotic use was significantly associated with an increased risk of any falls [RR 1.74 (95% CI: 1.13, 2.68)]; for recurrent falls, the relative risk was 1.61 (95% CI: 0.62, 4.13). For the subset of men who had previously fallen, there was no evidence of an association between use of nonbenzodiazepine sedative-hypnotics and risk of subsequent falls in age-adjusted or multivariable models.
Figure 2. Association between Non-benzodiazepine Sedative-hypnotic Use and Risk of Falls among men without a history of falls.
*model adjusted for age, site, GDS score, educational status, BMI, comorbidity index, ability to stand from a chair, PASE score, self-reported health status, IADL impairments, alcohol use, and MMSE.
Abbreviations: IADL, Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; BMI, body mass index; PASE, Physical Activity Scale for the Elderly
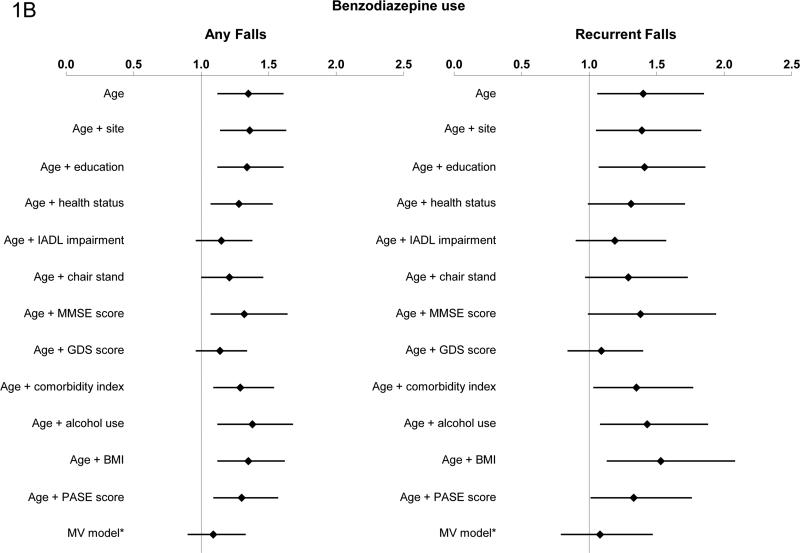
Benzodiazepine use and falls
In age-adjusted models, benzodiazepine use was associated with an increased risk of any falls (RR 1.35; 95% CI 1.12, 1.61) and recurrent falls (RR 1.40; 95% CI 1.06, 1.85). The addition of IADL impairments, ability to stand from a chair, and GDS score each attenuated the association between benzodiazepine use and risk of any and recurrent falls (Figure 1b). Use of benzodiazepines was not associated with risk of any falls [RR 1.09 (95% CI: 0.90, 1.33)] or risk of recurrent falls [RR 1.08 (95% CI: 0.79, 1.47)] in the full multivariable model. Addition of the PSQI to the multivariable model in the subset of men with this measurement did not significantly alter the results. There was no evidence of an interaction between benzodiazepine use and history of falls (p=0.41 for any falls and p=0.70 for recurrent falls).
DISCUSSION
In this cohort of older men, use of non-benzodiazepine sedative-hypnotics and use of benzodiazepines were each associated with an increase in the age-adjusted risk of falling. The association between benzodiazepine use and falls appeared to be largely explained by greater disability, poorer physical performance and greater level of depressive symptoms among men taking benzodiazepines. In contrast, the association between non-benzodiazepine sedative-hypnotic use and falls was only modestly attenuated by consideration of potential confounders. Furthermore, this association depended on fall history and was most pronounced among men without a fall history. These findings are consistent with the limited existing epidemiologic data. In a case-control study of patients aged 65 years and older, investigators found a significantly increased risk of hip fracture in users of zolpidem (adjusted OR 1.95; CI 1.09-3.51)[9], a rate similar for benzodiazepines. A recent retrospective cohort study found that the risk of nonvertebral fractures and dislocations was higher in the 90 days following an initial prescription for zolpidem than the risk prior to the prescription in members 65 years of age and older; this increased risk for zolpidem was similar to or higher than that associated with several benzodiazepines.[10]
Because these medications act via the benzodiazepine receptor-GABA complex, a similar side effect profile for non-benzodiazepine sedative-hypnotics and benzodiazepines is plausible. Indeed, clinical trials have reported comparable rates of CNS side effects, such as drowsiness, fatigue, impaired cognitive and motor function, postural sway, and ataxia.[6-8,21-24]
Due to its observational nature, our analysis has several limitations, most notably confounding by indication. These medications are typically prescribed for sleep disturbances. Because sleep disturbances have been linked to an increased risk of falls[25], the observed association between use of non-benzodiazepine sedative-hypnotics and risk of falls may be due to the underlying sleep disturbances that prompted their initiation. To address this issue, we controlled for the PSQI in multivariable models and did not observe any significant change in our results. However, the PSQI was not available at Visit 3 in the cohort and thus we utilized a PSQI measurement from an earlier visit in a subset of our original analytic cohort.
Another potential source of confounding is the possibility of channeling bias – i.e., if these drugs were preferentially prescribed to patients with a higher risk of falls due to the perception that these drugs have a safer side effect profile than benzodiazepines. We attempted to address these issues by controlling for multiple potential confounders, as well as by excluding subjects who had reported falls in the year prior to the study visit. Nevertheless, the potential for unmeasured confounding remains a limitation of this analysis.
Our analysis is also limited by a lack of detailed information about the reported falls. Information regarding time of day of the falls or the mechanism of the falls is not available in the cohort. We also do not have information about dose of medication or frequency of use; it is possible than subjects were not taking these medications at the time of a fall.
We found a weaker association between benzodiazepine use and risk of falls than previously reported. This finding may be due to temporal trends in benzodiazepine prescription patterns, due to growing recognition of the adverse effects of benzodiazepines in the elderly.[26] We also found that the association between use of non-benzodiazepine sedative hypnotics and risk of falls was more pronounced for men who had not fallen in the previous year. This finding may be due to the strong association between previous falls and risk of subsequent falls: the additional risk of a sedative hypnotic may be negligible for men already falling, whereas for men not previously falling, the addition of one of these medications may “tip the balance.”
Despite these limitations, this work adds to the growing body of literature suggesting that use of the non-benzodiazepine sedative-hypnotics may be associated with adverse effects such as falls. Future randomized clinical trials of this class of medications should include falls as a safety outcome. In addition, non-pharmacologic approaches to sleep disturbances, such as cognitive behavioral therapy, may represent the safest approach to this common problem in older adults.
ACKNOWLEDGMENTS
Results were presented at the Gerontological Society of America annual meeting on November 15, 2012 in San Diego, CA.
FUNDING
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
REFERENCES
- 1.Darcourt G, Pringuey D, Salliere D, Lavoisy J. The safety and tolerability of zolpidem--an update. J Psychopharmacol. 1999;13:81–93. doi: 10.1177/026988119901300109. PMID:10221362. [DOI] [PubMed] [Google Scholar]
- 2.Allain H, Bentue-Ferrer D, Polard E, Akwa Y, Patat A. Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: a comparative review. Drugs Aging. 2005;22:749–765. doi: 10.2165/00002512-200522090-00004. PMID:16156679. [DOI] [PubMed] [Google Scholar]
- 3.Parrino L, Terzano MG. Polysomnographic effects of hypnotic drugs. A review. Psychopharmacology (Berl) 1996;126:1–16. doi: 10.1007/BF02246405. PMID:8853211. [DOI] [PubMed] [Google Scholar]
- 4.Terzano MG, Rossi M, Palomba V, Smerieri A, Parrino L. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem and zaleplon. Drug Saf. 2003;26:261–282. doi: 10.2165/00002018-200326040-00004. PMID:12608888. [DOI] [PubMed] [Google Scholar]
- 5.Scott MA, Stigleman S, Cravens D. Clinical inquiries. What is the best hypnotic for use in the elderly? J Fam Pract. 2003;52:976–978. PMID:14653987. [PubMed] [Google Scholar]
- 6.Troy SM, Lucki I, Unruh MA, Cevallos WH, Leister CA, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam on memory, learning, and psychomotor performance. J Clin Psychopharmacol. 2000;20:328–337. doi: 10.1097/00004714-200006000-00007. PMID:10831020. [DOI] [PubMed] [Google Scholar]
- 7.Swainston Harrison T, Keating GM. Zolpidem: a review of its use in the management of insomnia. CNS Drugs. 2005;19:65–89. doi: 10.2165/00023210-200519010-00008. PMID:15651908. [DOI] [PubMed] [Google Scholar]
- 8.Frey DJ, Ortega JD, Wiseman C, Farley CT, Wright KP., Jr. Influence of zolpidem and sleep inertia on balance and cognition during nighttime awakening: a randomized placebo-controlled trial. J Am Geriatr Soc. 2011;59:73–81. doi: 10.1111/j.1532-5415.2010.03229.x. PMID:21226678. [DOI] [PubMed] [Google Scholar]
- 9.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. J Am Geriatr Soc. 2001;49:1685–1690. doi: 10.1111/j.1532-5415.2001.49280.x. PMID:11844004. [DOI] [PubMed] [Google Scholar]
- 10.Finkle WD, Der JS, Greenland S, Adams JL, Ridgeway G, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59:1883–1890. doi: 10.1111/j.1532-5415.2011.03591.x. PMID:22091502. [DOI] [PubMed] [Google Scholar]
- 11.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. PMID:16084776. [DOI] [PubMed] [Google Scholar]
- 12.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. PMID:16085466. [DOI] [PubMed] [Google Scholar]
- 13.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. PMID:7843344. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. PMID:6639693. [DOI] [PubMed] [Google Scholar]
- 15.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;1:1–115. PMID:3672938. [PubMed] [Google Scholar]
- 16.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. PMID:3611032. [PubMed] [Google Scholar]
- 17.Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. http://dx.doi.org/10.1300/J018v05n01_09. [Google Scholar]
- 18.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. PMID:8437031. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. PMID:2748771. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, III, Monk TH, Hoch CC, Yeager AL, et al. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991;14:331–338. PMID:1947597. [PubMed] [Google Scholar]
- 21.Allain H, Bentue-Ferrer D, Tarral A, Gandon JM. Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo. Eur J Clin Pharmacol. 2003;59:179–188. doi: 10.1007/s00228-003-0591-5. PMID:12756510. [DOI] [PubMed] [Google Scholar]
- 22.Berlin I, Warot D, Hergueta T, Molinier P, Bagot C, et al. Comparison of the effects of zolpidem and triazolam on memory functions, psychomotor performances, and postural sway in healthy subjects. J Clin Psychopharmacol. 1993;13:100–106. PMID:8463441. [PubMed] [Google Scholar]
- 23.Danjou P, Paty I, Fruncillo R, Worthington P, Unruh M, et al. A comparison of the residual effects of zaleplon and zolpidem following administration 5 to 2 h before awakening. Br J Clin Pharmacol. 1999;48:367–374. doi: 10.1046/j.1365-2125.1999.00024.x. PMID:10510148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. PMID:9832970. [DOI] [PubMed] [Google Scholar]
- 25.Stone KL, Ancoli-Israel S, Blackwell T, Ensrud KE, Cauley JA, et al. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168:1768–1775. doi: 10.1001/archinte.168.16.1768. PMID:18779464. [DOI] [PubMed] [Google Scholar]
- 26.Mamdani M, Rapoport M, Shulman KI, Herrmann N, Rochon PA. Mental health-related drug utilization among older adults: prevalence, trends, and costs. Am J Geriatr Psychiatry. 2005;13:892–900. doi: 10.1176/appi.ajgp.13.10.892. PMID:16223968. [DOI] [PubMed] [Google Scholar]