Abstract
Low-dose IL-2 administration suppresses unwanted immune responses in mice and humans, thus evidencing the potential of IL-2 to treat autoimmune disorders. Increased Tregs activity is one of the potential mechanisms by which low-dose IL-2 immunotherapy induces immunosuppression. In addition, recent data indicate that IL-2 may contribute to prevent unwanted self-reactive responses by preventing the developing of T-follicular helper cells, a CD4+ T-cell subset that expands in autoimmune disease patients and promotes long-term effector B-cell responses. Here we discuss the mechanisms underlying the clinical benefits of low-dose IL-2 administration, focusing on the role of this cytokine in promoting Treg-mediated suppression and preventing self-reactive T-follicular helper cell responses.
Keywords: autoimmune disease, germinal center B cells, IL-2, immunotherapy, T follicular helper cells, Tregs
When first discovered in 1976, IL-2 was characterized as a soluble factor with the unique ability to promote clonal expansion of T cells in vitro [1]. Follow-up studies confirmed this property [2] and further demonstrated the capacity of IL-2 to augment NK [3] cell activity and to promote tumor regression following in vivo administration [4]. Based on these studies, the US FDA granted the use of high-dose bolus IL-2 regimens for the treatment of metastatic renal cancer in 1992, and for the treatment of metastatic melanoma in 1998. Although less successfully, IL-2 has been evaluated to boost T-cell responses in patients with chronic viral infections, such as HIV [5], to increase anti-tumor responses following vaccination with tumor antigens [6] and to enhance the efficacy of antitumor adoptive T-cell transfer therapies [7,8]. Nevertheless, despite it being demonstrated that high doses of intravenous IL-2 induces tumor regression in a small fraction of patients with metastatic melanoma and metastatic renal cell carcinoma, the clinical benefits of high-dose IL-2-based immunotherapies were lower than initially expected [4].
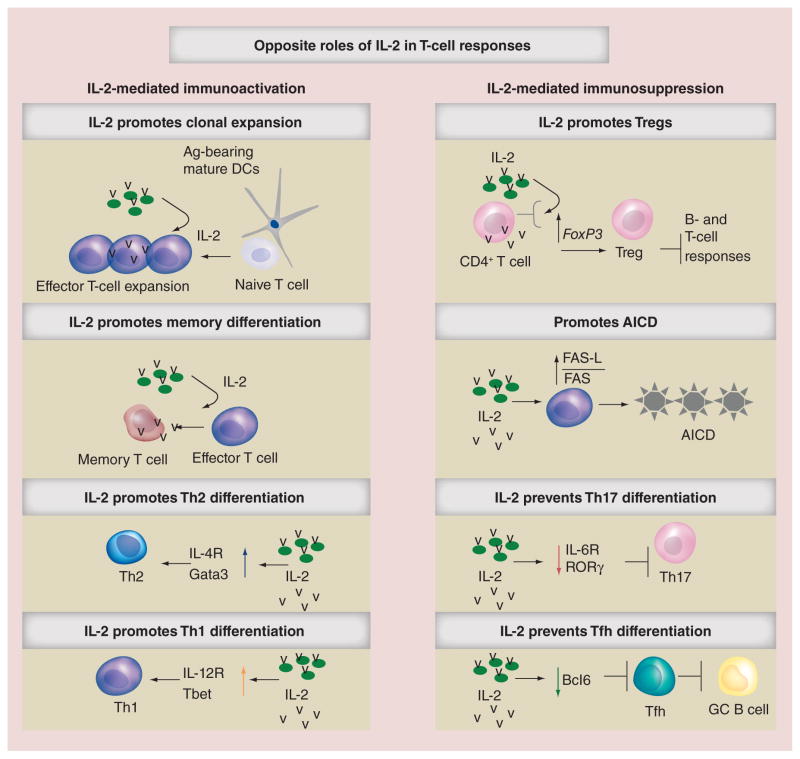
The identification of regulatory T cells (Tregs) in 1995 [9] contributed to a better understanding of the biological effects of IL-2, and provided a plausible explanation for the low efficacy of the majority of IL-2-based therapies. Given that Tregs have the capacity to suppress T- and B-cell responses [10], and that IL-2 is required for their development and function [10], the expansion of suppressor Tregs following IL-2 administration has been suggested to be the primary cause for the limited effectiveness of IL-2 therapies. Notably, in addition to suppress immune responses by promoting Treg-mediated immunosuppression, IL-2 also prevents effector immune responses by multiple Treg-independent mechanisms. For example, although IL-2 signaling during the primary response to pathogens is required for the differentiation of functional memory cells [11], excessive IL-2 signaling in T cells promotes terminal differentiation [12,13], precludes memory formation [12,13] and enhances the susceptibility of T cells to activation-induced cell death [14]. Similarly, although required for Th1 and Th2 differentiation [15], IL-2 signaling inhibits the development of Th17 cells [16] and prevents T-follicular helper (Tfh) cell differentiation [17–21], a CD4+ T-cell subset required for the generation of protective B-cell responses to pathogens [22]. Thus, IL-2/IL-2 receptor interactions can both promote and suppress immune responses depending on the dose and type of target cell activated by IL-2, highlighting the complexity of the pleiotropic nature of this cytokine (Figure 1). More Importantly and contrary to what was initially predicted, self-reactive T and B cells undergo uncontrolled expansion in IL-2- and IL-2 receptor-deficient mice, which develop a severe autoimmune syndrome [23–25]. Similarly, autoimmune disease development is linked to IL-2 deficiencies in humans [26–28], most likely due to the expansion of immature nonfunctional Tregs in the absence of IL-2 signals [29,30]. The close association between IL-2 deficiency and autoimmune disorders supports the important role of IL-2 in suppressing unwanted immune responses and maintaining immune tolerance.
Figure 1. The pleiotropic roles of IL-2 in controlling T-cell responses.
Ag: Antigen; AICD: Activation-induced cell death; DC: Dendritic cell; Tfh: T-follicular helper; Th: T helper.
Recent studies show that low-dose IL-2 regimens can be safely administered to patients [31–34] and have potent immunosuppressive effects [31,32,35], thus evidencing the potential of low-dose IL-2-based immunotherapies to treat autoimmune disorders. Here, we review our current knowledge about low-dose IL-2-based immunotherapies, and discuss the potential mechanisms underlying the clinical benefits of these treatments, focusing on the potential role of IL-2-based immunotherapies in Treg and Tfh cells.
IL-2 receptor & IL-2 signaling
IL-2 is a 15.5 kDa four-bundle, α-helical protein member of the common cytokine receptor γ-chain family of cytokines, which includes the immunoregulatory cytokines IL-4, IL-7, IL-9, IL-15 and IL-21 [36]. Antigen (Ag)-experienced activated T cells, particularly effector CD4+ T cells (Teff), are considered the principal source of IL-2 in vivo, although other immune cells, such as dendritic cells (DCs) [37] and B cells [38], also produce significantly large amounts of IL-2 following activation. IL-2 signaling is transmitted into the cell through distinct membrane-bound heterotrimeric and heterodimeric IL-2 receptor (IL-2R) complexes, consisting of the non-covalent association of the cell surface receptors IL-2Rα (CD25), IL-2Rβ (CD122), also part of the IL-15R; and the common γ chain (CD132) [39,40], shared by the members of the common receptor γ-chain cytokine family. The heterodimeric association of the IL-2Rβ chain and the common γ chain forms the intermediate-affinity IL-2R (binding affinity Kd ≈ 1 nM), present in resting T cells, memory CD8+ T cells and NK cells [36]. By contrast, the IL-2Rαβγ hetero trimeric complex, also known as the high-affinity IL-2R receptor (Kd ≈ 10 pm), is transiently expressed by activated immune cells, including T and B lymphocytes, NK cells, and DCs even though it is constitutively expressed on FoxP3-expressing CD4+ Tregs [9].
Although IL-2Rα alone is not directly involved in signal transduction due to its short cytoplasmic domain, the binding of IL-2 to IL-2Rα facilitates IL-2/IL-2Rβ interactions and subsequent IL-2Rαβγc complex assembly [39,40]. In this regard, the formation of the IL-2/L-2Rα binary complex induces a conformational change in the IL-2 helix C that relocates IL-2 residue Asn88 into hydrogen bonding distance with IL-2Rβ residue Arg42, stabilizing a complementary binding site for presentation of IL-2 to IL-2Rβ [39]. This conformational change enables IL-2/ IL-2Rβ interactions, the formation of an IL-2Rα-IL-2Rβ-IL-2 ternary complex, and the subsequent recruitment of the γ chain to form the IL-2Rαβγc heterotrimeric complex. Following IL-2 binding, the JAK1 and the JAK3 phosphorylate tyrosine residues within the cytoplasmic domain of the β and γ chains, creating docking sites for the recruitment of the transcription factor Stat5 (Stat5a and Stat5b) and the adaptor Shc into the cytoplasmic tails of the β and γ chains (reviewed in [36,41]). Stat5 phosphorylation by the JAKs facilitates the dissociation of the Stat5 protein from their docking sites and induces the formation of heterodimeric Stat5a/b complexes that translocate into the nucleus and regulate a broad range of target genes by binding to target DNA sequences. On the other hand, phosphorylation of the adaptor Shc leads to the activation of the Ras–Raf MAP kinase and PI-3K pathways in response to IL-2 [42]. Together, Stat5a/b, Ras-Raf MAP kinase and PI-3K signaling pathways allow rapid delivery of IL-2 signals from the membrane to the nucleus and control, in a direct and indirect manner, the expression and function of multiple master regulator transcription factors implicated in several differentiation and immunoregulatory pathways. For example, IL-2 signaling induces the expression of the FoxP3 [10] transcription factor, required for Treg development, and the expression of the transcription factors T-bet [15,18] and GATA-3 [15], which are necessary for Th1 and Th2 differentiation. On the contrary, sustained IL-2 signaling prevents the expression of RORγ [16] and Bcl6 [17–20,43], which are required for Th17 and Tfh cell development, respectively.
Although it is generally considered that IL-2 acts as a soluble factor that typically engages membrane-bound CD25, alternative IL-2/IL-2R interactions have been described. For example, DCs can upregulate CD25 in response to certain stimuli [44] and may use their CD25 to capture IL-2 and present it in trans to CD4+ T cells expressing intermediate-affinity IL-2Rβγ complexes [45]. This model is consistent with data showing that T cells [46,47] and DCs [45,48,49] directionally secrete IL-2 towards the T cell-DC interface. In this scenario, local concentration of IL-2 at the particular microenvironment of the DC-T cell contact may facilitate CD25/IL-2 binding by DCs and transpresentation to CD4+ T cells, despite the rapid on/off dissociation rates and the low affinity of CD25 for IL-2. In agreement with this model, selective blockade of CD25 on DCs by daclizumab, a humanized anti-CD25 monoclonal antibody, prevents T cell expansion by activated DCs [45]. Recent studies have also demonstrated the immunoregulatory properties of a soluble form of CD25 (sCD25) that has been detected in the serum of patients with systemic lupus erythematosus (SLE) [50–53], multiple sclerosis (MS) [54,55] and type I diabetes (T1D) [54]. Although the exact role played by sCD25 is still unclear, recent work suggests that sCD25 prevents IL-2 signaling by competing with membrane-bound CD25 for circulating IL-2, and exacerbates experimental autoimmune encephalitis (EAE) by allowing aberrant Th17 responses [56].
IL-2 & Treg homeostasis
Two different populations of Tregs are defined based on their anatomical origin, thymus-derived Tregs (tTregs) and peripherally derived Tregs (pTregs) [10,57,58]. tTregs arise from the thymus, whereas pTregs are generated in the periphery from conventional naive CD4+ T cells. Both populations cooperate to enforce tolerance by several direct and indirect mechanisms of suppression, including inhibition of the stimulatory capacity of antigen-presenting cells, secretion of immunosuppressive cytokines, and competition with activated T cells for available IL-2 [10]. The important role played by tTregs and pTregs in maintaining immune tolerance is evidenced by the uncontrolled expansion of autoreactive T and B cells and the development of catastrophic autoimmune syndromes observed in humans [59,60] and mice [61,62] that carry genetic deficiencies in the FoxP3 gene, the signature transcription factor required for the development of Tregs [10].
Numerous studies over the last years have demonstrated that IL-2 plays a critical role in immune tolerance by enforcing FoxP3 expression. At the earliest stage of the development, IL-2 engagement in the thymus promotes expression of Foxp3 in developing CD4+ T cells, contributing to the development of mature and functional tTregs [29,63,64]. In the periphery, further IL-2/IL-2R interactions are required to maintain the competitive fitness of the tTreg pool [65–67]. In a similar way, IL-2 signaling in conjunction with TGF-β is required to convert naive CD4+ T cells to pTregs [68,69]. The principal mechanism by which IL-2 contributes to tTreg and pTreg development and function is by inducing Stat5 activation, which in turn facilitates FoxP3 expression [64,70,71]. Interestingly, some Foxp3-expressing cells are still detected in IL-2- and IL-2rα-deficient mice despite the lack of IL-2 signaling [65,66], most likely due to the redundant capacity of IL-7 and IL-15 to activate Stat5 and partially compensate for the absence of IL-2/IL-2R interactions [72,73]. However, these cells express lower levels of Foxp3 and other key Treg functional molecules, including CTLA-4, and demonstrate poor competitive fitness and function when compared with Tregs developing in wild-type animals [29,65,66]. This supports an essential and non-redundant role for IL-2 in promoting Treg homeostasis and function. In agreement with this, IL-2- and IL-2R-deficient mice develop severe autoimmune disease associated with uncontrolled expansion of autoreactive effector T and B cells [26–28]. However, adoptive transfer of Tregs from wild-type, but not Cd25−/− mice, restores immune tolerance and prevents autoimmune pathology [63,74]. Importantly, single nucleotide polymorphisms (SNPs) [55,75–81] and genetic deficiency in the α chain of the IL-2R genes [26–28] are linked to autoimmune disease development in humans. Furthermore, impaired IL-2 production by T cells is observed in SLE [82], T1D [75,83] and RA patients [84]. Given the essential role for IL-2 in Treg homeostasis, auto immune disease development in these patients has been suggested to be a consequence of Treg malfunction due to impaired IL-2 signaling [75].
In agreement with the capacity of Treg to suppress self-reactive T and B-cell responses, adoptive transfer of Tregs prevents autoimmune disease development in several experimental models [85]. Hence, the targeting of Tregs numbers and/or function in vivo may be a good strategy for treating autoimmune diseases. The generation of functional Tregs for adoptive transfer, however, requires laborious and expensive laboratory procedures, which compromises the clinical feasibility of this approach. Furthermore, since FoxP3 expression is intracellular, purification of viable Tregs for adoptive transfer without Teff contamination is virtually impossible. Studies performed in the past few years indicate that the administration of low doses of recombinant IL-2 (rIL2) selectively targets Treg expansion [32,86,87] and has therapeutic benefits in the treatment of several forms of autoimmune disease [31,88,89] and graft rejection after transplantation [32,87]. Thus, low-dose IL-2-based immunotherapies may represent a good alternative to Treg-adoptive transfer approaches in order to boost Treg-mediated immunosuppression in vivo and to treat autoimmune disease.
Low-dose IL-2 therapy to treat autoimmune disease
The potential clinical benefits of low-dose rIL-2 immunotherapy have been recently evaluated in pre-clinical studies using non-obese diabetic (NOD) mice [86,90–92]. NOD mice spontaneously develop Type I (T1D) diabetes due to progressive immune-mediated destruction of insulin-producing β cells in the pancreatic islets of Langerhans [93]. Disease progression in this animal model is closely associated with reduced production of IL-2 [75,83,93], a defect mapped to the congenic T1D locus Idd3 on chromosome 3 [75], which correlates with defective Treg survival in the pancreatic islets [93]. Based on these data, it is generally believed that impaired intra-islet Treg homeostasis due to IL-2 shortage contributes to tolerance breakdown, self- reactive Teff cell expansion, and development of diabetes in NOD mice [93]. In support of this view, adoptive transfer of functional Tregs into NOD mice restores immune tolerance and prevents the development of diabetes [90,91]. SNPs in the Il-2 and Il-2rα receptor genes are also linked to T1D in humans [54,80,83], suggesting an important role for IL-2 in determining diabetes susceptibility in humans. Importantly, T1D-associated polymorphisms in Il-2rα gene correlate with altered CD25 expression, IL-2 hyporesponsiveness and diminished suppressor activity by Tregs [94,95].
Given the apparent causative relationship between local IL-2 deprivation, unbalance Teff:Treg responses and diabetes onset in NOD mice, this experimental model provides a valuable tool to evaluate the potential of IL-2-based immunotherapy to promote Treg suppression in vivo and to study its role in preventing disease progression. An elegant study by Qizhi Tang and colleagues [93] demonstrated that the administration of low doses of human rIL2 (25,000 IU) three times per week prevented the onset of diabetes in young prediabetic-female NOD mice. In this work, low-dose IL-2 immunotherapy significantly increased intra-islet Treg survival without significant effects in NK or Teff cells. In another study, low-dose IL-2 treatment starting at the time of T1D onset induced long-lasting diabetes remission, suggesting that IL-2 immunotherapy has the potential, not only to prevent, but also to reverse established disease [86]. The potential benefits of IL-2-based immunotherapy have also been demonstrated using the (NZBxNZW)F1 murine model of lupus [89]. Similarly to what was observed in the context of diabetes, T cells from lupus-prone mice [89,96] and human SLE patients [75,82,97,98] had impaired IL-2 production, suggesting a cause and effect relationship between IL-2 shortage and autoimmune disease progression that might be corrected by exogenous IL-2 administration.
While low-dose rIL-2 administration selectively targets pancreatic Tregs and prevents diabetes development, treatment with high doses of rIL-2 (250,000–500,000 IU) enhances pathogenic effector responses and precipitates the onset of T1D in pre-diabetic NOD mice [35,93,99]. The opposite effects of high and low doses of IL-2 illustrate the differential effects of IL-2 dosage in regulating the Treg:Teff balance. The current consensus is that treatment with relatively low levels of IL-2 selectively targets Treg expansion and subsequently prevents Teff responses, while treatment with high doses of IL-2 promotes effector T cell differentiation that may contribute to an accelerated tolerance breakdown [93]. The selective effect of low-dose IL-2 regimens in targeting Tregs may be explained by the fact that low IL-2R signaling is sufficient to support Treg development, while it is not sufficient to support Teff responses [100]. Moreover, Tregs constitutively express CD25 and may compete more efficiently with Teff cells for IL-2 when the physiological availability of this cytokine is limited [21,101].
In addition to promoting unwanted Teff responses, high-dose IL-2 administration also induces vascular leak syndrome [4], a life-threatening condition that results in a hypovolemic state and fluid accumulation in the extravascular space. Although some studies indicate that low-dose IL-2 regimens can be safely administered to patients [31–34,102], it may be difficult to deduce the appropriate dose of IL-2 for a heterogeneous populations of patients. Therefore, the multi-factorial potential toxicity of IL-2 could compromise the applicability of IL-2-based immunotherapies for the treatment of autoimmune disease, especially if administered for an extended period of time. Notably, when IL-2 is infused in complex with the anti-IL-2 monoclonal antibody JES6–1, the IL-2/JES6-1 complex selectively targets CD25-expressing cells and expands Tregs more efficiently than IL-2 administration alone [103]. This data suggest that the IL-2/anti-IL-2 complex may provide a valuable tool to improve IL-2-based immunotherapies, primarily by inducing similar biological effects to IL-2 monotherapy, but at lower concentration, which would potentially prevent unwanted IL-2-related side-effects. The benefits of IL-2/JES6-1 complex-administration have been demonstrated in several preclinical models. In this regard, several studies demonstrate that administration of low doses of IL-2/JES6-1 complexes prevents T1D development in NOD mice [86,92,93]. Interestingly, in the study from Diaz-de-Durana and colleagues [92], IL-2/ JES6-1 treatment correlated with increased in vivo β cell proliferation, suggesting a potential effect of IL-2 immunotherapy in β-cell regeneration [92]. However, the underlying mechanism behind this observation is unclear and further work needs to be done in order to better understand the potential role of IL-2/ JES6-1 complexes in β-cell regeneration. In another set of studies, IL-2/anti-IL-2 complex treatment suppresses collagen-induced arthritis [104], induces long-term acceptance of isles allografts [105], and suppresses experimental myasthenia in mice [106], further supporting the potential of the IL-2/JES6-1 complex in the treatment of autoimmune disorders. Interestingly, pretreatment of mice for 3 days with the IL-2/anti-IL-2 complexes expands Tregs and induces resistance to EAE, a model for multiple sclerosis [105]. However, when the IL-2/JES6-1 complex was administered to mice after EAE onset, no clinical benefit was observed, perhaps due to the concomitant expansion of preexisting CD25-expressing Teff following IL-2/JES6-1 complex injection [105].
In addition to a IL-2/IL-2R complex therapy, some groups have explored the potential benefits of combining IL-2 with rapamycin administration to more selectively target IL-2 to Tregs [35,105,107]. Rapamycin is an inhibitor of the PI3K/AKT/mTOR pathway that has the ability to prevent IL-2-dependent expansion of Teff without affecting Treg function [108]. This differential effect is due to the fact that Tregs express high levels of the PTEN and, unlike effector CD4+ and CD8+ T cells, do not activate the downstream targets of the PI3K pathway in response to IL-2 [108]. Although combinational therapies have shown positive synergistic effects in some experimental models [105,107], further studies are needed to elucidate the overall clinical benefits and to explain the detrimental effects observed in other studies [35,109].
Low-dose immunotherapy in humans
Two recent studies have evaluated the potential clinical benefits of low-dose rIL-2-based immunotherapy to treat patients with immune mediated disorders [31,87]. In one study, David Saadoun and colleagues [31] evaluated the effect of low-dose IL-2 administration in ten patients with hepatitis C virus-induced vasculitis (HCV). Patients who failed to respond to either antiviral therapy, Rituximab or both, received 1.5 million International Units (IU) of rIL2 per day for 5 days, followed by an additional 3 million IU per day at weeks 3, 6 and 9. Following 9 weeks of treatment, rIL-2 induced a significant increase in Tregs in all patients, and improved vasculitis in 8 out of 10 patients. In this study, low-dose IL2 immunotherapy did not induce significant adverse effects. In a study by Koreth et al. [87], a cohort of 29 patients with chronic graft-versus-host disease (GVHD) refractory to glucocorticoid therapy received rIL-2 at three dose levels (300,000, 1 million and 3 million IU) daily for a period of eight weeks. The numbers of Tregs increased in all patients, and approximately 50% of patients evaluated demonstrated an objective partial response. While IL-2 was tolerated at the lower doses, the highest dose of 3 million IU induced serious adverse effects. Furthermore, a more recent study showed that an ultra-low dose of IL-2 was well tolerated and may be associated with a lower incidence of GVHD after allogeneic hematopoietic stem cell transplantation in pediatric patients [34].
As aforementioned, preclinical studies using NOD mice demonstrated the potential of low-dose IL-2-based immunotherapy in T1D. Importantly, a recent clinical study indicates that low doses of rIL-2 can administrated to adults with established T1D with no serious side adverse effects [33]. Although the clinical benefits of IL-2 treatment have not been extensively characterized in this work, this study lays the basis for future clinical trials to study the effect of IL-2 immunotherapy in T1D patients.
The current consensus is that treatment with low-doses of IL-2 reestablishes immune tolerance by promoting Treg expansion. Interestingly, the therapeutic effect of IL-2 does not absolutely correlate with Treg expansion. In fact, IL-2 treatment of NOD mice starting at the time of T1D onset prevents disease progression and induces long-lasting diabetes remission without significantly altering the frequency of pancreatic Tregs [86]. Similarly, although treatment of HCV vasculitis and GVDH patients with low doses of IL-2 induced Tregs expansion in all subjects, not all patients showed clinical benefits after IL-2 administration [31,87]. Moreover, relative changes in the frequency of Tregs observed in the individual HCV vasculitis patients did not necessarily correlate with the relative reduction in cryoglobulinemia [31]. Thus, although it is clear that IL-2 boosts Treg-mediated immunosuppression in vivo, other additional Treg-independent mechanisms might also contribute in suppressing unwanted immune responses following IL-2 treatment.
IL-2-based immunotherapies to prevent self-reactive Tfh cell responses
While IL-2 signaling promotes Treg expansion and function, the same signaling pathway prevents the development of Tfh cells [17–20], a differentiated CD4+ T cell subset that plays a central role in the development and maintenance of the germinal center (GC) B cells [22,110]. Tfh cells are typically identified by the expression of the signature transcription factor Bcl6, the chemokine receptor CXCR5, and the inhibitory receptor PD-1 [22]. Bcl6 represses the expression of other lineage-specific transcription factors, particularly Blimp-1, and promotes the expression of genes implicated in Tfh cell development and function [22]. In fact, CD4+ T cells fail to differentiate into Tfh cells in the absence of Bcl6 and, as a consequence, GCs and long-term antibody responses are impaired in mice in which T cells fail to express Bcl6 [111–113]. On the other hand, expression of CXCR5 instructs homing of recently activated Tfh cells into the B cell follicles. Once in the B cell follicles, Tfh cells interact with activated B cells and provide them with unique survival and differentiation signals required for the generation and maintenance of GC B cells, including CD40 ligand [114,115] and IL-21 [116–118]. In the highly selective environment of the GC, activated B cells interacting with Tfh cells and follicular dendritic cells undergo extensive clonal expansion, somatic hyper-mutation and affinity maturation, and differentiate into memory B cells and long-lived plasma cells [119]. In the absence of Tfh cells, GCs do not form, memory B cells and long-lived PC are not generated, and long-term antibody responses are impaired. This highlights the importance of Tfh cells in the development of antigen-specific B cell immunity [17,111–113].
The balance between the transcription factors Bcl6 and Blimp-1, which respectively enforce and repress Tfh cell development, largely influences the commitment of Ag-experienced CD4+ T cells into the Tfh cell differentiation pathway [22]. Recent works demonstrate that IL-2 regulates this balance by directly promoting Blimp-1 expression via Stat5 [19,20], which in turn represses Bcl6 and precludes Tfh cell development. In addition to repress Bcl6 expression, IL-2 signaling also influences Tfh cell differentiation by preventing the biological activity of Bcl6 via T-bet [18]. In this regard, an elegant study by Oestreich et al. shows that high levels of IL-2 increase the T-bet/Bcl6 ratio and favors the formation of T-bet-Bcl6 complexes that mask the Bcl-6 DNA-binding domain and prevent it from binding to its target genes [18].
In agreement with the inhibitory role of IL-2 in Tfh cell differentiation, we have recently shown that IL-2 administration prevents the development of Tfh cells following influenza virus infection, indicating that the physiological availability of IL-2 is a critical factor for the regulation of Tfh cell responses in vivo [17]. IL-2 treatment also precluded influenza-specific GCs and long-lived PC responses in this work. However, these observations were not due to a direct effect of IL-2 on B cells, but instead due to the absence of help from Tfh cells. More importantly, high IL-2 levels suppressed Tfh cell responses in the absence of Tregs [17,21], suggesting that Tregs are not required for the ability of IL-2 to repress Tfh cell responses. Collectively, these studies demonstrate an immunosuppressive function of IL-2 that is independent on its role on Treg homeostasis, and provide and alternative mechanism to explain the clinical benefits of IL-2 immunotherapies to treat antibody-mediated autoimmune disorders. Furthermore, these data offer new insights into how polymorphisms in the IL-2 and IL-2R genes can affect self-reactive Tfh and B cell responses and influence the development of autoimmune disease manifestations.
Conclusion & future perspective
GCs with self-reactive B cells are often detected in human patients [120–124] and in animal models with several forms of autoimmune disease, including SLE [125–127] and diabetes [126,128]. Some studies also indicate that ectopic GC formation in non-lymphoid tissues could contribute to pathogenesis of lupus tubulointerstitial inflammation in human SLE patients [120] and to synovial inflammation in rheumatoid arthritis [124,129]. Although the exact immunological mechanisms by which self-reactive GC B cells may contribute to disease pathogenesis is not clear, production of auto-antibodies from PC arising from self-reactive GCs may be one of the mechanisms by which autoreactive GCs might contribute to autoimmune disease pathogenesis and progression. In addition, to promote Ab responses, GC B cells also efficiently present Ag to CD4+ T cells [130,131], particularly when Ag availability is limited, secrete proinflammatory cytokines, and facilitate DC:T encounters by regulating DC and T-cell positioning within the secondary lymphoid organs [132,133]. Therefore, self-reactive GC responses might also contribute to autoimmune disease pathogenesis by several antibody-independent mechanisms [134].
In humans, B-cell depletion therapy is approved for the treatment of RA, and has been evaluated for the treatment of other forms of autoimmune diseases, including T1D [135] and SLE [136–138], although the clinical benefits of B-cell depletion are lower than initially predicted [139]. Importantly, although existing B-cell depleting agents efficiently deplete the majority of circulating naive B cells, mature B cells and memory B cells, they have limited impact on GC B cells and long-lived PCs [140–142]. Thus, survival of long-lived PCs and continuous replenishment of long-lived PCs from the GCs provides a plausible explanation for the long-term stability of some autoantibody species observed after B-cell depletion and the limited efficacy of these therapies.
Notably, self-reactive Tfh cells expand and contribute to autoimmune disease development in several mouse models [143,144]. Likewise, expansion of Tfh-like CD4+ T cells is observed in autoimmune patients [145–151], and circulating Tfh cells and circulating CCR7loPD-1hiCX-CR5hi Tfh precursors, have been suggested as a potential biomarker of disease in various autoimmune disorders [143,144,152]. Based on the central role of Tfh cells in controlling GC B-cell maintenance and their putative role in autoimmune disease development, it is reasonable to speculate that depletion of Tfh cells may disrupt pre-existing self-reactive GC structures and preclude the repopulation of the long-lived PC compartment with self-reactive PCs. Supporting the potential therapeutic benefits of Tfh depletion-based therapies, in vivo blockade of factors implicated in Tfh homeostasis and function, such as CD40 [123,126] or IL-21 [153–155], prevents disease progression in several preclinical models. Importantly, given that elevated physiological levels of IL-2 preclude Tfh cell responses, it is possible that, in addition to promoting Tregs expansion, treatment with IL-2 also prevents self-reactive Tfh cell responses in vivo, thereby hindering the maintenance of self-reactive GC B cells and subsequent immunopathology. Although, the dose-effect of IL-2 in Tfh cell differentiation has not been formally addressed, we found that treatment of influenza-infected mice with relatively low doses of IL-2 prevented influenza-specific Tfh cell responses, without necessary affecting the expansion of influenza-specific Teff cells [17]. Therefore, the potential effects of IL-2-based immunotherapies on Tfh cells should now be considered when using low IL-2 doses in preclinical studies and clinical trials.
The data summarized in this review support the potential of low IL-2-based immunotherapies to treat antibody-mediated autoimmune disorders. However, the toxicity and pleiotropic effects of IL-2 limit the clinical applicability of these therapies. New therapeutic approaches to prevent undesirable adverse effects, and specifically target IL-2 to Tregs and/or Tfh cells, will significantly improve the efficacy of these therapies. One potential way to achieve these goals is to explore the potential synergistic effects of combining IL-2 administration with blockade of cytokine pathways that prevent Tregs function and/or promote Tfh cell development. For example, while IL-2 signaling promotes Treg expansion and function, IL-6 suppresses Treg activity [156]. Thus, one could speculate that co-administration of low doses of IL-2 (or IL-2/anti IL-2 complexes) together with anti-IL6R (currently FDA-approved for the treatment of RA) might synergize to promote Tregs-mediated suppression. Notably, IL-6 also favors Bcl6 expression and Tfh cell differentiation [157–160]. Thus, in addition to enhancing Treg activity, combined IL-2 and anti-IL-6R administration could prevent unwanted Tfh cell responses, thereby precluding self-reactive GC B-cell responses.
The use of IL-2/anti-IL-2 complexes to target CD25-expressing cells and expand Tregs also represent a promising strategy to promote IL-2 mediated immunosuppression without inducing IL-2-mediated expansion of autoreactive Teff cells. However, no clinical studies have yet been conducted to evaluate the safety and the clinical benefits of IL-2/anti-IL-2 complex-based immunotherapies when administered to human patients. Therefore, future studies need to be done in order to further validate the results obtained with the animal experimental models, and to determine the appropriate dose of IL-2/anti-human IL-2 complexes that can be safely administered to patients.
Executive summary.
IL-2-based immunotherapy
IL-2 can promote and inhibit immune responses depending on the dose and type of target cells.
Low-dose IL-2 immunotherapy selectively favors the immunosuppressive functions of IL-2 and shows clinical benefits in the treatment of some autoimmune disorders.
IL-2 promotes regulatory T cell mediated immunosuppression
Regulatory T cells (Tregs) prevent self-reactive responses by multiple direct and indirect mechanisms of suppression.
The transcription factor Foxp3 is required for the development and function of Tregs.
IL-2 plays a critical role in immune tolerance by enforcing FoxP3 expression.
Impaired IL-2 signaling is linked to Treg malfunction and autoimmune disease development in human patients.
Low-doses of IL-2 therapy selectively targets Treg expansion.
Low-dose IL-2-based immunotherapies may prevent unwanted self-reactive responses by promoting Treg-mediated immunosuppression.
IL-2/anti-IL-2 complex immunotherapy
IL-2 infused in complex with the anti-IL-2 monoclonal antibody JES6-1 selectively targets CD25-expressing cells and expands Tregs more efficiently than IL-2 administration alone.
IL-2/anti-IL-2 complex induce similar biological effects to IL-2 monotherapy, but at lower concentration.
The benefits of IL-2/JES6-1 complex administration have been demonstrated in several preclinical models.
T-follicular helper cells & autoimmune disorders
T-follicular helper (Tfh) cells help germinal center (GC) B-cell responses.
Self-reactive Tfh cells expand and contribute to autoimmune disease development in preclinical models and humans with autoimmune disorders.
Self-reactive GC B cells are present in human patients and animal models with several forms of autoimmune disease.
Survival of GC B cells provides a plausible explanation for the long-term stability of some autoantibodies species observed after B-cell depletion and the limited efficacy of these therapies.
Depletion of Tfh cells may disrupt self-reactive GC structures and prevent B-cell dependent immunopathology.
IL-2 inhibits Tfh cell development
The transcription factor Bcl6 is required for the Tfh cell development and function.
IL-2 represses Bcl6 expression and biological activity.
IL-2 treatment prevents the development of Tfh cells, thereby hindering GC B-cell responses in vivo.
Tregs are not required for the ability of IL-2 to repress Tfh cell responses.
The effect of IL-2 in Tfh cells demonstrates an immunosuppressive function of IL-2 that is independent of its role on Treg homeostasis.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work supported by a subaward of AR 048311 to A Ballesteros-Tato and by an NIH grant AI109962 (director: T Randall). The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 2.Wagner H, Hardt C, Heeg K, Rollinghoff M, Pfizenmaier K. T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature. 1980;284(5753):278–278. doi: 10.1038/284278a0. [DOI] [PubMed] [Google Scholar]
- 3.Henney CS, Kuribayashi K, Kern DE, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4(127):127ps128. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pett SL, Kelleher AD, Emery S. Role of interleukin-2 in patients with HIV infection. Drugs. 2010;70(9):1115–1130. doi: 10.2165/10898620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86(15):1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. First study characterizing Tregs. [PubMed] [Google Scholar]
- 10.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441(7095):890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32(1):79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32(1):91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353(6347):858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 15.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23(5):598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 17•.Ballesteros-Tato A, Leon B, Graf BA, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36(5):847–856. doi: 10.1016/j.immuni.2012.02.012. Demonstrates that IL-2 administration prevents the development of T-follicular helper (Tfh) cells following influenza virus infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. 2012;13(4):405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209(2):243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurieva RI, Podd A, Chen Y, et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012;287(14):11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon B, Bradley JE, Lund FE, Randall TD, Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun. 2014;5:3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor beta chain. J Exp Med. 1997;185(3):499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadlack B, Lohler J, Schorle H, et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25(11):3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268(5216):1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 26.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1997;94(7):3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119(2):482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Roifman CM. Human IL-2 receptor alpha chain deficiency. Pediatr Res. 2000;48(1):6–11. doi: 10.1203/00006450-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Cheng G, Yu A, Dee MJ, Malek TR. IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol. 2013;190(4):1567–1575. doi: 10.4049/jimmunol.1201218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan X, Cheng G, Malek TR. The importance of regulatory T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259(1):103–114. doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365(22):2067–2077. doi: 10.1056/NEJMoa1105143. Low-dose rIL-2 treatment significantly increases Tregs and improves vasculitis in patients with hepatitis C virus-induced vasculitis. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra143. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartemann A, Bensimon G, Payan CA, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a Phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1(4):295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. Ultra Low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20(8):2215–2225. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeyens A, Perol L, Fourcade G, et al. Limitations of IL-2 and rapamycin in immunotherapy of type 1 diabetes. Diabetes. 2013;62(9):3120–3131. doi: 10.2337/db13-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 37.Zelante T, Fric J, Wong AY, Ricciardi-Castagnoli P. Interleukin-2 production by dendritic cells and its immunoregulatory functions. Front Immunol. 2012;3:161. doi: 10.3389/fimmu.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1(6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310(5751):1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 40.Rickert M, Wang X, Boulanger MJ, Goriatcheva N, Garcia KC. The structure of interleukin-2 complexed with its alpha receptor. Science. 2005;308(5727):1477–1480. doi: 10.1126/science.1109745. [DOI] [PubMed] [Google Scholar]
- 41.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7(5):679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 43.Walker SR, Nelson EA, Frank DA. STAT5 represses BCL6 expression by binding to a regulatory region frequently mutated in lymphomas. Oncogene. 2007;26(2):224–233. doi: 10.1038/sj.onc.1209775. [DOI] [PubMed] [Google Scholar]
- 44.Fukao T, Koyasu S. Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur J Immunol. 2000;30(5):1453–1457. doi: 10.1002/(SICI)1521-4141(200005)30:5<1453::AID-IMMU1453>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 45.Wuest SC, Edwan JH, Martin JF, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med. 2011;17(5):604–609. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabatos CA, Doh J, Chakravarti S, et al. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29(2):238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7(3):247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 48.Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J Immunol. 2003;170(10):5075–5081. doi: 10.4049/jimmunol.170.10.5075. [DOI] [PubMed] [Google Scholar]
- 49.Kulhankova K, Rouse T, Nasr ME, Field EH. Dendritic cells control CD4+CD25+ Treg cell suppressor function in vitro through juxtacrine delivery of IL-2. PLoS ONE. 2012;7(9):e43609. doi: 10.1371/journal.pone.0043609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokano Y, Murashima A, Takasaki Y, Hashimoto H, Okumura K, Hirose S. Relation between soluble interleukin 2 receptor and clinical findings in patients with systemic lupus erythematosus. Ann Rheum Dis. 1989;48(10):803–809. doi: 10.1136/ard.48.10.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawada S, Hashimoto H, Iijma S, et al. Increased soluble IL-2 receptor in serum of patients with systemic lupus erythematosus. Clin Rheumatol. 1993;12(2):204–209. doi: 10.1007/BF02231527. [DOI] [PubMed] [Google Scholar]
- 52.Ward MM, Dooley MA, Christenson VD, Pisetsky DS. The relationship between soluble interleukin 2 receptor levels and antidouble stranded DNA antibody levels in patients with systemic lupus erythematosus. J Rheumatol. 1991;18(2):235–240. [PubMed] [Google Scholar]
- 53.Ter Borg EJ, Horst G, Limburg PC, Kallenberg CG. Changes in plasma levels of interleukin-2 receptor in relation to disease exacerbations and levels of anti-dsDNA and complement in systemic lupus erythematosus. Clin Exp Immunol. 1990;82(1):21–26. doi: 10.1111/j.1365-2249.1990.tb05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maier LM, Lowe CE, Cooper J, et al. IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PLoS Genet. 2009;5(1):e1000322. doi: 10.1371/journal.pgen.1000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maier LM, Anderson DE, Severson CA, et al. Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol. 2009;182(3):1541–1547. doi: 10.4049/jimmunol.182.3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell SE, Moore AC, Fallon PG, Walsh PT. Soluble IL-2Ralpha (sCD25) exacerbates autoimmunity and enhances the development of Th17 responses in mice. PLoS ONE. 2012;7(10):e47748. doi: 10.1371/journal.pone.0047748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 58.Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14(4):307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 59.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 60.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 61.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 62.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 63.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17(2):167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 64.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28(1):100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 66.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6(11):1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 67.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178(7):4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 68.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178(4):2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 69.Davidson TS, Dipaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178(7):4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 70.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178(1):280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 71.Yao Z, Kanno Y, Kerenyi M, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109(10):4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181(5):3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bayer AL, Lee JY, De La Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181(1):225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furtado GC, Curotto De Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196(6):851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamanouchi J, Rainbow D, Serra P, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39(3):329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brand OJ, Lowe CE, Heward JM, et al. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves’ disease using a multilocus test and tag SNPs. Clin Endocrinol. 2007;66(4):508–512. doi: 10.1111/j.1365-2265.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- 77.Matesanz F, Fedetz M, Collado-Romero M, et al. Allelic expression and interleukin-2 polymorphisms in multiple sclerosis. J NeuroImmunol. 2001;119(1):101–105. doi: 10.1016/s0165-5728(01)00354-x. [DOI] [PubMed] [Google Scholar]
- 78.Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vella A, Cooper JD, Lowe CE, et al. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76(5):773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lowe CE, Cooper JD, Brusko T, et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet. 2007;39(9):1074–1082. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 81.Hafler DA, Compston A, Sawcer S, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357(9):851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 82.Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J BioMed Biotechnol. 2010;2010:740619. doi: 10.1155/2010/740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hulme MA, Wasserfall CH, Atkinson MA, Brusko TM. Central role for interleukin-2 in type 1 diabetes. Diabetes. 2012;61(1):14–22. doi: 10.2337/db11-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitas GD, Salmon M, Farr M, Gaston JS, Bacon PA. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin Exp Immunol. 1988;73(2):242–249. [PMC free article] [PubMed] [Google Scholar]
- 85.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 86.Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87••.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. Demonstrates that patients with chronic graft-versus-host disease respond to low-dose IL-2 treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Churlaud G, Jimenez V, Ruberte J, et al. Sustained stimulation and expansion of Tregs by IL2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin Immunol. 2014;151(2):114–126. doi: 10.1016/j.clim.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 89•.Humrich JY, Morbach H, Undeutsch R, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci USA. 2010;107(1):204–209. doi: 10.1073/pnas.0903158107. Indicates that IL-2 shortage and autoimmune disease progression in lupus prone mice can be prevented by exogenous IL-2 administration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199(11):1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diaz-De-Durana Y, Lau J, Knee D, et al. IL-2 immunotherapy reveals potential for innate beta cell regeneration in the non-obese diabetic mouse model of autoimmune diabetes. PLoS ONE. 2013;8(10):e78483. doi: 10.1371/journal.pone.0078483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28(5):687–697. doi: 10.1016/j.immuni.2008.03.016. Demonstrates that administration of low doses of rIL2 prevented the onset of diabetes in young prediabetic-female non-obese diabetic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dendrou CA, Plagnol V, Fung E, et al. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat Genet. 2009;41(9):1011–1015. doi: 10.1038/ng.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garg G, Tyler JR, Yang JH, et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J Immunol. 2012;188(9):4644–4653. doi: 10.4049/jimmunol.1100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wofsy D, Dauphinee MJ, Kipper SB, Talal N. Interleukin-2 deficiency in murine systemic lupus erythematosus. Transact Assoc Am Phys. 1981;94:341–348. [PubMed] [Google Scholar]
- 97.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol. 2001;166(6):4216–4222. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- 98.Linker-Israeli M, Bakke AC, Kitridou RC, Gendler S, Gillis S, Horwitz DA. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE) J Immunol. 1983;130(6):2651–2655. [PubMed] [Google Scholar]
- 99.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R. Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes. 2002;51(3):638–645. doi: 10.2337/diabetes.51.3.638. [DOI] [PubMed] [Google Scholar]
- 100.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30(2):204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 102.Ito S, Bollard CM, Carlsten M, et al. Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol Ther. 2014;22(7):1388–1395. doi: 10.1038/mt.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103•.Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci USA. 2010;107(26):11906–11911. doi: 10.1073/pnas.1002569107. Demonstrates that antibody JES6-1, as the IL-2/JES6-1 complex, selectively targets CD25-expressing cells and expands Tregs more efficiently than IL-2 administration alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee SY, Cho ML, Oh HJ, et al. Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunol. 2012;137(4):305–316. doi: 10.1111/imm.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206(4):751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu R, Zhou Q, La Cava A, Campagnolo DI, Van Kaer L, Shi FD. Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol. 2010;40(6):1577–1589. doi: 10.1002/eji.200939792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shapiro AM, Suarez-Pinzon WL, Power R, Rabinovitch A. Combination therapy with low dose sirolimus and tacrolimus is synergistic in preventing spontaneous and recurrent autoimmune diabetes in non-obese diabetic mice. Diabetologia. 2002;45(2):224–230. doi: 10.1007/s00125-001-0745-x. [DOI] [PubMed] [Google Scholar]
- 108.Walsh PT, Buckler JL, Zhang J, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116(9):2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Long SA, Rieck M, Sanda S, et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs beta-cell function. Diabetes. 2012;61(9):2340–2348. doi: 10.2337/db12-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ballesteros-Tato A, Randall TD. Priming of T follicular helper cells by dendritic cells. Immunol Cell Biol. 2014;92(1):22–27. doi: 10.1038/icb.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johnston RJ, Poholek AC, Ditoro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11(8):681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 115.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7–2 in established germinal centers. J Immunol. 1995;155(2):556–567. [PubMed] [Google Scholar]
- 116.Linterman MA, Beaton L, Yu D, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207(2):353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vogelzang A, Mcguire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29(1):127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 118.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179(12):8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 119.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang A, Henderson SG, Brandt D, et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol. 2011;186(3):1849–1860. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pugh-Bernard AE, Silverman GJ, Cappione AJ, et al. Regulation of inherently autoreactive VH4–34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108(7):1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167(4):2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 123.Grammer AC, Slota R, Fischer R, et al. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003;112(10):1506–1520. doi: 10.1172/JCI19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann NY Acad Sci. 2003;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 125.Ding Y, Li J, Wu Q, et al. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J Immunol. 2013;191(4):1614–1624. doi: 10.4049/jimmunol.1300479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luzina IG, Atamas SP, Storrer CE, et al. Spontaneous formation of germinal centers in autoimmune mice. J leukocyte Biol. 2001;70(4):578–584. [PubMed] [Google Scholar]
- 127.Wang JH, New JS, Xie S, et al. Extension of the germinal center stage of B cell development promotes autoantibodies in BXD2 mice. Arthritis Rheum. 2013;65(10):2703–2712. doi: 10.1002/art.38059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kendall PL, Yu G, Woodward EJ, Thomas JW. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J Immunol. 2007;178(9):5643–5651. doi: 10.4049/jimmunol.178.9.5643. [DOI] [PubMed] [Google Scholar]
- 129.Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93(1):221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baumjohann D, Preite S, Reboldi A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38(3):596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 131.Deenick EK, Chan A, Ma CS, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33(2):241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13(7):681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Angeli V, Ginhoux F, Llodra J, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 134.Leon B, Ballesteros-Tato A, Misra RS, Wojciechowski W, Lund FE. Unraveling effector functions of B cells during infection: the hidden world beyond antibody production. Infect Disord Drug Targets. 2012;12(3):213–221. doi: 10.2174/187152612800564437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, Phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Merrill J, Buyon J, Furie R, et al. Assessment of flares in lupus patients enrolled in a Phase II/III study of rituximab (EXPLORER) Lupus. 2011;20(7):709–716. doi: 10.1177/0961203310395802. [DOI] [PubMed] [Google Scholar]
- 138.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 139.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6(6):326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dilillo DJ, Hamaguchi Y, Ueda Y, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180(1):361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- 141.Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci USA. 2008;105(12):4802–4807. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bekar KW, Owen T, Dunn R, et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol. 2013;92(1):64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- 144.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nat Rev Immunol. 2013;13(6):412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 145.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 146.Luo C, Li Y, Liu W, et al. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. J NeuroImmunol. 2013;256(1–2):55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 147.Zhu C, Ma J, Liu Y, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metabol. 2012;97(3):943–950. doi: 10.1210/jc.2011-2003. [DOI] [PubMed] [Google Scholar]
- 148.Liu R, Wu Q, Su D, et al. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res Ther. 2012;14(6):R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma J, Zhu C, Ma B, et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin Dev Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang J, Shan Y, Jiang Z, et al. High frequencies of activated B cells and T follicular helper cells are correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Exp Immunol. 2013;174(2):212–220. doi: 10.1111/cei.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu X, Shi Y, Cai Y, et al. Inhibition of increased circulating Tfh cell by anti-CD20 monoclonal antibody in patients with type 1 diabetes. PLoS ONE. 2013;8(11):e79858. doi: 10.1371/journal.pone.0079858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39(4):770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 153.Rankin AL, Guay H, Herber D, et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012;188(4):1656–1667. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bubier JA, Sproule TJ, Foreman O, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA. 2009;106(5):1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R. Fc reduces disease progression. J Immunol. 2007;178(6):3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
- 156.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 157.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. 2013;190(7):3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334(6057):825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nakayamada S, Poholek AC, Lu KT, et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J Immunol. 2014;192(5):2156–2166. doi: 10.4049/jimmunol.1300675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]