Abstract
Human herpesvirus-8 (HHV-8) replication in the oropharynx may play an important role in HHV-8 transmission and contribute to the development of Kaposi sarcoma (KS) in some individuals. Studies in the United States and Europe report high rates of HHV-8 DNA detection in saliva of HHV-8 infected men, but little is known about the natural history of HHV-8 among persons in sub-Saharan Africa, where prevalence of HHV-8 infection and KS is greatest. To address this gap, this study evaluated oral HHV-8 replication in a cohort of 40 HHV-8 seropositive Kenyan women. Study clinicians collected daily oral swabs from participants for up to 30 consecutive days, and swab samples were tested for HHV-8 DNA using quantitative, real-time polymerase chain reaction. HHV-8 was detected at least once in 27 (68%) participants, and the overall shedding rate was 23%. On days with HHV-8 detection, mean HHV-8 quantity was 4.5 log10 copies/ml. Among HIV-infected women, CD4 count ≥500 cells/mm3 versus <500 cells/mm3 was associated with higher HHV-8 copy number (4.8 log10 copies/ml vs. 3.4 log10 copies/ml; coef 1.2 [95% CI, 0.5–1.9]; P=0.001) and a higher HHV-8 shedding rate (49% vs.12%; RR, 4.2 [95% CI, 0.8–21.4]; P=0.08). No other factors were associated with HHV-8 shedding rate or copy number. The study demonstrates high rates and quantity of HHV-8 in the oropharynx of HHV-8 seropositive African women. These findings support the observation that oral replication is an essential feature of HHV-8 infection, with likely implications for HHV-8 transmission and KS pathogenesis.
Keywords: human herpesvirus 8, Kaposi sarcoma-associated herpesvirus, viral replication, Kaposi sarcoma, HIV-1
INTRODUCTION
Human herpesvirus-8 (HHV-8) is the oncogenic virus that causes all forms of Kaposi sarcoma (KS). HHV-8 seroprevalence varies considerably by geography; estimates of HHV-8 infection rates range from 1% to 8% of adults in the United States and Europe compared with 40–80% of adults in sub-Saharan Africa [Whitby et al., 1995; Gao et al., 1996; Simpson et al., 1996; Kedes et al., 1997; Angeloni et al., 1998; Bestetti et al., 1998; Calabro et al., 1998; Mayama et al., 1998; Gessain et al., 1999; Antman and Chang, 2000; Wawer et al., 2001; Eltom et al., 2002; Lavreys et al., 2003]. While the factors leading from HHV-8 infection to the development of KS are not fully understood, HHV-8 replication in the oropharynx is believed to play an essential role in HHV-8 transmission and KS pathophysiology [Corey et al., 2002]. HHV-8 is most consistently detected in the oropharynx, with studies reporting HHV-8 DNA in 15–57% of salivary samples of asymptomatic HHV-8 seropositive persons [Boldogh et al., 1996; Koelle et al., 1997; Blackbourn et al., 1998; Lucht et al., 1998; Pauk et al., 2000; Casper et al., 2004; Gandhi et al., 2004; Taylor et al., 2004; Miller et al., 2006; Johnston et al., 2009]. These high rates of oropharyngeal replication suggest that salivary transmission may be an important route of infection and that oral replication likely serves as an important reservoir of virus that contributes to systemic dissemination and ultimately the development of KS.
Most HHV-8 shedding studies to date have been conducted among men in the United States and Europe. Little is known about the natural history of HHV-8 in sub-Saharan Africa, where the burden of HHV-8 and KS is greatest. A few studies in African populations demonstrate high rates of HHV-8 detection in oral and plasma samples, but these reports are limited by cross-sectional design and small sample size [Taylor et al., 2004; Johnston et al., 2009]. In addition, few studies have focused on women, who have a different epidemiology of KS and may have different clinical manifestations of KS compared to men [Benedetti et al., 1991; Lassoued et al., 1991; Cooley et al., 1996; Nasti et al., 1999; Lampinen et al., 2000; Phipps et al., 2010]. Because African women represent a unique and important population for the study of HHV-8 transmission and KS pathophysiology, this study sought to characterize the frequency and quantity of HHV-8 detection in the oropharynx of Kenyan women, and to identify factors associated with frequent and high quantity HHV-8 replication.
PATIENTS AND METHODS
Participants and Procedures
HHV-8 seropositive, KS negative women participating in 2005 in the Mombasa Cohort, an ongoing prospective cohort study of women at risk or infected with HIV-1 in Mombasa, Kenya [Martin et al., 1998], were included in the study. Women were recruited into the cohort if they reported exchanging sex for money or gifts. As part of the cohort study procedures, participants presenting to the municipal study clinic were administered a standardized questionnaire concerning recent sexual practices, contraception methods, alcohol and tobacco use, and current symptoms of illness. They also received a physical examination, HIV antibody testing, and screening for genital tract infections, including bacterial vaginosis and syphilis as described elsewhere [Wang et al., 2001; McClelland et al., 2007]. The protocol was approved by the institutional review boards at Kenyatta National Hospital and the University of Washington. All participants provided written informed consent.
Study clinicians collected daily swabs of the oral mucosa by vigorously swabbing the buccal mucosa, gums, tongue, and hard palate for up to 30 consecutive days. Each swab was placed in a vial containing 1ml of digestion buffer [Ryncarz et al., 1999] and stored at room temperature for up to 4 hr, then sent to the laboratory for storage at −80°C. Samples were shipped to the University of Washington in liquid nitrogen dry shippers.
Laboratory Methods
Serum samples were tested for HHV-8 antibodies at the University of Washington using an immunofluorescence assay (IFA) to detect antibodies to latent and lytic HHV-8 proteins [Chandran et al., 1998]. Swab samples were evaluated for HHV-8 DNA by quantitative, real-time PCR as described previously [Pauk et al., 2000; Casper et al., 2007]. Samples with ≥150 copies/ml of HHV-8 DNA were considered positive [Magaret et al., 2007].
Statistical Analysis
Oral HHV-8 shedding rate and HHV-8 copy number were examined as outcomes. The HHV-8 shedding rate was defined as the number of days on which HHV-8 was detected by PCR on oral swabs, divided by the total number of days with PCR results. Quantity of shedding was measured by HHV-8 copy number as defined by the log10 copies of HHV-8 DNA per milliliter of digestion buffer in samples with HHV-8 detected.
Age, HIV status, age at sexual debut, years engaged in transactional sex, contraception methods, pregnancies and live births, alcohol and tobacco use, and concurrent infections were evaluated as potential covariates. CD4 counts were included in the analysis if obtained within 6 months of the HHV-8 oral sample collection. Parameterization of CD4 count was explored and best fit grouping was determined to be <500 and ≥500 cells/mm3. Women were classified as having bacterial vaginosis with Nugent score ≥7.
Risk factors for oral shedding were evaluated using Poisson regression. Univariate predictors with two-sided P-values ≤0.1 were included in multivariate models and backward elimination was applied to obtain a final model; two-sided P-valued ≤0.05 were considered statistically significant. Generalized estimating equations (GEE) with Gaussian distribution were used to evaluate risk factors for HHV-8 quantity. Correlation between HHV-8 shedding rate and quantity was measured with a Spearman-rank correlation coefficient. All statistical calculations were performed using Stata 9.1 (StataCorp, College Station, TX).
RESULTS
Forty HHV-8 seropositive women were enrolled in the study. The median age was 37 years (range, 26–55 years) and most participants were Kenyan (95%; Table I). Nineteen (47%) were HIV seronegative and 21 (53%) were HIV seropositive. Among women who were HIV-infected, the median CD4 count was 526 cells/mm3 (IQR, 380, 599 cells/mm3). All were naïve to antiretroviral therapy (ART). The median duration of transactional sex was 9 years (range, 2–26 years) and median number of sex partners per week was 1 (range, 0–4 partners). Most women did not smoke cigarettes (36 of 40; 90%), but 31 (77%) reported drinking alcohol. During the study observation period, 3 (8%) women had a maculopapular rash and 11 (28%) women were diagnosed with bacterial vaginosis. No other intercurrent STD’s were noted, including trichomoniasis, gonorrhea, syphilis, or genital ulcer disease.
TABLE I.
Demographic and Clinical Characteristics of Study Cohort
| Characteristic | Total cohort (N = 40) |
HIV negative (N = 19) |
HIV positive (N = 21) |
|---|---|---|---|
| Age, in years (median, range) | 37 (26, 55) | 39 (26, 55) | 36 (31, 48) |
| CD4 T-cell count (within 6 months) (median, IQR) (N = 11) | 526 (380, 599) | — | 526 (380, 599) |
| ART Naïve (N = 21) | 21 (100%) | — | 21 (100%) |
| Nationality | |||
| Kenyan | 38 (95%) | 19 (100%) | 19 (90%) |
| Tanzanian | 1 (2.5%) | 0 (0%) | 1 (5%) |
| Ugandan | 1 (2.5%) | 0 (0%) | 1 (5%) |
| Marital status | |||
| Never married | 10 (25%) | 3 (16%) | 7 (33%) |
| Married | 1 (2.5%) | 1 (5%) | 0 (0%) |
| Widowed/divorced | 29 (72.5%) | 15 (79%) | 14 (67%) |
| Education, in years (median, range) | 7 (0, 13) | 7 (0, 13) | 8 (3, 13) |
| Age at first sex (median, range) | 16.5 (13, 25) | 16 (14, 20) | 17 (13, 25) |
| Years engaged in transactional sex (median, range) | 9 (2, 26) | 10 (3, 25) | 8 (2, 26) |
| Frequency of sexual intercourse per week (median, range) | 1 (1, 7) | 2 (1, 7) | 1 (1, 4) |
| Number of sex partners per week (median, range) | 1 (0, 4) | 1 (1, 4) | 1 (0, 4) |
| Contraception method | |||
| None | 23 (58%) | 14 (74%) | 9 (43%) |
| Condoms only | 1 (2.5%) | 1 (5%) | 0 (0%) |
| OCP | 5 (12.5%) | 2 (11%) | 3 (14%) |
| Depo | 9 (22%) | 2 (11%) | 7 (33%) |
| Tubal ligation | 2 (5%) | 0 (0%) | 2 (10%) |
| Months on Depo (median, range) (N = 18) | 43 (0, 115) | 40 (0, 87) | 51 (11, 115) |
| Pregnancies (median, range) | 3 (0, 7) | 3 (0, 6) | 3 (0, 7) |
| Live births (median, range) | 2 (0, 7) | 2 (0, 6) | 2 (0, 7) |
| Smoke cigarettes | |||
| No | 36 (90%) | 17 (89%) | 19 (90%) |
| Yes | 4 (10%) | 2 (11%) | 2 (10%) |
| Drink alcohol | |||
| No | 9 (23%) | 2 (11%) | 7 (33%) |
| Yes | 31 (77%) | 17 (89%) | 14 (67%) |
| Maculopapular rash | 3 (8%) | 0 (0%) | 3 (14%) |
| Bacterial vaginosis (Nugent score ≥7) | 11 (28%) | 4 (21%) | 7 (33%) |
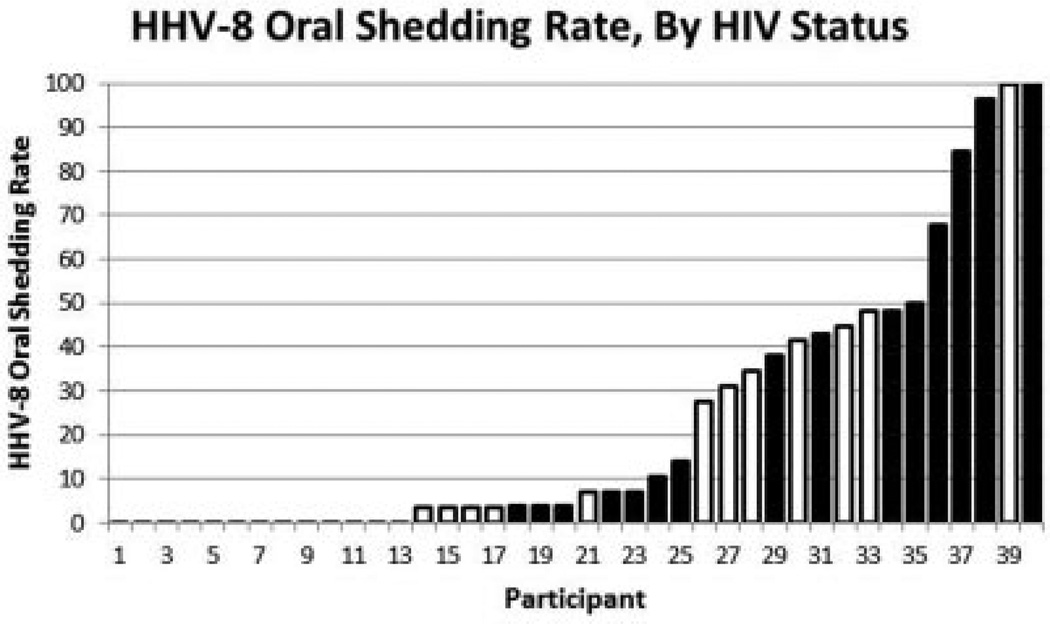
Oral swabs were collected for a median of 29 days (range, 8–29 days). HHV-8 was detected at least once in 27 (68%) participants (Fig. 1). A total of 1,122 oral swabs were included in the analysis, and the overall percentage of oral swabs with HHV-8 DNA was 23% (263/1,122). Among women in whom HHV-8 was detected, two had HHV-8 detected in every swab, four in 50–99% of the swabs, nine in 25–49% of the swabs, and 12 in 1–24% of the swabs she contributed. The mean oral HHV-8 detection rate was 27% (IQR, 0, 48) among women with HIV infection compared to 18% (IQR, 0, 34) among women without HIV infection (RR, 1.5 [95% CI, 0.7–3.4]; P=0.3). Among HIV-infected women, CD4 count ≥500 cells/mm3 was associated with a higher oral shedding rate compared with women who had CD4 counts less than 500 cells/mm3 (49% vs. 12%; RR, 4.2 [95% CI, 0.8–21.4]; P=0.08) near statistical significance. No other factors, including age, years engaged in transactional sex, contraception method, number of pregnancies and live births, smoking, or alcohol use were found to impact the oral HHV-8 shedding rate (Table II).
Fig. 1.
Distribution of percentage of days with HHV-8 oral shedding, sorted by participant from lowest to highest rate. Participant 1 contributed eight swabs; all other participants contributed 26–29 swabs. Black=HIV+; White=HIV−.
TABLE II.
Factors Associated With HHV-8 Oral Shedding Rate
| Univariate |
|||
|---|---|---|---|
| Factor | N (%) | RR (95% CI) | P-value |
| Age: Each 10 years increase | 40 (100) | 1.5 (0.8, 2.7) | 0.24 |
| HIV status: Positive versus negative | 21 (53) | 1.5 (0.7, 3.4) | 0.32 |
| CD4 count: ≥500/<500 (among HIV+ pts only) | 6 (29)a | 4.2 (0.8, 21.4) | 0.08 |
| Years of transactional sex: >10 years versus ≤10 years | 18 (45) | 1.6 (0.7, 3.7) | 0.25 |
| Method of contraception | |||
| None | 23 (58) | Ref | — |
| Hormonal | 14 (35) | 1.0 (0.4, 2.2) | 0.90 |
| Condoms only | 1 (3) | 1.7 (0.3, 11.8) | N/A |
| Tubal ligation | 2 (5) | 0.1 (0.0002, 75.5) | N/A |
| Pregnancies: 2+ versus <2 | 28 (70) | 1.8 (0.6, 4.9) | 0.27 |
| Live births: 1+ versus none | 33 (83)b | 1.9 (0.5, 7.2) | 0.36 |
| Smoking: Yes versus no | 4 (10) | 0.03 (0.0, 14.8) | 0.28 |
| Drink alcohol: Yes versus no | 31 (78) | 1.2 (0.4, 3.6) | 0.68 |
| Maculopapular rash | 3 (8) | 2.3 (0.8, 6.9) | 0.12 |
| Nugent score ≥7 | 11 (28) | 1.4 (0.6, 3.2) | 0.46 |
CD4 count not available within 6 months for 10 (48%) HIV+ participants.
Number of live births missing for 1 (3%) participant.
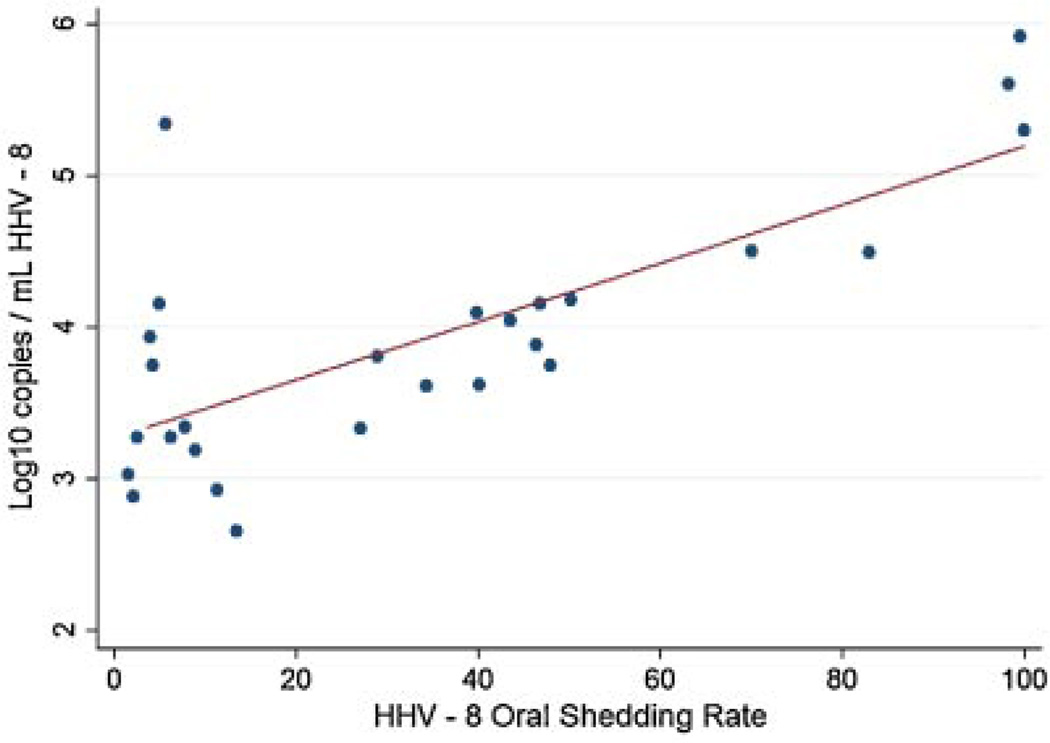
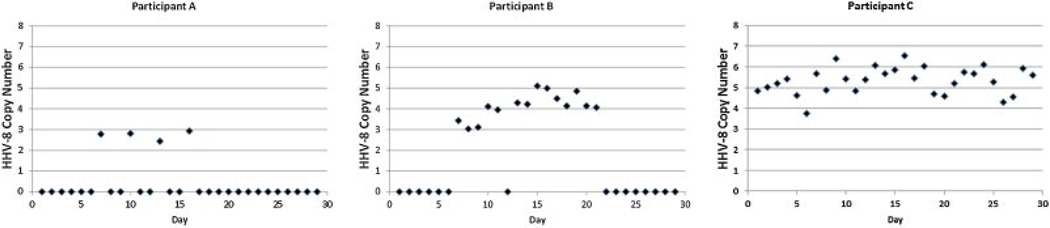
Of the 263 oral samples in which HHV-8 DNA was detected, the mean HHV-8 copy number was 4.5 log10 copies/ml (range, 2.3–7.3 log10 copies/ml). The quantity of HHV-8 DNA detected was highly correlated with the rate of HHV-8 shedding (R=0.6, P=0.001; Fig. 2). Representative viral shedding patterns for participants with low and high mean HHV-8 copy number are shown in Figure 3. The mean HHV-8 copy number was 4.6 log10 copies/ml among women with HIV infection compared to 4.3 log10 copies/ml among women without HIV infection (coef −0.1 [95% CI, −0.7–0.6]; P=0.9). The quantity was higher among HIV-infected women with CD4 count ≥500 cells/mm3 compared to HIV-infected women with CD4 counts less than 500 cells/mm3 (4.8 log10 copies/ml vs. 3.4 log10 copies/ml; coef 1.2 [95% CI, 0.5–1.9]; P=0.001) in univariate analysis (Table III). No other variables were found to be significant predictors of HHV-8 copy number in univariate or multivariate analysis.
Fig. 2.
Correlation of HHV-8 oral shedding rate and HHV-8 quantity in oral swabs on days with HHV-8 detected. Each point represents the HHV-8 shedding rate and the average HHV-8 copy number for each participant with any oral HHV-8 detected (N=27).
Fig. 3.
Day-level HHV-8 shedding and copy number patterns of three representative participants with oral HHV-8 DNA detected.
TABLE III.
Factors Associated With Oral HHV-8 Copy Number*
| Univariate |
|||
|---|---|---|---|
| Factor | N (%) | β (95% CI) | P-value |
| Age: Each 10 years increase | 263 (100) | −0.2 (−0.7, 0.4) | 0.50 |
| HIV status: Positive versus negative | 162 (62) | −0.1 (−0.7, 0.6) | 0.87 |
| CD4 count: ≥500/<500 (among HIV+ pts only) | 82 (51)a | 1.2 (0.5, 1.9) | 0.001 |
| Years of transactional sex: >10 years versus ≤10 years | 152 (58) | −0.01 (−0.64, 0.63) | 0.99 |
| Method of contraception | |||
| None | 158 (60) | Ref | — |
| Hormonal | 92 (35) | −0.3 (−1.0, 0.3) | 0.34 |
| Condoms only | 12 (5) | 0.02 (−0.4, 0.4) | 0.93 |
| Tubal ligation | 1 (0.4) | −1.2 (−1.6, −0.8) | N/A |
| Pregnancies: 2+ versus <2 | 210 (80) | −0.7 (−1.6, 0.2) | 0.13 |
| Live births: 1+ versus none | 239 (91) | −0.3 (−1.0, 0.5) | 0.52 |
| Smoking: Yes versus no | 1 (0.4) | 0.2 (−0.1, 0.5) | N/A |
| Drink alcohol: Yes versus no | 217 (83) | 0.3 (−0.3, 1.0) | 0.36 |
| Maculopapular rash | 42 (16) | 0.5 (−0.9, 1.9) | 0.50 |
| Nugent score ≥7 | 86 (33) | 0.6 (−0.2, 1.4) | 0.12 |
Includes oral swabs with detectable HHV-8 DNA (N = 263).
CD4 count not available within 6 months for 63 (39%) swabs among HIV+ participants.
DISCUSSION
High rates and quantities of oral HHV-8 shedding were observed in this cohort of Kenyan women. The percentage of women with detectable HHV-8 in saliva was nearly double what has been observed in prior studies in the US and Africa [Boldogh et al., 1996; Koelle et al., 1997; Blackbourn et al., 1998; Lucht et al., 1998; Pauk et al., 2000; Casper et al., 2004; Gandhi et al., 2004; Taylor et al., 2004; Johnston et al., 2009]. These higher rates may in part be due to the longer sampling duration, documented HHV-8 seropositivity as a criterion for study inclusion, or the exclusive female sex of the cohort. The study also demonstrated high quantities of HHV-8 in salivary samples, consistent with other reports of mean salivary HHV-8 copy numbers which have ranged between 3.8 and 5.2 log10 copies/ml [Koelle et al., 1997; Taylor et al., 2004; Johnston et al., 2009]. Importantly, the mean HHV-8 copy number in this study of 4.5 log10 copies/ml is likely a sufficient quantity to transmit infection [Corey et al., 2002]. Together, these findings support the hypothesis that oral replication is an essential feature of HHV-8 infection and that saliva is an important mode of HHV-8 transmission.
The finding that higher CD4 counts are associated with more frequent and higher quantity HHV-8 replication among HIV-infected women is consistent with previously reported studies. Three US studies found higher HHV-8 detection rates in persons with higher CD4 T-cell counts, with one of these studies reporting a nearly fivefold higher HHV-8 rate among HIV-infected men who have sex with men (MSM) with CD4 T-cell counts over 200 cells/mm3 compared to those with counts under 200 cells/mm3 [Casper et al., 2004; Gandhi et al., 2004; Miller et al., 2006]. The mechanism explaining the association between HHV-8 detection and high CD4 counts is unclear, but data suggest that host inflammation may be an important driver of HHV-8 replication. Interferon-gamma has been shown to induce lytic HHV-8 replication in vitro [Chang et al., 2000; Mercader et al., 2000], suggesting that HHV-8 production is stimulated by the interferon-gamma producing Th1 helper T-cell responses that may be more effective at higher CD4 counts. Clinically, KS-immune reconstitution inflammatory syndrome (IRIS) is characterized by clinical worsening and enlargement of KS lesions and occurs when the CD4 T-cell count is increased and immune response restored [Achenbach et al., 2012]. These observations suggest the preservation or restoration of an inflammatory immune response may in fact potentiate HHV-8 replication and consequently the development of KS and KS-IRIS in susceptible individuals.
These findings may have important implications for timing of ART among persons with HIV and HHV-8 co-infection. Emerging evidence suggests that ART may reduce oral HHV-8 shedding [Casper et al., 2004; Cattamanchi et al., 2011]. Consequently, if HHV-8 replication is greater at high CD4 counts, early ART initiation may be warranted to prevent HHV-8 transmission and to decrease likelihood of KS development. However, a higher HHV-8 pathogen load in the setting of high replication may increase likelihood of KS-IRIS development. Further studies are needed to determine the effect of ART on HHV-8 replication and the ideal timing of ART initiation among persons co-infected with HIV and HHV-8.
This study is notable for several factors that were not associated with HHV-8 replication. Because KS was historically a disease of men prior to the HIV epidemic, gender-related factors, including hormonal, environmental, and genetic factors, have been posited to protect women against the cancer. In particular, human chorionic gonadotrophin (hCG) has been hypothesized to be a protective factor in KS development based on its inhibition of KS cell line growth [Lunardi-Iskandar et al., 1995]. Yet this study found no association between the rate or quantity of oral HHV-8 replication and hormone-related factors, including pregnancy, live births, or hormonal contraception use. Additional natural history studies in Africa comparing men and women directly are needed to explore other gender-related factors that may influence KS development. There was also no association between HIV infection and oral HHV-8 replication. A similar study of HHV-8-infected adults in Uganda also did not find an increased likelihood of HHV-8 shedding with HIV infection or an association with plasma HIV RNA level [Johnston et al., 2009]. However, an earlier study of women in Kenya found that HIV infection was associated with a twofold increased likelihood of detecting HHV-8 on any mucosal surface, including the oropharynx, but the study did not assess rate or quantity of HHV-8 detection [Taylor et al., 2004]. Given the potential complexity of HIV and HHV-8 interactions in the context these discrepant findings, it remains unclear whether HIV serostatus is a predictor of oral HHV-8 replication.
Strengths of this study include evaluating HHV-8 in a well-characterized longitudinal cohort of African women with detailed information on participants’ demographic, behavioral, and clinical characteristics. The longer duration of samples permitted a more thorough description of viral activity than most similar studies. Along with these strengths, there are several limitations of the study. The study had a relatively small sample size, so it is possible that it did not have power to observe some factors that are actually associated with HHV-8 replication. CD4 data within 6 months of the sampling period was not available for all of the HIV-infected participants, limiting the power to examine the relationship between CD4 count and HHV-8 replication. The range of CD4 counts in the cohort was narrow, potentially obscuring the association between immune function and viral replication; other cohorts that include individuals with greater immunosuppression may give additional insight into the relationship between CD4 count and HHV-8 activity. In addition, the study only evaluated oral samples and did not include other potential sites of HHV-8 replication, such as plasma or PBMCs, so it does not provide an assessment of systemic HHV-8 activity. Finally, the study is limited to Kenyan women who report transactional sex and may therefore be at higher risk for HIV and HHV-8 infection than the general population. Consequently, the findings may not be generalizable to all women or to other populations outside of Kenya. Despite these limitations, this study provides a comprehensive description of oral HHV-8 replication in an important and understudied population and offers novel data with potential clinical and policy significance.
In summary, the study demonstrates high rates and quantities of HHV-8 replication in the oropharynx of African women, particularly among HIV-infected women with high CD4 counts. These findings have important implications for HHV-8 transmission and KS pathogenesis and may warrant strategies such as early ART initiation to prevent development of KS in HIV/HHV-8 co-infected women.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: CA-86795; K24 AI-071113; AI-030731.; Grant sponsor: International AIDS Research and Training Program; Grant sponsor: Fogarty International Center; Grant number: D43-TW00007.
Footnotes
Conflicts of Interest: All authors report no conflicts.
REFERENCES
- Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clin Infect Dis. 2012;54:424–433. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeloni A, Heston L, Uccini S, Sirianni MC, Cottoni F, Masala MV, Cerimele D, Lin SF, Sun R, Rigsby M, Faggioni A, Miller G. High prevalence of antibodies to human herpesvirus 8 in relatives of patients with classic Kaposi’s sarcoma from Sardinia. J Infect Dis. 1998;177:1715–1718. doi: 10.1086/517429. [DOI] [PubMed] [Google Scholar]
- Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342:1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- Benedetti P, Greco D, Figoli F, Tirelli U. Epidemic Kaposi’s sarcoma in female AIDS patients—A report of 23 Italian cases. AIDS. 1991;5:466–467. doi: 10.1097/00002030-199104000-00025. [DOI] [PubMed] [Google Scholar]
- Bestetti G, Renon G, Mauclere P, Ruffie A, Mbopi Keou FX, Eme D, Parravicini C, Corbellino M, de The G, Gessain A. High seroprevalence of human herpesvirus-8 in pregnant women and prostitutes from Cameroon [letter] AIDS. 1998;12:541–543. [PubMed] [Google Scholar]
- Blackbourn DJ, Lennette ET, Ambroziak J, Mourich DV, Levy JA. Human herpesvirus 8 detection in nasal secretions and saliva. J Infect Dis. 1998;177:213–216. doi: 10.1086/517356. [DOI] [PubMed] [Google Scholar]
- Boldogh I, Szaniszlo P, Bresnahan WA, Flaitz CM, Nichols MC, Albrecht T. Kaposi’s sarcoma herpesvirus-like DNA sequences in the saliva of individuals infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:406–407. doi: 10.1093/clinids/23.2.406. [DOI] [PubMed] [Google Scholar]
- Calabro ML, Sheldon J, Favero A, Simpson GR, Fiore JR, Gomes E, Angarano G, Chieco-Bianchi L, Schulz TF. Seroprevalence of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy. J Hum Virol. 1998;1:207–213. [PubMed] [Google Scholar]
- Casper C, Redman M, Huang ML, Pauk J, Lampinen TM, Hawes SE, Critchlow CW, Morrow RA, Corey L, Kiviat N, Wald A. HIV infection and human herpesvirus-8 oral shedding among men who have sex with men. J Acquir Immune Defic Syndr. 2004;35:233–238. doi: 10.1097/00126334-200403010-00003. [DOI] [PubMed] [Google Scholar]
- Casper C, Krantz E, Selke S, Kuntz SR, Wang J, Huang ML, Pauk JS, Corey L, Wald A. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J Infect Dis. 2007;195:30–36. doi: 10.1086/509621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattamanchi A, Saracino M, Selke S, Huang ML, Magaret A, Celum C, Corey L, Wald A, Casper C. Treatment with valacyclovir, famciclovir, or antiretrovirals reduces human herpesvirus-8 replication in HIV-1 seropositive men. J Med Virol. 2011;83:1696–1703. doi: 10.1002/jmv.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran B, Smith MS, Koelle DM, Corey L, Horvat R, Goldstein E. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology. 1998;243:208–217. doi: 10.1006/viro.1998.9055. [DOI] [PubMed] [Google Scholar]
- Chang J, Renne R, Dittmer D, Ganem D. Inflammatory cytokines and the reactivation of Kaposi’s sarcoma-associated herpesvirus lytic replication. Virology. 2000;266:17–25. doi: 10.1006/viro.1999.0077. [DOI] [PubMed] [Google Scholar]
- Cooley TP, Hirschhorn LR, O’Keane JC. Kaposi’s sarcoma in women with AIDS. AIDS. 1996;10:1221–1225. doi: 10.1097/00002030-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Corey L, Brodie S, Huang ML, Koelle DM, Wald A. HHV-8 infection: A model for reactivation and transmission. Rev Med Virol. 2002;12:47–63. doi: 10.1002/rmv.341. [DOI] [PubMed] [Google Scholar]
- Eltom MA, Mbulaiteye SM, Dada AJ, Whitby D, Biggar RJ. Transmission of human herpesvirus 8 by sexual activity among adults in Lagos, Nigeria. AIDS. 2002;16:2473–2478. doi: 10.1097/00002030-200212060-00014. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Koelle DM, Ameli N, Bacchetti P, Greenspan JS, Navazesh M, Anastos K, Greenblatt RM. Prevalence of human herpesvirus-8 salivary shedding in HIV increases with CD4 count. J Dent Res. 2004;83:639–643. doi: 10.1177/154405910408300811. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo CR, Saah A, Phair J, Detels R, Chang Y, Moore PS. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- Gessain A, Mauclere P, van Beveren M, Plancoulaine S, Ayouba A, Essame-Oyono JL, Martin PM, de The G. Human herpesvirus 8 primary infection occurs during childhood in Cameroon, Central Africa. Int J Cancer. 1999;81:189–192. doi: 10.1002/(sici)1097-0215(19990412)81:2<189::aid-ijc4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Johnston C, Orem J, Okuku F, Kalinaki M, Saracino M, Mbidde E, Sande M, Ronald A, McAdam K, Huang M, Drolette L, Selke S, Wald A, Corey L, Casper C. Impact of HIV infection and Kaposi Sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS ONE. 2009;4:e4222. doi: 10.1371/journal.pone.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes DH, Ganem D, Ameli N, Bacchetti P, Greenblatt R. The prevalence of serum antibody to human herpesvirus 8 (Kaposi sarcoma- associated herpesvirus) among HIV-seropositive and high-risk HIV- seronegative women. JAMA. 1997;277:478–481. [PubMed] [Google Scholar]
- Koelle DM, Huang ML, Chandran B, Vieira J, Piepkorn M, Corey L. Frequent detection of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: Clinical and immunologic correlates. J Infect Dis. 1997;176:94–102. doi: 10.1086/514045. [DOI] [PubMed] [Google Scholar]
- Lampinen TM, Kulasingam S, Min J, Borok M, Gwanzura L, Lamb J, Mahomed K, Woelk GB, Strand KB, Bosch ML, Edelman DC, Constantine NT, Katzenstein D, Williams MA. Detection of Kaposi’s sarcoma-associated herpesvirus in oral and genital secretions of Zimbabwean women. J Infect Dis. 2000;181:1785–1790. doi: 10.1086/315426. [DOI] [PubMed] [Google Scholar]
- Lassoued K, Clauvel JP, Fegueux S, Matheron S, Gorin I, Oksenhendler E. AIDS-associated Kaposi’s sarcoma in female patients. AIDS. 1991;5:877–880. doi: 10.1097/00002030-199107000-00013. [DOI] [PubMed] [Google Scholar]
- Lavreys L, Chohan B, Ashley R, Richardson BA, Corey L, Mandaliya K, Ndinya-Achola JO, Kreiss JK. Human herpesvirus 8: Seroprevalence and correlates in prostitutes in Mombasa, Kenya. J Infect Dis. 2003;187:359–363. doi: 10.1086/367703. [DOI] [PubMed] [Google Scholar]
- Lucht E, Brytting M, Bjerregaard L, Julander I, Linde A. Shedding of cytomegalovirus and herpesviruses 6, 7 and 8 in saliva of human immunodeficiency virus type 1-infected patients and healthy controls. Clin Infect Dis. 1998;27:137–141. doi: 10.1086/514604. [DOI] [PubMed] [Google Scholar]
- Lunardi-Iskandar Y, Bryant JL, Zeman RA, Lam VH, Samaniego F, Besnier JM, Hermans P, Thierry AR, Gill P, Gallo RC. Tumorigenesis and metastasis of neoplastic Kaposi’s sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature. 1995;375:64–68. doi: 10.1038/375064a0. [DOI] [PubMed] [Google Scholar]
- Magaret AS, Wald A, Huang M-L, Selke S, Corey L. Optimizing PCR positivity criterion for detection of Herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HL, Jr, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, Jackson DJ, Ndinya-Achola JO, Kreiss J. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- Mayama S, Cuevas LE, Sheldon J, Omar OH, Smith DH, Okong P, Silvel B, Hart CA, Schulz TF. Prevalence and transmission of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77:817–820. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, Ndinya-Achola J, Jaoko W, Baeten JM. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000;156:1961–1971. doi: 10.1016/S0002-9440(10)65069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Berger JR, Mootoor Y, Avdiushko SA, Zhu H, Kryscio RJ. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J Clin Microbiol. 2006;44:2409–2415. doi: 10.1128/JCM.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasti G, Serraino D, Ridolfo A, Antinori A, Rizzardini G, Zeroli C, Nigro L, Tavio M, Vaccher E, Tirelli U. AIDS-associated Kaposi’s sarcoma is more aggressive in women: A study of 54 patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:337–341. doi: 10.1097/00042560-199904010-00003. [DOI] [PubMed] [Google Scholar]
- Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, Celum C, Selke S, Corey L. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343:1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- Phipps W, Ssewankambo F, Nguyen H, Saracino M, Wald A, Corey L, Orem J, Kambugu A, Casper C. Gender differences in clinical presentation and outcomes of epidemic Kaposi sarcoma in Uganda. PLoS ONE. 2010;5:e13936. doi: 10.1371/journal.pone.0013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryncarz AJ, Goddard J, Wald A, Huang ML, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol. 1999;37:1941–1947. doi: 10.1128/jcm.37.6.1941-1947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GR, Schulz TF, Whitby D, Cook PM, Boshoff C, Rainbow L, Howard MR, Gao SJ, Bohenzky RA, Simmonds P, Lee C, de Ruiter A, Hatzakis A, Tedder RS, Weller IV, Weiss RA, Moore PS. Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Chohan B, Lavreys L, Hassan W, Huang ML, Corey L, Ashley Morrow R, Richardson BA, Mandaliya K, Ndinya-Achola J, Bwayo J, Kreiss J. Shedding of human herpesvirus 8 in oral and genital secretions from HIV-1-seropositive and -seronegative Kenyan women. J Infect Dis. 2004;190:484–488. doi: 10.1086/421466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, McClelland RS, Reilly M, Overbaugh J, Emery SR, Mandaliya K, Chohan B, Ndinya-Achola J, Bwayo J, Kreiss JK. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183:1017–1022. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Eng SM, Serwadda D, Sewankambo NK, Kiwanuka N, Li C, Gray RH. Prevalence of Kaposi sarcoma-associated herpesvirus compared with selected sexually transmitted diseases in adolescents and young adults in rural Rakai District, Uganda. Sex Transm Dis. 2001;28:77–81. doi: 10.1097/00007435-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Whitby D, Boshoff C, Hatzioannou T, Weiss RA, Schulz TF, Howard MR, Brink NS, Tedder RS, Tenant-Flowers M, Copas A, Suggett FE, Aldam DM, Denton AS, Miller RF, Weller IVD. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]