Abstract
Zebrafish, Danio rerio, are frequently handled during husbandry and experimental procedures in the laboratory, yet little is known about the physiological responses to such stressors. We measured the whole-body cortisol levels of adult zebrafish subjected to net stress and air exposure at intervals over a 24 h period; cortisol recovered to near control levels by about 1 h post-net-stress (PNS). We then measured cortisol at frequent intervals over a 1 h period. Cortisol levels were more than 2-fold higher in net stressed fish at 3 min PNS and continued to increase peaking at 15 min PNS, when cortisol levels were 6-fold greater than the control cortisol. Mean cortisol declined from 15 to 60 min PNS, and at 60 min, net-stressed cortisol was similar to control cortisol. Because the age of fish differed between studies, we examined resting cortisol levels of fish of different ages (3, 7, 13, and 19 months). The resting cortisol values among tanks with the same age fish differed significantly but there was no clear effect of age. Our study is the first to report the response and recovery of cortisol after net handling for laboratory-reared zebrafish.
Keywords: Age, Biomedical model, Danio rerio, Fish husbandry, Stressors
1. Introduction
Cultured fishes are confined, crowded, captured, handled and transported during routine husbandry (Piper et al., 1992; Klontz, 1995). These procedures are stressful and elicit physiological changes in response to perceived threats (Schreck, 1981; Davis, 2006). Changes may be rapid or delayed, long-term or short-term depending upon numerous factors including the species and life stage of the fish, the nature of the stressor, and other environmental factors (Schreck, 2000; Barton, 2000, 2002).
Stress has been described as a cascade of physiological events that occurs after perception of a threat, during which an organism attempts to restore homeostatic norms (Schreck et al., 2001). The hormone cortisol is a primary stress indicator in fishes, which typically increases during exposure to acute or chronic stressors (Schreck, 1981; Wendelaar Bonga, 1997; Barton, 2002; Ramsay et al., 2006). Plasma cortisol levels are typically used as an indicator in larger fish species (Barton and Iwama, 1991; Wendelaar Bonga, 1997), whereas whole-body cortisol is often used for smaller fishes, due to inadequate blood volume to accurately measure plasma cortisol (Feist and Schreck, 2002; Pottinger et al., 2002; Ramsay et al., 2006). Short-term increases in cortisol are believed to be adaptive, whereas repeated or chronic increases in cortisol are maladaptive resulting in decreased growth, impaired reproduction, and increased susceptibility to infectious diseases (Maule et al., 1989; Schreck et al., 2001; Saeij et al., 2003; Jentoft et al., 2005).
Handling of fishes typically elicits a cortisol response, but the nature of this response is quite variable. The cortisol response to common husbandry stressors such as crowding and handling has been described for numerous species, particularly salmonids (Schreck, 1981, 1982; Barton, 2000). After a 30 s acute handling stress, salmonids can elevate cortisol levels 20 to 100 fold within 1 h (Schreck, 1981; Barton, 2000); however, the magnitude of this response and recovery time to control levels varies greatly with species (Barton, 2002). Juvenile white suckers, Catostomus commersoni, elevate plasma cortisol levels after 5 min of chasing with a net and recover to control levels 6 h later (Bandeen and Leatherland, 1997). In contrast, juvenile pallid sturgeon, Scaphirhynchus albus, exhibit a low cortisol response to acute handling stress (Barton et al., 2000) even though this is the predominant steroid produced by this species in response to stress (Webb et al., 2007).
Although the cortisol response to husbandry stressors has been described for many aquatic species, less is known about the response of fishes commonly used for biomedical research. Zebrafish, Danio rerio, are widely used as vertebrate research organisms, primarily for developmental genetics, and increasingly for toxicological, cancer, behavioral, and disease studies (Trede et al., 2004; Parng, 2005; Dahm and Geisler, 2006; Wright et al., 2006; Beckman, 2007). Few studies have examined the effects of husbandry stress on zebrafish (Ramsay et al., 2006; Lawrence, 2007). Stress is often implicated when breeding is poor and during outbreaks of disease (Westerfield, 2007) suggesting a further need to measure and elucidate how husbandry stressors affect zebrafish. Additionally, zebrafish may be developed as a model for stress in other aquaculture species which may be particularly relevant to species reared for the aquarium trade (Dahm and Geisler, 2006).
Pottinger and Calder (1995) described recovery times of zebrafish whole-body corticosteroids after transport; whole-body corticosteroids were highest upon arrival in the laboratory but recovered significantly 1 h following transfer to aquaria. Increases in the whole-body cortisol of zebrafish, in response to direct and visual contact with a predator, have also been reported (Barcellos et al., 2007). We have recently described the whole-body cortisol response of zebrafish to crowding; zebrafish increase whole-body cortisol after crowding (Ramsay et al., 2006). However, the cortisol response to net handling and air exposure has not been examined.
The aim of this study was to describe the whole-body cortisol response of adult zebrafish to an acute handling stressor. Determining how zebrafish respond to acute stressors will allow researchers to understand the nature of the cortisol response in this species and allow improvements to fish handling, health and fitness while minimizing husbandry-associated sources of variation in experiments (Kent et al., 2009).
2. Methods
Zebrafish (adult, AB wild types) were provided by the Zebrafish International Resource Center (ZIRC) at the University of Oregon in Eugene, Oregon, USA. Acrylic tanks (4 L, Thoren Aquatics®, 91007) were each stocked with 10 fish, maintained in a recirculation system at the ZIRC (Ramsay et al., 2006; Westerfield, 2007). Husbandry conditions were identical to those used by Ramsay et al. (2006) including temperature (28.5 °C) and photoperiod (14 h light:10 h dark). A total of 260 fish (age: 7 months) were used for the 24 h Study; 200 fish (age: 13 months) were used for the 1 h Study; 120 fish (ages: 3, 7, 13, and 19 months) were used for the Age Study. An acclimation period of 7 to 10d was used prior to initiating experiments. Fish were fed twice daily (ZIRC Mastermix and brine shrimp nauplii, Artemia sp.) during the acclimation and experimental periods according the ZIRC feeding protocols (Westerfield, 2007). Feed was withheld on the afternoon the day before sampling. Fish were not fed for the duration of either the 24 h or 1 h Studies.
2.1. Net stress protocol and sampling
All sampling commenced at approximately the same time of day (10 00–11 00 h) in order to minimize the possibility of fluctuations in cortisol due to natural circadian rhythms (Schreck, 1981).
2.1.1. 24 hour Study
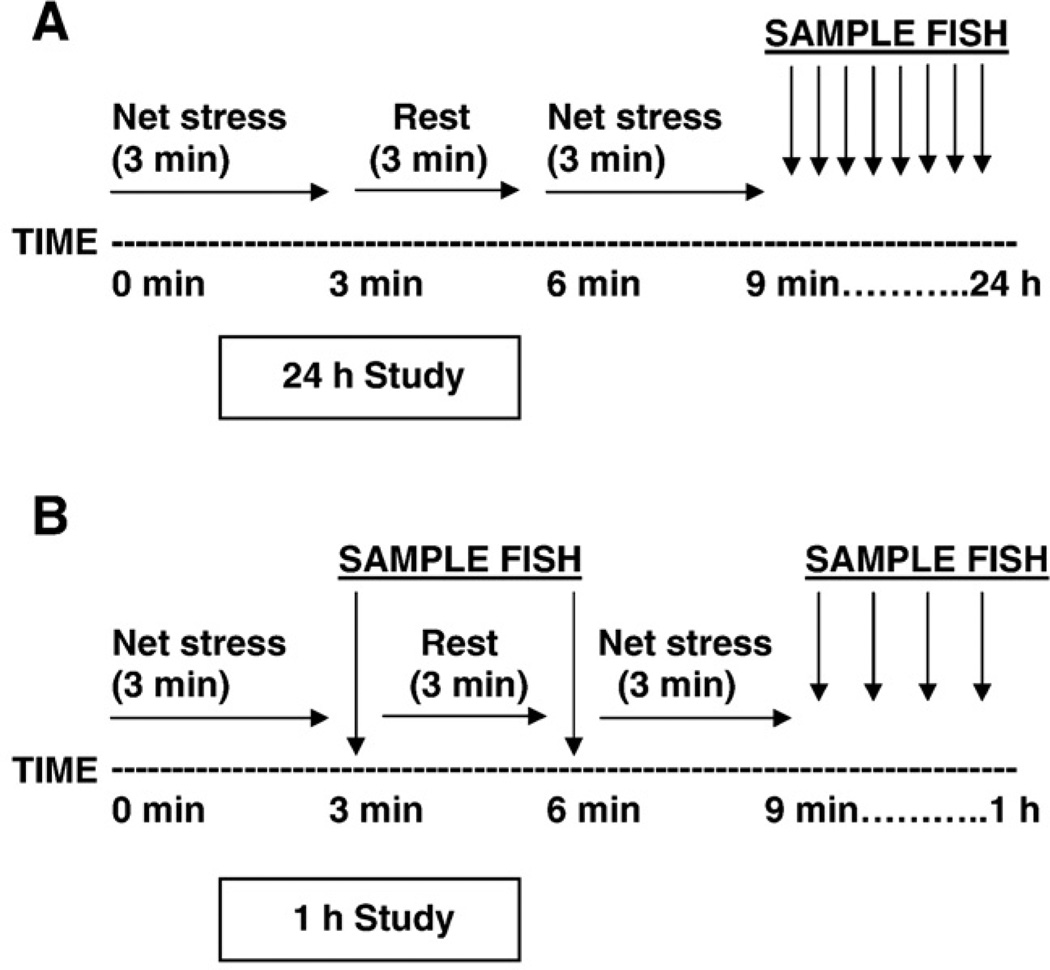
Net stress was administered to each stress treatment tank. Both the fish and the water contained in the tank were poured into a net leaving the fish suspended in the net. All fish were netted simultaneously, suspended in the air for 3 min, returned to their original tanks for 3 min, and suspended in the air for an additional 3 min. When the stressed fish were returned to their original tanks they were allowed to swim freely in the tank until sampled. Duplicate tanks of fish were sampled at 9 min, 39 min, 69 min, 3 h, 8 h, 12 h, and 24 h post-net-stress (PNS; Fig. 1A). Duplicate control tanks, to which no net stress had been administered, were sampled at 9 min, 39 min, 69 min, 3 h, 12 h, and 24 h PNS.
Fig. 1.
Timeline of net stress protocol. Fish were netted from their tanks, suspended in the air for 3 min, returned to their tanks to rest for 3 min and subjected to a second 3 min net stressor. For the 24 h Study, replicate tanks (n = 10) were sampled at 9 min, 39 min, 69 min, 3 h, 8 h, 12 h, and 24 h after the netting. For the 1 h Study, replicate tanks (n = 10) were sampled 3, 6, 9, 15, 22, 30, and 60 min after netting.
2.1.2. One hour Study
The dynamics of the cortisol response following net stress was examined over more frequent intervals during a 1 h period. The same net stress protocol was administered as for the 24 h Study. Duplicate treatment tanks of 10 fish were sampled at 3, 6, 9, 15, 22, 30, and 60 min PNS (Fig. 1B).Duplicate control tanks were sampled at 0, 15, and 60 min PNS.
2.1.3. Age Study
We used 7 month old fish for the 24 h Study and 13 month old fish for 1 h Study. Differences in control cortisol levels between the Studies suggested that age may affect resting cortisol levels. To investigate this further, triplicate tanks of fish aged 3, 7, 13, or 19 months were held under similar control conditions (10 fish in a 4 L acrylic tank) for a period of 2 weeks after which they were sampled for whole-body cortisol.
2.1.4. Sampling procedures
Sampling of fish, including euthanasia and freezing, was performed as described by Ramsay et al. (2006). Upon sampling, fish were euthanized with buffered tricaine methane sulfonate (500 mg/L MS-222), blotted on paper towels to remove excess water, immediately frozen in liquid nitrogen, and stored at −80 °C until analyzed. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Oregon (Protocol # 03-01R).
2.2. Whole-body cortisol extraction and measurement
Whole-body cortisol was extracted using the method of Ramsay et al. (2006). Whole zebrafish were thawed, weighed, homogenized, and extracted twice with 8 mL of diethyl ether. Extraction efficiency was determined for each extraction by adding tritiated cortisol to homogenized samples (n = 4) and extracting the samples as described above. Average extraction efficiency was 73 ± 0.7%, similar to previous studies that examined whole body cortisol levels in zebrafish (Ramsay et al., 2006), Chinook salmon, Oncorhynchus tshawytscha (Feist and Schreck, 2002), and chum salmon, O. keta (de Jesus and Hirano, 1992).
Cortisol in the whole-body extracts of individual fish was measured using a radioimmunoassay (RIA) from the method of Redding et al. (1984), modified and validated by Ramsay et al. (2006). Consistency between assays was verified by measuring cortisol in whole-body extracts spiked with known concentrations of cortisol. Intra-assay and inter-assay variation was accepted at no more than 10%. The detection limit of the cortisol RIA was 3.125 pg/tube. All whole-body cortisol values were above the detection limit. Cortisol values (pg/tube) were corrected for dilution factors, weight of fish, and extraction efficiency.
2.2.1. Statistical analyses
Statistical analyses were performed using the statistical software program S-PLUS 7 (Insightful Corp., 2005, Seattle, WA, USA). For each study, the weights of fish in each treatment were examined and compared using an analysis of variance (ANOVA).
For the 24 and 1 h Studies, replicates of each treatment time PNS were compared using a Welch's modified t-test. The cortisol data from treatment replicates were pooled if there was no significant difference. For the 24 h Study, an ANOVA was used to compare control cortisol values over time to determine any diurnal patterns. Additionally, for the 24 h Study, at each time PNS control and net stressed cortisol values were compared using t-tests. Control cortisol and net stressed cortisol were each compared over time using an ANOVA.
For the 1 h Study, the net stressed cortisol values were compared over time using an ANOVA. Additionally, control cortisol values were compared to net stressed cortisol at 15 and 60 min PNS using t-tests. Control cortisol levels were also compared over time using an ANOVA. Subsequently, relationships between cortisol and time PNS were evaluated using a linear regression model (y = mx + b; where y = cortisol, x = time PNS) to describe the increase in cortisol and another linear regression model to describe the decrease in cortisol.
For the Age Study, an ANOVA was used to compare mean cortisol values between triplicate tanks within an age group. Treatment tanks were pooled if there was no significant difference. If the data can be pooled, we will compare different age groups using an ANOVA.
Significance differences were reported at α = 0.05.
3. Results
There was no observed morbidity or mortality of the fish during the experiments. Comparisons of the cortisol values of replicate tanks subjected to the same treatment indicated no significant differences in either the 24 h or 1 h Study. For the Age Study, significant differences between triplicate tanks of the same age group did not allow us to pool data from replicate tanks.
3.1. 24 h Study
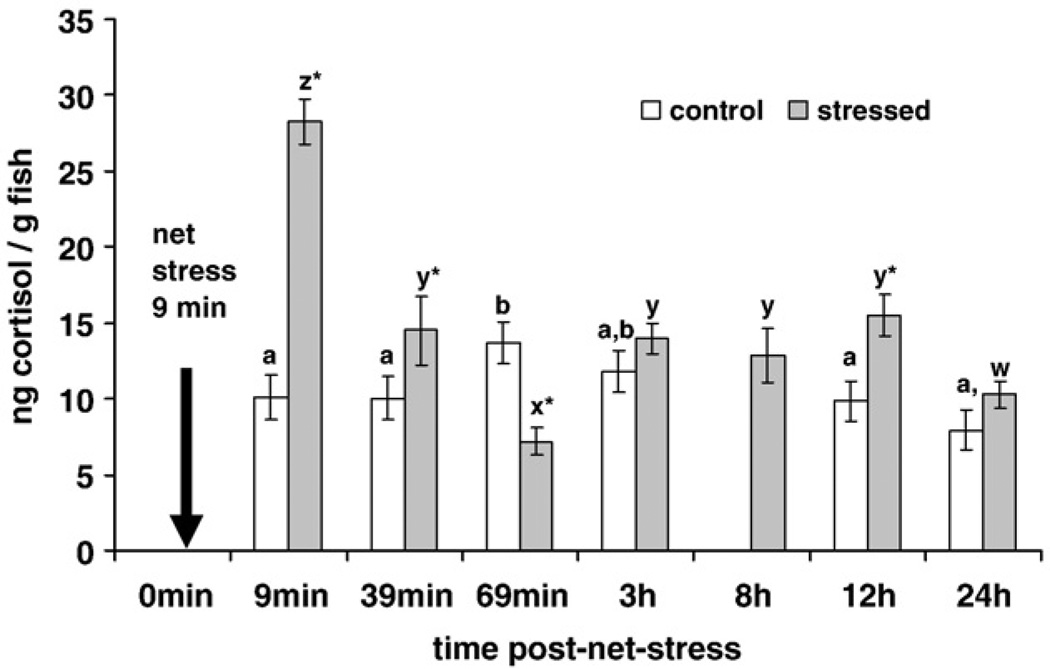
At 9 min PNS, mean cortisol increased almost 3-fold in net-stressed fish compared to the control fish (Fig. 2). By 39 min cortisol of the net-stressed fish had declined but was still significantly greater than the control group. Mean net-stressed cortisol was significantly lower compared to controls at 69 min PNS. The mean cortisol 12 h after net stress was significantly higher in controls. Cortisol did not differ between the net-stressed and control groups at 3 h or 24 h PNS. Among net-stressed groups over time, net-stressed cortisol was significantly higher at 9 min PNS than all other times PNS (Fig. 2). At 69 min PNS net-stressed cortisol was significantly lower than all other net-stressed time intervals. There was no difference between net-stressed cortisol at 39 min, 3 h and 12 h PNS. At 69 min PNS, control cortisol was significantly higher than all other groups but otherwise control cortisol did not differ significantly over time. There were no significant differences in the weights of any of the fish in the treatment groups (mean of control groups = 202 ± 4 mg; mean of net-stressed groups = 195 ± 4 mg).
Fig. 2.
Mean whole-body cortisol (ng/g fish weight; ± SEM; n = 20) over time post-net stress (24 h Study). Net-stress groups (gray bars) were held suspended in a net for 3 min, allowed to recover for 3 min and suspended in a net for 3 min (9 min total net stress). Control groups (white bars) received no net stress prior to sampling. T-tests were used to compare the cortisol of control and net-stressed groups at each time post-net stress. Significant differences between control and net-stressed groups at each time are indicated by an asterisk over the net-stressed group. The control group was compared over time post-net stress using an analysis of variance (ANOVA). Significant differences between control groups are indicated by different letters (a,b,c). The net-stressed group was compared over time post-net stress using an ANOVA. Significant differences between net-stressed groups are indicated by different letters (z,y,x,w). Significance was reported at p < 0.05.
3.2. One hour Study
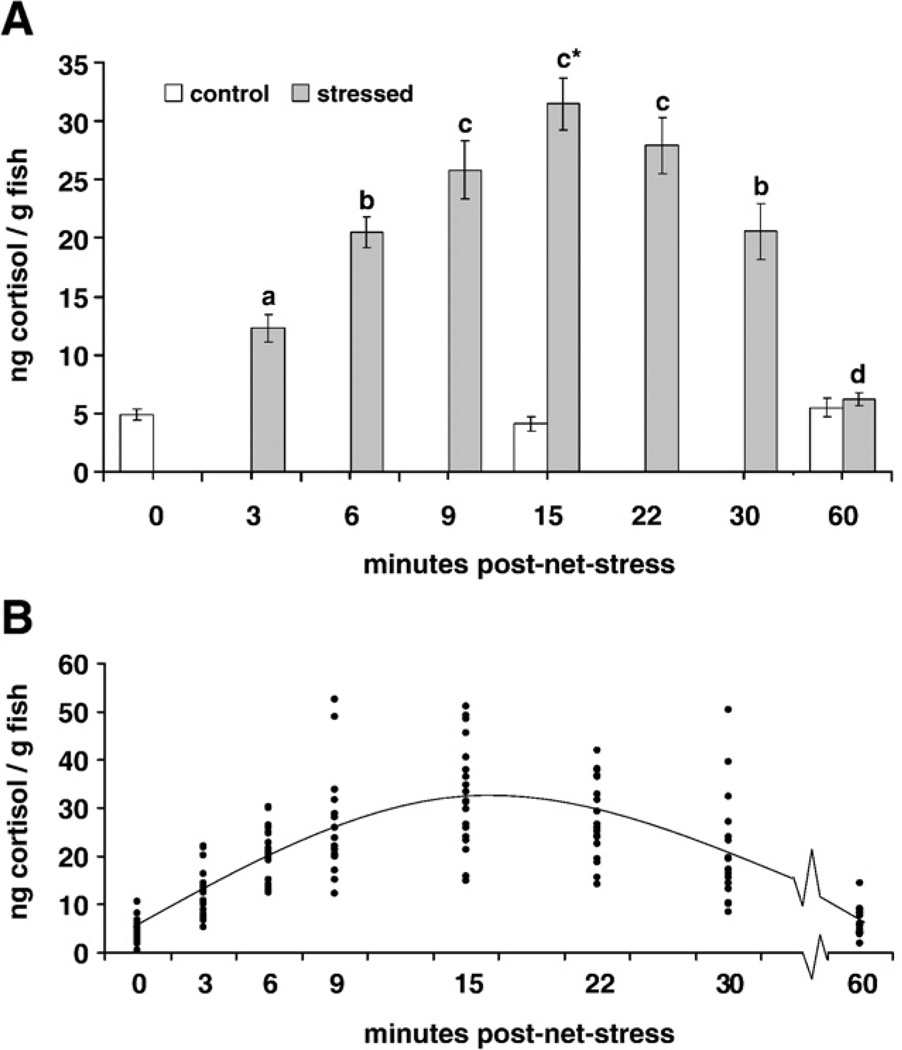
The mean cortisol values among net stressed fish were significantly greater than the control groups at all intervals except 60 min PNS when there was no significant difference between net stressed and control groups (Fig. 3A). Among the net-stressed groups over time, cortisol values at 3, 6, and 9 min PNS were significantly different from each other. There was no significant difference in mean cortisol at 9, 15 and 22 min PNS and cortisol values were similar at 6 and 30 min PNS. At 60 min PNS net-stressed cortisol was significantly lower than all other net-stressed time intervals. There was no significant difference among the control groups over time.
Fig. 3.
Whole-body cortisol values (ng/g fish weight; ± SEM; n = 20) over time (60 min) after acute net-stress (1 h Study). Net-stress groups (gray bars) were held suspended in a net for 3 min, allowed to recover for 3 min and suspended in a net for 3 min (9 min total net stress). Control groups (white bars) received no net stress prior to sampling. A) Mean cortisol over time among control and net stressed groups. T-tests were used to compare the cortisol of control and net-stressed groups at 15 min and 60 min post-net stress; a significant difference between control and net-stressed groups is indicated by an asterisk over the net-stressed group. The control group was compared over time post-net stress using an analysis of variance (ANOVA); there were no significant differences between control groups over time. The net-stressed group was compared over time post-net stress using an ANOVA. Significant differences between net-stressed cortisol are indicated by different letters (a,b,c,d). Significance was reported at p < 0.05. B) Individual cortisol (●) among net-stressed fish over time. Fish at time 0 min received no net stress prior to sampling (control). There was a positive linear relationship between cortisol and time from 0 to 15 min PNS (cortisol = 7.26 + 1.76 × time; R2 = 0.622; P = 5.5×10−22) and a negative linear relationship between cortisol and time from 15 to 60 min PNS (cortisol = 39.5−0.56×time; R2 = 0.516; p = 1.33×10−13).
We found that 3 to 15 min after netting stress, cortisol levels increased linearly from 2.5 to 6 fold higher than control levels. Then, cortisol decreased linearly to control levels. The positive (1) and negative (2) linear relationships between cortisol and time PNS were explained by the models:
cortisol = 7.26 + 1.76×time (R2 = 0.622; p = 5.5×10−22; Fig. 3B).
cortisol = 39.5−0.56×time (R2 = 0.516;p = 1.33×10−13; Fig.3B).
The control group (239 ± 6 mg) weighed significantly less than the net-stressed group (259 ± 5 mg; p = 0.03).
3.3. Age Study
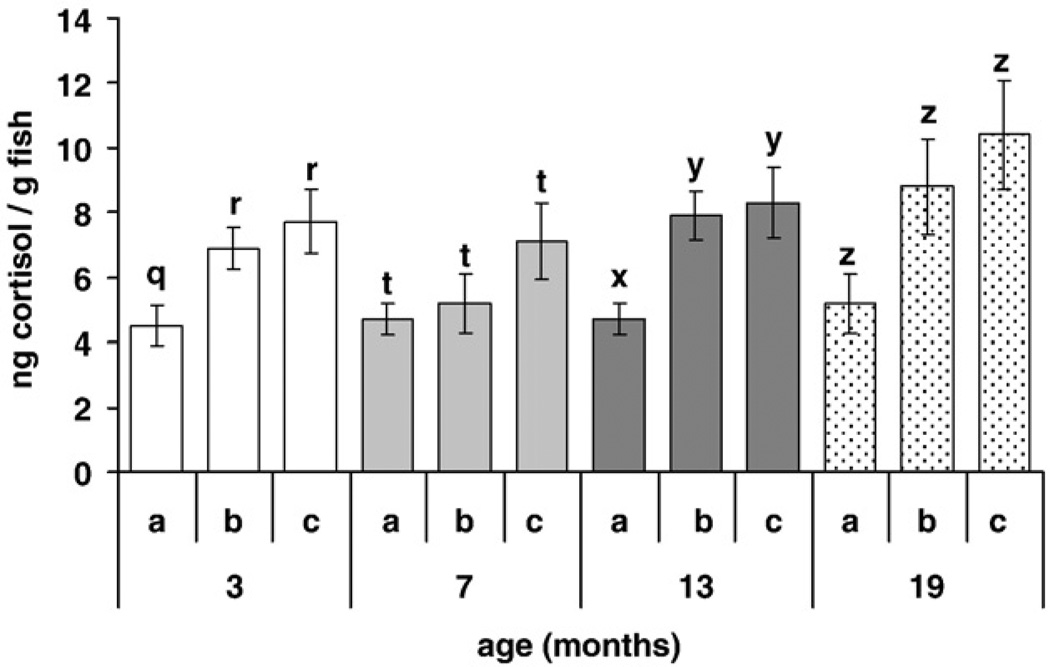
We did not pool cortisol values of replicate tanks of the same treatment because of significant differences between replicate tanks of 3 month old fish and 13 month old fish, although there were no significant differences between replicate tanks of the 7 month and 19 month old fish (Fig. 4). Three month old fish weighed significantly less (212 ± 14 mg) than fish at the other ages. There were no significant differences between the weights of the 7, 13, and 19 month old fish (367 ± 13 mg).
Fig. 4.
Mean whole-body cortisol (ng/g fish weight; ± SEM) of triplicate tanks (a,b,c) of different aged fish (3, 7, 13, 19 months) held at the same density (2.5 fish/L). Cortisol values were not pooled because of significant differences between triplicate tanks of the same age group. Different letter above the standard error bars indicate a significant difference (p < 0.05) between cortisol values within each age group (3 months: q,r; 7 months: t; 13 months: x,y; 19 months: z).
4. Discussion
Our study describes the cortisol response of adult zebrafish to acute handling stress; this complements our previous study that examined the cortisol response of zebrafish to chronic crowding stress (Ramsay et al., 2006). Despite the wide use of zebrafish as a research organism, there are few data regarding the stress response as it relates to husbandry (Lawrence, 2007). Better understanding how zebrafish respond to handling will allow researchers to create a rearing environment for optimal health and reproduction while minimizing husbandry-associated sources of variation (Kent et al., 2009). Additionally, zebrafish may be developed as a model of stress for aquaculture species (Dahm and Geisler, 2006; Alsop and Vijayan, 2008).
There was no mortality during or after netting. Similar stressors often result in mortality in other fishes, notably salmonids (Schreck, 1982; Schreck et al., 1995) and marine fishes (Davis et al., 2001; Suski et al., 2007). In addition, the whole-body cortisol levels of zebrafish increased rapidly following acute stress and recovered relatively rapidly, within about an hour. An extensive history of domestication and physiological adaptations favorable to laboratory culture may explain the ability of zebrafish to endure husbandry stressors without significant mortality (Crease, 1934; Eaton and Farley, 1974; Laale, 1977; Dahm and Geisler, 2006). Domesticated zebrafish have a reduced startle response compared to zebrafish obtained from wild populations (Robison and Rowland, 2005). Behavioral differences between wild and domesticated zebrafish reflect differences in genetic profiles (Wright et al., 2006). In addition, the cortisol response is highly heritable in fishes (Øverli et al., 1999; Pottinger and Carrick, 2001; Fevolden, et al., 2002). Continuous spawning and short generation time of zebrafish likely facilitated rapid selection of traits favorable to survival in a laboratory environment, including a rapid cortisol response and recovery from husbandry stressors such as net handling.
4.1. Linear increase in whole-body cortisol after net handling
Zebrafish increased whole-body cortisol levels upon exposure to net stress with peaks occurring 9 min after netting. When examined at more frequent intervals over a 1 h period, cortisol peaked at an even higher level at 15 min, confirming that zebrafish rapidly produce cortisol in response to net stress and air exposure. In a similar study examining transport stress in zebrafish, whole-body corticosteroids peaked 30 min after transfer from holding tanks to transport containers (Pottinger and Calder, 1995). This was the first measurement following transfer. Therefore, it is not possible to know whether or not this value would have been higher 15 min after transfer. Peak whole-body cortisol values in our study ranged from 28 to 30 ng/g. This is much greater than the peak values found by Pottinger and Calder (1995) which ranged from4 to 9 ng/g. The nature of the stressor (brief handling and transfer rather than severe net handling and air exposure) may have resulted in a greater cortisol response in our study. Additionally, we conducted our study at 28.5 °C whereas the previous study used 23 °C which may explain differences in the magnitude and timing of peak cortisol response (Schreck, 2000). Among fishes, lower temperatures tend to delay the cortisol response to and recovery from net stressors whereas higher temperatures tend to cause more rapid increases in cortisol (Das et al., 2002; Van Ham et al., 2003; Davis, 2004).
Zebrafish demonstrated a linear increase in whole-body cortisol over time. Davis and Small (2006) showed a similar linear increase in plasma cortisol values among sunshine bass, Morone chrysops × M. saxatilis, exposed to a 15 min low water stressor with plasma cortisol levels peaking at 20 to 30 min post-stressor. Differences in the nature and duration of the stressor as well as species and temperature differences may have resulted in differences in the timing and magnitude of the cortisol response. Nevertheless, the similar linear increase in cortisol suggests that a better understanding of the dynamics of the cortisol response following exposure to handling stressors could be examined to create husbandry protocols that minimize stress in aquaculture.
4.2. Recovery from net handling: clearance of cortisol
Whole-body cortisol levels recovered to that of the controls by 1 h after net stress during the 1 h Study. Similarly, whole-body corticosteroids of adult zebrafish had recovered to baseline values 1 h following transport (Pottinger and Calder, 1995). Pottinger and Calder (1995) also noted that zebrafish are less sensitive to changes in environment and recover relatively rapidly following transport stress compared to salmonids. Zebrafish are frequently handled during routine laboratory procedures (breeding, tank cleaning, etc.). Stress conditioning has been shown to attenuate the cortisol response of Chinook salmon, O. tshawytscha, to subsequent stressors (Schreck et al., 1995). Previous experience with similar handling stressors may have facilitated the rapid recovery of elevated cortisol to control levels.
Interestingly, during the 24 Study, at 69 min after net stress cortisol levels among the net stressed fish were significantly lower than controls suggesting an increased rate of cortisol clearance in the first 69 min after net stress. The clearance rate of cortisol was increased in coho salmon, Oncorhynchus kisutch, after netting and crowding stress or chronic administration of exogenous cortisol (Redding et al., 1984). Bradford et al. (1992) demonstrated evidence for ultra-short-loop negative feedback of cortisol secretion by the interrenal of coho salmon in the presence of elevated levels of exogenous cortisol. Elevated whole-body cortisol as a result of severe net stress may have increased the clearance rate of cortisol as well as suppressed cortisol secretion, resulting in significantly lower cortisol levels at 69 min after net stress. Alternatively, the control cortisol value at 69 min was higher than other control cortisol levels measured during this study. This may also have contributed to significant differences between control and net-stressed groups at 69 min PNS.
The cortisol of the net stressed group at 12 h PNS was significantly higher than the control cortisol range. The 12 h group was sampled at approximately 22 00 h, during which time the facility is typically void of activity and people. Barcellos et al. (2007) have demonstrated increased cortisol in zebrafish upon visual contact with a predator. It is possible that unanticipated visual contact with the sampler just prior to sampling elicited a slight but significant elevation in the cortisol of previously stressed zebrafish, perhaps in anticipation of another net stressor.
The relationship between cortisol and time from 15 to 60 min was linear. Davis and Small (2006) also demonstrated a decrease in plasma cortisol among channel catfish, Ictalurus punctatus, and sunshine bass of a similar linear nature. After a 30 s handling stressor, coho salmon showed a linear decrease in plasma cortisol from 1 to 3 h post-stress; however, subsequent measurements at 6, 12, and 24 h post-stress did not reflect a linear decrease (Patiño et al., 1987). The nature of stressor, species of fish and environmental conditions of the experiment all influence the recovery of cortisol levels to baseline.
4.3. Resting cortisol: effects of age
Cortisol values varied widely between tanks of fish of the same age, indicating a possible tank effect. We were not able to discern any effect of tank position as demonstrated in other studies (e.g. Speare et al., 1995) but could not pool the data from replicate tanks for comparison. Further investigation of the effects of age on resting cortisol is needed to unequivocally determine this relationship in zebrafish. As animals age, basal cortisol secretion typically increases and is associated with decreased health and increased disease problems (Sapolsky et al., 1986; Butcher and Lord, 2004). Furthermore, agonistic behavior among many fishes occurs at low as opposed to high densities and often increases with age (Fleming and Johansen, 1984).
Differences in the cortisol levels of the control groups between the 24 h and 1 h studies may also be the result of slight differences in sampling procedures during each experiment. During the 24 h study, sampling activities were performed in closer proximity to the tanks than during the 1 h study. The transparent acrylic tanks allowed the fish to see activity outside of the tank, which may have been perceived as a threat. Zebrafish rely on visual cues to avoid threats such as predators (Fleisch and Neuhauss, 2006; Barcellos et al., 2007; Spence et al., 2008). The close proximity of treatment tanks to control tanks allowed control fish to see distressed treatment fish which may have elicited a slight but significant increase in whole-body cortisol among the control fish. Although there were slight differences in control cortisol levels between the 24 h and 1 h Studies, the cortisol levels of the net stressed fish were much greater than the control groups.
5. Conclusions
Zebrafish responded and recovered rapidly to the net stress protocol used in our study. Extensive domestication suggests selection of traits favorable for survival in a laboratory environment, including the ability to endure net handling and air exposure. Despite the ability of zebrafish to recover rapidly from handling stressors, increasing the frequency and duration of handling may alter the dynamics of the cortisol response and necessitate longer recovery periods. Differences in handling procedures among laboratories may increase the husbandry-associated sources of variation in experiments. Additionally, reproduction and disease resistance may be affected long after cortisol levels have recovered. We were unable to discern any statistically significant differences in resting cortisol levels among fish of different ages. However, we believe that age may be an important factor in the dynamics of the cortisol response of this species and should be further investigated. Better understanding how handling stressors and age affect whole-body cortisol, reproduction, and diseases of zebrafish may allow improvements in the husbandry, health, and consistency of studies that use this important model.
Acknowledgements
The authors wish to thank the ZIRC staff, particularly Dr. Jennifer Matthews, April Freeman, Carrie Carmicheal, Beth Murrill and David Lains for their assistance with the experiments. Thanks to Drs. John Leatherland and Dixon Landers for their critical review of the manuscript. The research was supported by the National Institutes of Health (NIH grants#: R24RRO17386-01 A1 and P40RR12546). JMR was supported by a Post-graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada, the Departments of Fisheries and Wildlife and Microbiology at Oregon State University and the Zebrafish International Resource Center at the University of Oregon. Mention of a brand name does not imply endorsement of the product by the U.S. Federal Government.
References
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R711–R719. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- Bandeen J, Leatherland JF. Transportation and handling stress of white suckers raised in cages. Aquac. Int. 1997;5:385–396. [Google Scholar]
- Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, da Silva LB, Bedin AC, Finco J, Cericato L. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture. 2007;272:774–778. [Google Scholar]
- Barton BA. Salmonid fishes differ in their cortisol and glucose responses to handling and transport stress. N. Am. J Aquac. 2000;62:12–18. [Google Scholar]
- Barton BA. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002;42:517–525. doi: 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- Barton BA, Iwama GK. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann. Rev. Fish Dis. 1991;1:3–26. [Google Scholar]
- Barton BA, Bollig H, Hauskins BL, Jansen CR. Juvenile pallid (Scaphirhynchus albus) and hybrid pallid × shovelnose (S. albus x platorynchus) sturgeons exhibit low physiological responses to acute handling and severe confinement. Comp. Biochem. Physiol. 2000;126A:125–134. doi: 10.1016/s1095-6433(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Beckman M. Zebrafish take the stage in cancer research. J. Natl. Cancer Inst. 2007;99:500–501. doi: 10.1093/jnci/djk156. [DOI] [PubMed] [Google Scholar]
- Bradford CS, Fitzpatrick MS, Schreck CB. Evidence for ultra-short-loop feedback in ACTH-induced interrenal steroidogenesis in coho salmon: acute self suppression of cortisol secretion in vitro. Gen. Comp. Endocrinol. 1992;87:292–299. doi: 10.1016/0016-6480(92)90034-h. [DOI] [PubMed] [Google Scholar]
- Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3:151–160. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- Crease CW. The technic of handling the zebra fish (Brachydanio rerio) for the production of eggs which are favorable for embryological research and are available at any specified time throughout the year. Copeia. 1934;1934:159–161. [Google Scholar]
- Dahm R, Geisler R. Learning from small fry: the zebrafish as a genetic model organism for aquaculture fish species. Mar. Biotech. 2006;8:329–345. doi: 10.1007/s10126-006-5139-0. [DOI] [PubMed] [Google Scholar]
- Das MK, Dutta T, Acharya S, Bhowmick S. Sublethal temperature stress in juvenile Labeo rohita (Ham-Buch.) and Rita rita (Ham.): some physiological changes. Indian J. Exp. Biol. 2002;40:589–593. [PubMed] [Google Scholar]
- Davis KB. Temperature affects physiological stress responses to acute confinement in sunshine bass (Morone chrysops × Morone saxatilis) Comp. Biochem. Physiol. 2004;139:433–440. doi: 10.1016/j.cbpb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Davis KB. Management of physiological stress in finfish aquaculture. N. Am. J. Aquac. 2006;68:116–121. [Google Scholar]
- Davis KB, Small BC. Rates of cortisol increase and decrease in channel catfish and sunshine bass exposed to an acute confinement stressor. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006;143:134–139. doi: 10.1016/j.cbpc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Davis MW, Olla BL, Schreck CB. Stress induced by hooking, net towing, elevated sea water temperature and air in sablefish: lack of concordance between mortality and physiological measures of stress. J. Fish Biol. 2001;58:1–15. [Google Scholar]
- de Jesus EGT, Hirano T. Changes in whole body concentrations of cortisol, thyroid hormones and sex steroids during early development of the chum salmon, Oncorhynchus keta. Gen. Comp. Endocrinol. 1992;85:55–61. doi: 10.1016/0016-6480(92)90171-f. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Farley RD. Spawning cycle and egg production of zebrafish, Brachydanio rerio, in the laboratory. Copeia. 1974;1974:195–204. [Google Scholar]
- Feist G, Schreck CB. Ontogeny of the stress response in chinook salmon, Oncorhynchus tshawytscha. Fish Physiol. Biochem. 2002;25:31–40. [Google Scholar]
- Fevolden SE, Røed KH, Fjalestad KT. Selection response of cortisol and lysozyme in rainbow trout and correlation to growth. Aquaculture. 2002;205:61–75. [Google Scholar]
- Fleisch VC, Neuhauss SCF. Visual behavior in zebrafish. Zebrafish. 2006;3:191–201. doi: 10.1089/zeb.2006.3.191. [DOI] [PubMed] [Google Scholar]
- Fleming IA, Johansen PH. Density and agnostic behaviour of young-of-the-year largemouth bass (Micropterus salmoides) Can. J. Zool. 1984;62:1454–1455. [Google Scholar]
- Jentoft S, Aastveit AH, Torjesen PA, Andersen Ø. Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss) Comp. Biochem. Physiol. Part A. 2005;141:353–358. doi: 10.1016/j.cbpb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kent ML, Feist SW, Harper C, Hoogstraten-Miller S, Law JM, Sánchez-Morgado JM, Tanguay RL, Sanders GE, Spitsbergen JM, Whipps CM. Recommendations for control of pathogens and infectious diseases in fish research facilities. Comp. Biochem. Physiol. Part C. 2009;140:240–248. doi: 10.1016/j.cbpc.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klontz GW. Care of fish in biological research. J. Anim. Sci. 1995;73:3485–3492. doi: 10.2527/1995.73113485x. [DOI] [PubMed] [Google Scholar]
- Laale HW. The biology and use of zebrafish, Brachydanio rerio in fisheries research: a literature review. J. Fish. Biol. 1977;10:121–173. [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. [Google Scholar]
- Maule AG, Tripp RA, Kaattari SL, Schreck CB. Stress alters immune function and disease resistance in Chinook salmon (Oncorhynchus tshawytscha) J. Endocrinol. 1989;120:135–142. doi: 10.1677/joe.0.1200135. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Olsen RE, Løvik F, Ringø E. Dominance hierarchies on Arctic charr, Salvelinus alpinus L.: differential cortisol profiles of dominant and subordinate individuals after handling stress. Aquac. Res. 1999;30:259–264. [Google Scholar]
- Parng C. In vivo zebrafish assays for toxicity testing. Curr. Opin. Drug Discov. Dev. 2005;8:100–106. [PubMed] [Google Scholar]
- Patiño R, Redding JM, Schreck CB. Interrenal secretion of corticosteroids and plasma cortisol and cortisone concentrations after acute stress and during seawater acclimation in juvenile coho salmon (Oncorhynchus kisutch) Gen. Comp. Endocrinol. 1987;68:431–439. doi: 10.1016/0016-6480(87)90082-7. [DOI] [PubMed] [Google Scholar]
- Piper RG, McElwain IB, Orme LE, McCraren JP, Fowler LG, Leonard JR. Fish Hatchery Management. 5th Ed. Washington, D.C. USA.: United States Fish and Wildlife Service; 1992. [Google Scholar]
- Pottinger TG, Calder GM. Physiological stress in fish during toxicological procedures: a potentially confounding factor. Environ. Toxicol. Water Qual. 1995;10:135–146. [Google Scholar]
- Pottinger TG, Carrick TR. Stress responsiveness affects dominant-subordinate relationships in rainbow trout. Horm. Behav. 2001;40:419–427. doi: 10.1006/hbeh.2001.1707. [DOI] [PubMed] [Google Scholar]
- Pottinger TG, Carrick TR, Yeomans WE. The three-spined stickleback as an environmental sentinel: effects if stressors on whole-body physiological indices. J. Fish Biol. 2002;61:207–229. [Google Scholar]
- Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture. 2006;258:565–574. [Google Scholar]
- Redding JM, Patiño R, Schreck CB. Clearance of corticosteroids in yearling coho salmon, Oncorhynchus kisutch, in freshwater and seawater after stress. Gen. Comp. Endocrinol. 1984;54:433–443. doi: 10.1016/0016-6480(84)90159-x. [DOI] [PubMed] [Google Scholar]
- Robison BD, Rowland W. A potential model system for studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio) Can. J. Fish. Aquat. Sci. 2005;62:2046–2054. [Google Scholar]
- Saeij JP, Verburg-van Kemenade LB, van Muiswinkel WB, Wiegertjes GF. Daily handling stress reduces resistance of carp to Trypanoplasma borreli: in vitro modulatory effects of cortisol on leukocyte function and apoptosis. Dev. Comp. Immunol. 2003;27:233–245. doi: 10.1016/s0145-305x(02)00093-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen B. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schreck CB. Stress and compensation in teleostean fishes: response to social and physical factors. In: Pickering AD, editor. Stress and Fish. London: Academic Press; 1981. pp. 295–321. [Google Scholar]
- Schreck CB. Stress and rearing of salmonids. Aquaculture. 1982;28:241–249. [Google Scholar]
- Schreck CB. Accumulation and long-term effects of stress in fish. In: Moberg GP, Mench JA, editors. The Biology of Animal Stress: Assessment and Implications for Animal Welfare. Wallingford: CAB International; 2000. pp. 147–158. [Google Scholar]
- Schreck CB, Jonsson L, Feist G, Reno P. Conditioning improves performance of juvenile Chinook salmon, Oncorhynchus tshawytscha, to transportation stress. Aquaculture. 1995;135:99–110. [Google Scholar]
- Schreck CB, Contreras-Sánchez W, Fitzpatrick MS. Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture. 2001;197:3–24. [Google Scholar]
- Speare DJ, MacNair N, Hammell KL. Demonstration of tank effect on growth indices of juvenile rainbow trout (Oncorhynchus mykiss) during an ad libitum feeding trial. Am. J. Vet. Res. 1995;56:1372–1379. [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Suski CD, Cooke SJ, Danylchuk AJ, O'Connor CM, Gravel MA, Redpath T, Hanson KC, Gingerich AJ, Murchie KJ, Danylchuk SE, Koppelman JB, Goldberg TL. Physiological disturbance and recovery dynamics of bonefish (Albula vulpes), a tropical marine fish, in response to variable exercise and exposure to air. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007;148:664–673. doi: 10.1016/j.cbpa.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- Van Ham EH, Van Anholt RD, Kruitwagen G, Imsland AK, Foss A, Sveinsbø BO, FitzGerald R, Parpoura AC, Stefansson SO, Wendelaar Bongaa SE. Environment affects stress in exercised turbot. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003;136:525–538. doi: 10.1016/s1095-6433(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Webb MA, Allert JA, Kappenman KM, Marcos J, Feist GW, Schreck CB, Shackleton CH. Identification of plasma glucocorticoids in pallid sturgeon in response to stress. Gen. Comp. Endocrinol. 2007;154:98–104. doi: 10.1016/j.ygcen.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Wendelaar Bonga SE. The stress response in fish. Physiol. Rev. 1997;77:591–625. doi: 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5th Edition. Eugene, OR, USA.: University of Oregon Press; 2007. [Google Scholar]
- Wright D, Nakamichi R, Krause J, Butlin RK. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio) Behav. Genet. 2006;36:271–284. doi: 10.1007/s10519-005-9029-4. [DOI] [PubMed] [Google Scholar]