Abstract
C-type lectin like-receptor 2 (CLEC-2) has been reported to activate platelets through a lipid raft-dependent manner. Secreted ADP potentiates CLEC-2-mediated platelet aggregation. We have investigated whether the decrease in CLEC-2-mediated platelet aggregation, previously reported in platelets with disrupted rafts, is a result of the loss of agonist potentiation by ADP. We disrupted platelet lipid rafts with methyl–β-cyclodextrin (MβCD) and measured signaling events downstream of CLEC-2 activation. Lipid raft disruption decreases platelet aggregation induced by CLEC-2 agonists. The inhibition of platelet aggregation by the disruption of lipid rafts was rescued by the exogenous addition of epinephrine but not 2-Methylthioadenosine diphosphate (2MeSADP), which suggests that lipid raft disruption effects P2Y12-mediated Gi activation but not Gz. Phosphorylation of Syk (Y525/526) and PLCγ2 (Y759), were not affected by raft disruption in CLEC-2 agonist-stimulated platelets. Furthermore, tyrosine phosphorylation of the CLEC-2 hemi-ITAM was not effected when MβCD disrupts lipid rafts. Lipid rafts do not directly contribute to CLEC-2 receptor activation in platelets. The effects of disruption of lipid rafts in in vitro assays can be attributed to inhibition of ADP feedback that potentiates CLEC-2 signaling.
1. INTRODUCTION
Platelets play a critical role in hemostasis and thrombosis [1, 2]. Platelets contain two types of agonist receptors; G-protein coupled receptors (GPCRs) and Tyrosine kinase pathway receptors and ligand-gated ion channels which are important for their activation [3–7]. All tyrosine kinase pathway receptors GPVI, FcγRIIA and CLEC-2 are linked to activation of Syk and PLCγ2 [4, 8–12]. GPVI and FcγRIIA are ITAM containing receptors [13, 14], where as CLEC-2 is a hemi-ITAM receptor [15, 16].
C-type lectin like receptor -2 (CLEC-2) is highly expressed in platelets and at lower levels in neutrophils and dendritic cells [17]. CLEC-2 can be activated by podoplanin [18, 19], rhodocytin [20], a human CLEC-2 antibody [21], and fucoidan [22]. The crystal structure of rhodocytin shows that CLEC-2 receptors are activated through clustering by this tetrameric ligand [20]. The CLEC-2 receptor plays an important role in tumor metastasis [23], hemostasis and thrombosis [16, 24–26]. Unlike GPVI, which has an ITAM, CLEC-2 has a hemi-ITAM sequence that is phosphorylated by Src and Syk tyrosine kinases[21, 26], whereas phosphorylation of the ITAM is mediated solely by Src kinases[27, 28].
Lipid rafts are distinct areas of the plasma membrane implicated in the regulation of signaling in a variety of cells including platelets [29–33]. A previous study has shown that the CLEC-2 receptor is partially associated with lipid rafts in both resting and activated platelets [34]. It was also suggested that disruption of the rafts leads to direct impairment of CLEC-2 signaling [34]. Many agonists depend on secreted ADP [35, 36] and we have shown that there is reduced ADP signaling through the Gi-coupled P2Y12 receptor in platelets with disrupted lipid rafts as Gi requires lipid raft microdomains [32]. It is known that secreted secondary mediators, such as ADP and thromboxaneA2, play an important positive feedback role in platelet activation by CLEC-2 agonists [34]. Studies from our lab has also shown that Gi pathway play a crucial role in potentiation of secretion when platelets are stimulated with different agonists [37]. We wanted to determine whether or not the decrease in CLEC-2 signaling found in platelets with disrupted rafts was a result of loss of positive feedback by secreted ADP.
In this study we demonstrate that the primary signaling events downstream of CLEC-2 do not require a lipid raft environment and all the diminished functional responses seen with MβCD are because of the attenuated effects of Gi signaling.
2. MATERIALS AND METHODS
2.1 Reagents
Rhodocytin provided by Dr. Steve P Watson (University of Birmingham). 2MeSADP, epinephrine, Apyrase (type VII) and fucoidan were obtained from Sigma (St. Louis, MO). ARC69931MX was a gift from AstraZeneca (Longhborough, UK). ). Whatman protein nitrocellulose transfer membrane was obtained from Fisher Scientific (Pittsburg, PA), LI-COR Odyssey blocking buffer was purchased from LI-COR Biosciences (Lincoln, NE). Protein A/G PLUS-agarose was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-Syk (Tyr525/Tyr 526), PLCγ2 (Tyr759), and βactin were from Cell Signaling Technology (Beverly, MA). Monoclonal phosphotyrosine antibody (clone 4G10) was purchased from Upstate Biotechnologies (Lake Placid, NY). Monoclonal anti–CLEC-2 antibody was obtained from abnova and Goat anti-CLEC-2 antibody was obtained from R & D systems Inc. (Minneapolis, MN). Goat anti-mouse IgG (H+L) Dylight 680 and Donkey anti-Goat IgG (H+L) Dylight 800 secondary antibodies were from Thermo Scientific (Rockford, IL).
2.2 Preparation of human platelets
Blood was collected from informed healthy volunteers in to one-sixth volume of acid/citrate/dextrose (2.5g sodium citrate, 2 g glucose, and 1.5 g citric acid in 100 ml de-ionized water). Platelet rich plasma was obtained by centrifugation at 250g for 20 minutes at ambient temperature. Platelets were isolated from plasma by centrifugation at 980g for 10 minutes at ambient temperature and resuspended in Tyrode’s buffer pH 6.5 (138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 5 mM glucose, 10 mM PIPES (pH6.5) containing 20 nM PGE1, 10mM indomethacin, 500 mM EGTA and 0.2 U/ml apyrase). Platelets were isolated from Tyrode’s buffer pH 6.5 by centrifugation at 980g for 10 minutes and resuspended in Tyrode’s buffer, pH 7.4 (138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 5 mM glucose, 10 mM HEPES and 0.2 U/ml apyrase, pH 7.4). The platelet count was adjusted to 2–2.5 × 108/ml. Approval was obtained from the institutional review board of Temple University for these studies. Informed consent was provided prior to blood donation.
2.3 Platelet aggregation
Platelet aggregation was measured using a lumi-aggregometer (Chrono-Log, Havertown, PA) at 37°C under stirring conditions. A 0.5 ml sample of aspirin-treated washed platelets was stimulated with different agonists and change in light transmission was measured. Platelets were pre-incubated with different inhibitors where noted before agonist stimulation. The chart recorder was set for 0.2 mm/s.
2.4 Platelet secretion
Platelet secretion was determined by measuring the release of ATP using lumichrome reagent (Chronolog, Havertown, PA). The activation of platelets was performed in the lumi-aggregometer at 37° C with stirring (900 rpm) and secretion was measured.
2.5 Depletion/Repletion of platelet cholesterol
For cholesterol depletion, washed platelets were incubated with 5mM or 10mM methyl-β-cytodextrin (MβCD) for 1 hour at room temperature before stimulation. For cholesterol repletion washed platelets were incubated with 5mM MβCD and 200 μg/ml cholesterol, and were incubated at room temperature for 1 hour before stimulation with CLEC-2 agonists.
2.6 Immunoprecipitation
Washed human platelets (1 × 109/mL) were stimulated with fucoidan (50 μg/mL). Reactions were terminated by adding equal volume of 2× NP-40 lysis buffer (20 mM Tris-HCl, pH 7.6, 300 mM NaCl, 2 mM EGTA, 2 mM EDTA, 2% Nonidet P-40, 2 mM PMSF, 5 mM Na3VO4, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μg/ml pepstatin). Cell debris was removed by centrifugation at 15,000 × g for 10 min. Antibodies against CLEC-2 (5 μg/mL) or control IgG were added to the resultant supernatant and incubated overnight with Protein A/G agarose beads. Precipitated proteins were separated by SDS-PAGE and western blotted with the 4G10 antibody and monoclonal CLEC-2 antibody.
2.7 Western blot analysis
Platelets were stimulated with agonists in the presence of inhibitors or vehicles for the appropriate time under stirring conditions at 37°C and the reaction was stopped by the addition of 0.6 N HClO4. The resulting acid precipitate was collected and kept on ice. The samples were centrifuged at 13000g for 4 minutes followed by resuspending in 0.5 ml of deionized water. The protein was again pelleted by centrifugation at 13000 g for 4 minutes. The protein pellets were solubilized in sample buffer containing 0.1 M Tris, 2% SDS, 1% (v/v) glycerol, 0.1% bromophenol blue, and 100 mM DTT then boiled for 10 minutes. Proteins were resolved by SDS polyacrylamide gels and transferred to nitrocellulose membrane (Whatman Protran). Membranes were blocked with Odyssey blocking buffer for 1 hour at ambient temperature, incubated overnight at 4°C with the desired primary antibody and then washed 4 times with TBS-T. Membranes were then incubated with appropriate secondary infrared dye-labeled antibody for 60 minutes at room temperature and washed 4 times with TBS-T. Membranes were examined with a Li-Cor Odyssey infrared imaging system.
2.8 Statistical analysis
Each experiment was repeated at least 3 times. Results are expressed as means ± S.E.M. with number of observations (n). Data was analyzed using Kelidograph software. Significant differences were determined using Student’s t-test. Differences were considered significant at ***P 0.05 and non-significant (NS) at P≤ 0.05.
3 RESULTS
3.1 Disruption of lipid rafts inhibits platelet aggregation induced by CLEC-2 agonists
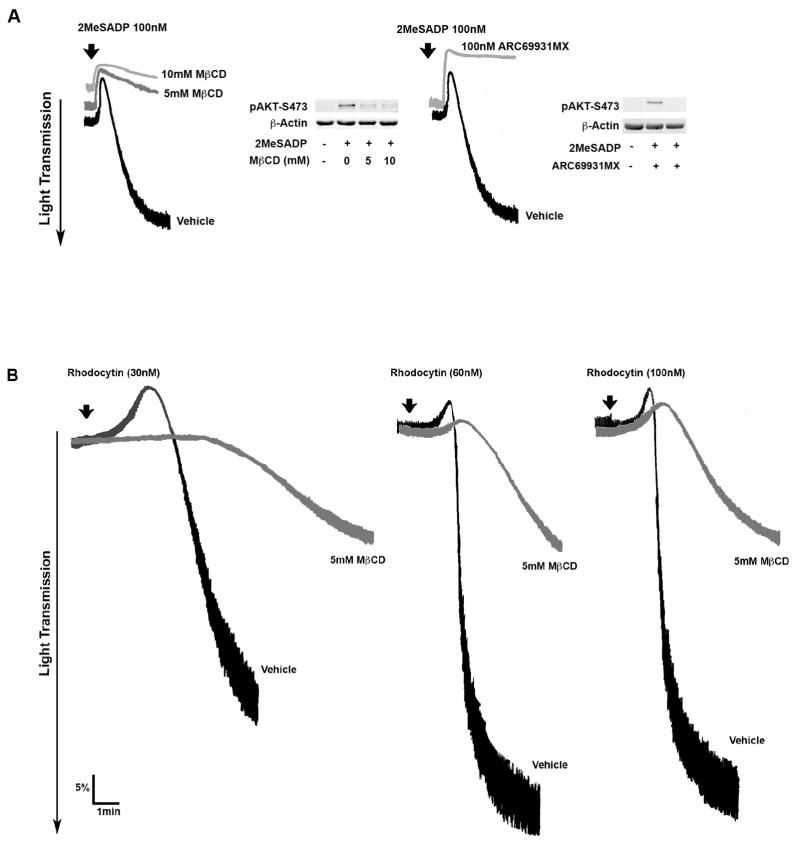
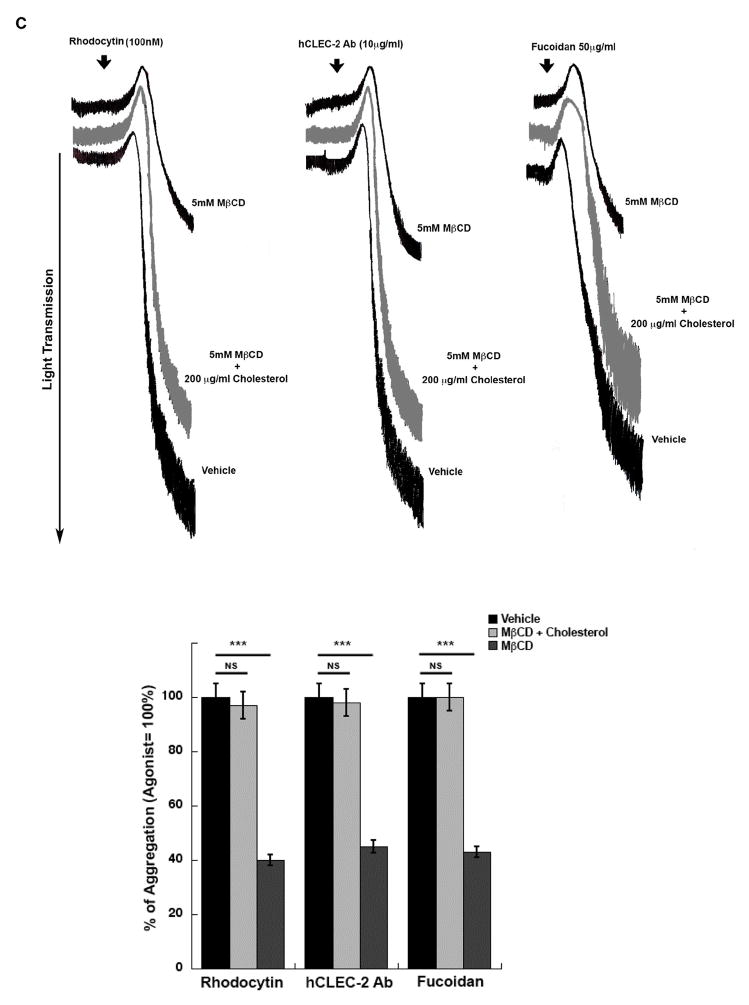
We investigated the requirement of lipid rafts in CLEC-2 signaling in platelets by using the cholesterol-lowering agent methyl-β-cytodextrin (MβCD). In order to standardize the conditions for disrupting lipid rafts in platelets, we examined the effect of MβCD on ADP-induced platelet aggregation and Akt phosphorylation as it was shown previously that Gi activation requires lipid rafts [32]. We used 2-methylthio-adenosine-5′-diphosphate (2MeSADP), a close analogue of endogenous agonist ADP. Pre-treatment of platelets with MβCD abolished 2MeSADP-induced platelet aggregation and Akt phosphorylation (Figure 1A) similar to platelets treated with AR-C69931MX [38], a P2Y12 receptor antagonist. Platelets pre-treated with MβCD and stimulated with CLEC-2 agonists (Rhodocytin, hCLEC-2 Ab and fucoidan) show a significant reduction in the extent of platelet aggregation (***P≤0.05), but not completely inhibition (Figure 1B & 1C). In order to evaluate the non-specific effect of MβCD, we evaluated whether platelets treated with cholesterol restores aggregation inhibited by MβCD. As shown in Figure 1C, rhodocytin-induced platelet aggregation was restored to control levels by the repletion of cholesterol to platelets. These data demonstrate that lipid rafts play an important role in CLEC-2 agonists induced platelet aggregation.
Figure 1. Lipid raft disruption decreases CLEC-2 mediated platelet aggregation.
1A) Washed aspirin-treated human platelets were incubated with the vehicle or MβCD (5 or 10 mM) for 1 hour at 37°C prior to stimulation with 100nM 2MeSADP for 3 minute at 37°C with stirring. Platelet aggregation and Akt phosphorylation were analysis. 1B) Washed aspirin-treated human platelets were incubated with or with 5mM MβCD for 1 hour at 37°C prior to stimulation with different concentrations of rhodocytin (30nM, 60nM and 100nM) for 10 minutes. 1C) Washed aspirin-treated human platelets were incubated with Vehicle or 5mM MβCD and 200 μg/ml cholesterol (control) or with indicated with 5mM MβCD for 1 hour at 37°C prior to stimulation with different CLEC-2 agonists of rhodocytin (100nM), human CLEC-2 Ab (10μg/ml)(incubated with IV.3 F(ab’)2 fragments (10μg/ml) for 5 min to block FcγRIIA receptor) and fucoidan (50μg/ml). Data are representation of at least three independent experiments. Graphs represent mean ± S.E. of % aggregation from at least three different experiments (***P 0.05).
3.2 Lipid raft disruption does not directly affect CLEC-2-mediated platelet aggregation
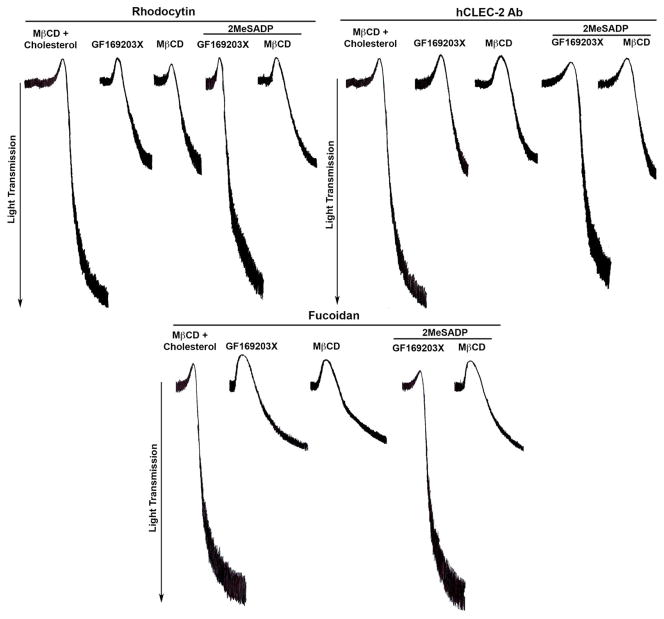
Secondary mediators, such as ADP and thromboxane A2, are known to play an important role in CLEC-2-mediated platelet aggregation [34]. It is known that Gi activation by ADP depends on lipid rafts and is required for thromboxaneA2 production and potentiation of secretion in platelets [32, 37]. Hence we hypothesized that the effects of MβCD on CLEC-2 agonists-induced platelet aggregation are due to the disruption of Gi signaling, which is stimulated by secreted ADP. We compared the effects of MβCD on CLEC2-induced platelet aggregation using a pan-PKC inhibitor, GF109203X, and a P2Y12 receptor antagonist, ARC69931MX. CLEC-2 agonist-induced platelet aggregation was similar in platelets treated with MβCD or GF109203X, which inhibits ADP secretion (Figure 2). However, exogenous addition of 2MeSADP to platelets pre-treated in MβCD and activated with CLEC-2 agonists did not restore aggregation, where as addition of 2MeSADP to GF109203X or cholesterol treated platelets rescued aggregation (Figure 2). These results suggest that inhibition of platelet aggregation induced by CLEC-2 agonists in raft-disrupted platelets is due to the disruption of Gi activation.
Figure 2. Lipid raft disruption does not directly affect CLEC-2-mediated platelet aggregation.
Washed human platelets were incubated with 5mM MβCD and 200 μg/ml cholesterol (control) for 1 hour or GF109203X (5μM), a pan-PKC inhibitor for 5 min or MβCD (10mM) for 1 hour prior to stimulation with rhodocytin (100nM), human CLEC-2 Ab (10μg/ml) and fucoidan (50μg/ml) along with or without 2MesADP (100nM) for 3 minute at 37°C under stirring conditions. All platelets were incubated with IV.3 F(ab’)2 fragments (10μg/ml) for 5 min to block FcγRIIA receptor. Aggregation tracings are representation of 3 independent experiments.
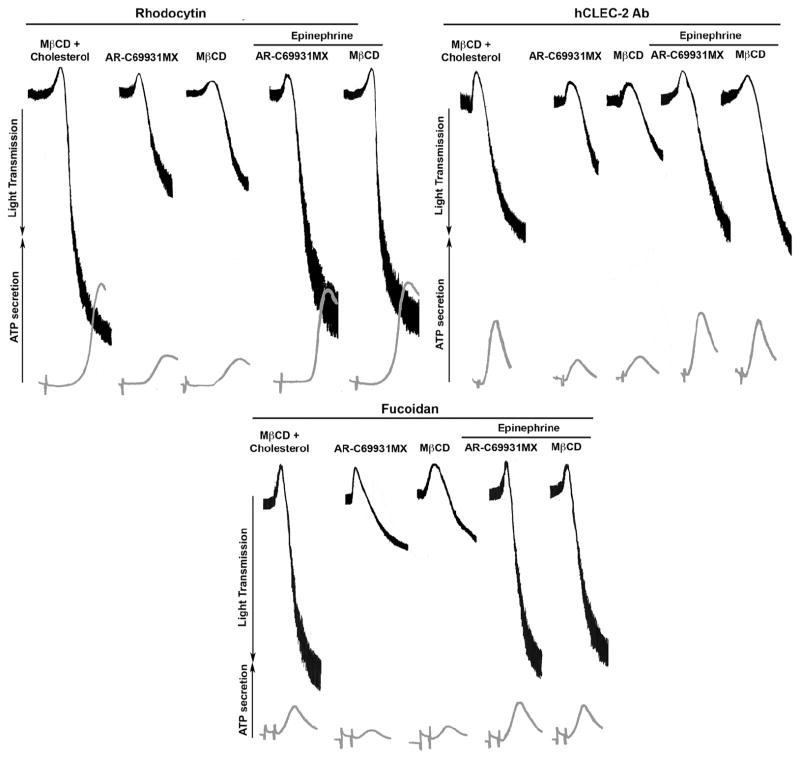
We also evaluated the role of the P2Y12 receptor that activates Gi pathway in CLEC-2 mediated signaling by using AR-C69931MX, a P2Y12 receptor antagonist. As shown in Figure 3, platelets pre-incubated with AR-C69931MX showed similar extent of reduction in CLEC-2-mediated platelet aggregation and secretion as observed with MβCD. As ADP plays a crucial role in platelet activation by CLEC-2 agonists, the inhibition in secretion in the presence of MβCD is due to disruption of Gi pathway, which has been shown previously to play an important role in potentiation of secretion by thromboxaneA2 [37]. It is known that epinephrine, an α2A receptor agonist, activates the Gi subclass member Gz, to activate similar pathways as the P2Y12 receptor [32, 39]. It is also known that Gz signaling is not affected by lipid raft disruption [32]. Hence we investigated whether the addition of epinephrine with CLEC-2 agonists to platelets pre-treated with ARC69931MX or MβCD restores platelet aggregation and secretion. As observed in Figure 3, stimulation with a combination of epinephrine and CLEC-2 agonists rescued aggregation and secretion in platelets pre-treated with ARC69931MX or MβCD. These results demonstrate that lipid raft disruption does not directly affect CLEC-2-mediated platelet aggregation, but affects Gi activation by secreted ADP.
Figure 3. Effect of lipid raft disruption upon Gi and Gz-dependent CLEC-2 mediated aggregation.
Washed human platelets were incubated with 5mM MβCD and 200 μg/ml cholesterol (control) for 1 hour or ARC69931MX (100nM), a P2Y12 antagonist for 2 min or MβCD (5mM) for 1 hour prior to stimulation with rhodocytin (100nM), human CLEC-2 Ab (10_g/ml) and fucoidan (50μg/ml) along with or without Epinephrine (10μM) for 3 minute at 37°C under stirring conditions. All platelets were incubated with IV.3 F(ab’)2 fragments (10μg/ml) for 5 min to block FcγRIIA receptor. Aggregation and secretion tracings are representation of 3 independent experiments.
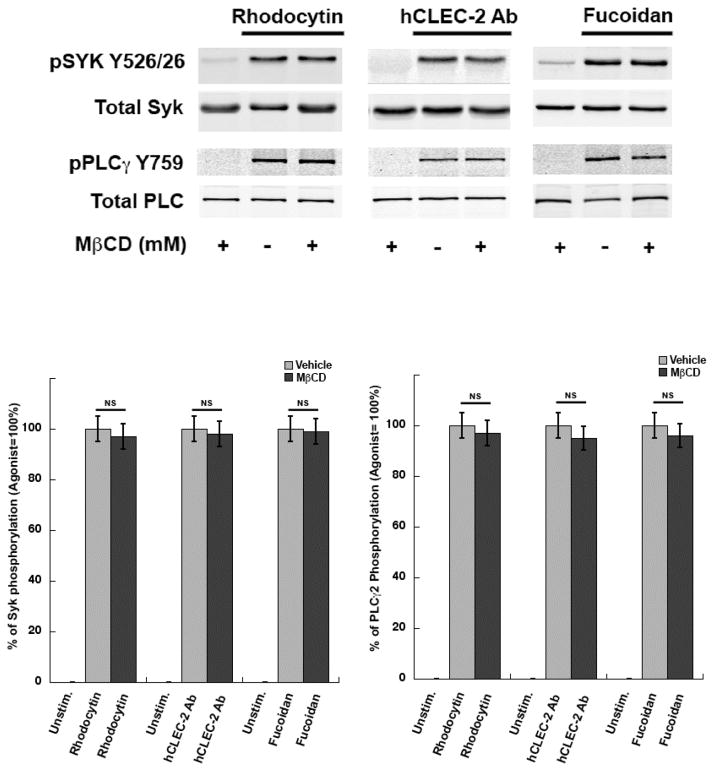
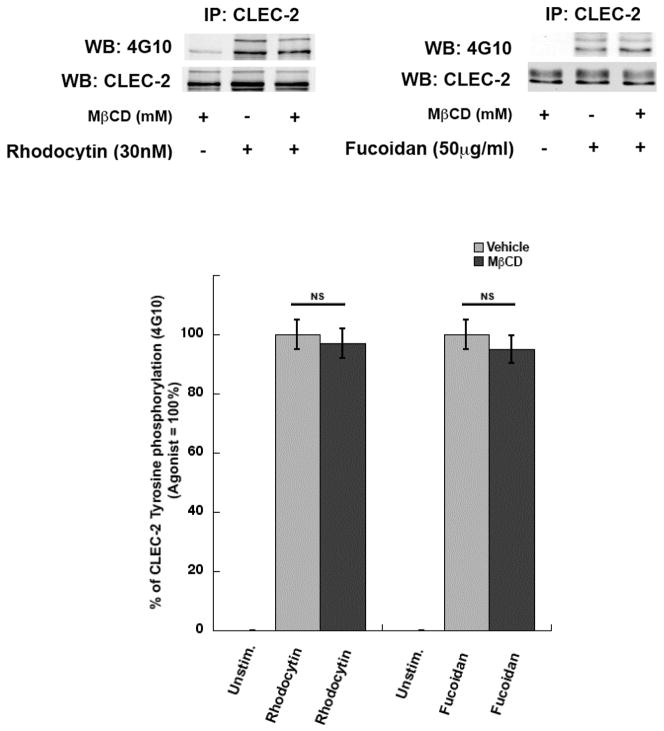
3.3 CLEC-2 mediated signaling is not affected by lipid raft disruption
Previous studies have shown that disruption of lipid rafts leads to inhibition in CLEC-2 signaling [34]. In order to evaluate the role of lipid rafts on CLEC-2 receptor activation, we measured protein phosphorylation in platelets activated by CLEC-2 agonists following MβCD treatment. We found no difference in the phosphorylation of Syk or PLCγ2 (Figure 4) (NS, P≥0.05) between MβCD-treated and untreated platelets following activation with CLEC-2 agonists. In agreement with these results, agonist-induced CLEC-2 receptor hemi-ITAM tyrosine phosphorylation was not inhibited by MβCD (Figure 5) (NS, P≥0.05). These results further establish that lipid raft disruption does not affect CLEC-2 receptor activation.
Figure 4. CLEC-2 receptor signaling in methyl-β-cyclodextrin (MβCD) treated and untreated platelets.
Washed human platelets treated with or without MβCD (5mM) was activated with rhodocytin (100nM), human CLEC-2 Ab (10μg/ml) and fucoidan (50μg/ml) for 1 min and samples were analyzed using SDS-PAGE/Western blotting and probed with a phospho-Syk and phospho-PLCγ2 antibody. Data are representation of 3 independent experiments. Graphs represent mean ± S.E. of % aggregation from at least three different experiments (NS, P≥0.05).
Figure 5. Effect of lipid raft disruption on CLEC-2 receptor phosphorylation.
CLEC-2 was immunoprecipitated from platelets pretreated with MβCD (5mM) and stimulated with rhodocytin (100nM) and fucoidan (50μg/ml) for 1 min and samples were analyzed using SDS-PAGE/Western blotting and probed for anti-phosphotyrosine (4G10) and anti-CLEC-2 antibody. Data are representation of 3 independent experiments. Graphs represent mean ± S.E. of % aggregation from at least three different experiments (NS, P≥0.05).
4 DISCUSSION
The CLEC-2 receptor has been postulated to signal in a lipid raft dependent manner by utilizing its hemi-ITAM containing cytoplasmic tail to activate downstream signaling molecules upon activation [8, 15, 34]. Disruption of the lipid rafts affects platelet aggregation and signaling by CLEC-2 agonists analogous to that of responses mediated by GP1b-IX-V and GPVI receptor activation [31, 40]. However, studies have reported that platelet aggregation mediated by GP1b-IX-V, GPVI and CLEC-2 agonists depends on secondary mediators such as ADP and thromboxaneA2 [34, 41, 42]. Our lab has demonstrated that lipid rafts are required for Gi activation in ADP-induced platelet activation [32]. Previous studies from our lab has also shown that Thromboxane A2 generated downstream of P2Y12 helps in potentiation of aggregation and secretion when stimulate with ADP. Therefore we hypothesize that platelets stimulated with CLEC-2 agonists upon lipid raft disruption affects Gi pathway activation thereby abolishing thromboxaneA2 generation, which is an important secondary mediator that helps in potentiation of CLEC-2-mediated platelet aggregation and secretion.
Our results contrast with Pollit et al, who have shown that 30 nM rhodocytin-induced platelet aggregation was abolished in MβCD-treated platelets. We observed that platelet aggregation induced by low concentrations (30 nM) of rhodocytin was delayed and inhibited but not abolished. The difference in CLEC-2-mediated platelet aggregation in MβCD treated platelets from these two studies could be attributed to the time points used to measure platelet aggregation. Pollit et al have stimulated raft-disrupted platelets with rhodocytin for 5 minutes, in contrast to 10 minutes in our experiments. The delay in aggregation was rescued by increasing rhodocytin concentration from 30 nM to 100 nM (Figure 1B). It is known that the lipid rafts help in bringing receptors and signaling molecules to close proximity, however in platelets treated with MβCD the membrane is more fluid and dynamic, which may result in delay what was observed in Figure 1B. We also used two other CLEC-2 agonists, fucoidan and hCLEC-2 antibody, to further demonstrate that platelet aggregation induced by CLEC-2 agonists was not abolished as previously shown; they are only delayed and inhibited. These results suggest that CLEC-2-mediated primary aggregation is not effected by raft disruption.
Even with high concentrations of rhodocytin, platelet aggregation was still inhibited in the presence of MβCD, suggesting that lipid raft disruption affects secondary mediator induced platelet aggregation. Secondary mediators such as ADP and TXA2 are shown to play important roles in CLEC-2-mediated platelet activation[34]. The inhibition of CLEC-2-mediated aggregation in the presence of MβCD, was similar to the conditions where ADP secretion or signaling were blocked. The rescue experiments with epinephrine, which activates Gz, and signals independent of lipid rafts, clearly demonstrate that the inhibition of CLEC-2-mediated aggregation in the presence of MβCD, is due to the effect of MβCD on Gi signaling. Consistent with this idea, MβCD did not affect downstream signaling events from CLEC-2, including phosphorylation of the hemi-ITAM, Syk, and PLCγ2.
Although previous studies have shown that a small portion (25%) of CLEC-2 moves to the lipid rafts and is phosphorylated upon stimulation with rhodocytin, a considerably large amount of (75%) CLEC-2 is still in the soluble fractions. T-cell receptor activation (TCR) in T-cells and GPVI receptor activation platelets have been shown to occur in a lipid raft independent manner [33, 43], suggesting a possibility that this may also be true of CLEC-2. The lipid rafts could be important in CLEC-2-mediated platelet activation because signaling proteins such as Src and LAT are enriched in lipid rafts. However recent work has also shown that Src and LAT can be activated in a lipid raft independent manner [44, 45]. Furthermore, CLEC-2-mediated platelet activation in LAT-/- platelets is not affected suggesting that lipid rafts are not important for CLEC-2-mediated platelet activation [46]. Previous studies also suggested that GPVI receptors and CLEC-2 move into the lipid rafts upon stimulation with the agonists[34].
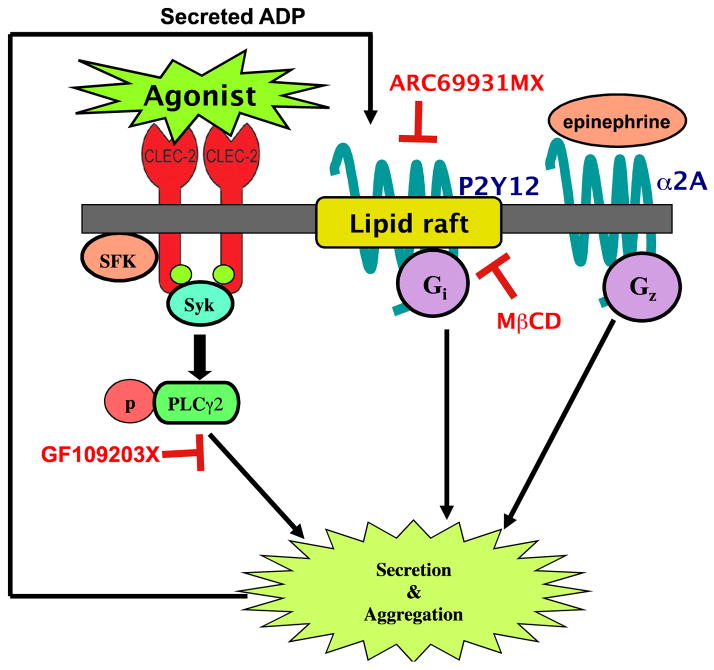
In conclusion, we have examined the dependence of CLEC-2 receptor activation on lipid rafts in human platelets and found that CLEC-2 activation was not affected by raft disruption. Furthermore, we also show that disruption of lipid rafts affects Gi activation there by diminishing CLEC-2 agonists induced platelet aggregation (Figure 6). Lipid rafts may be important membrane domains that regulate signaling pathways, but our work demonstrates that they are not directly involved in CLEC-2 receptor signaling in platelets.
Figure 6. Model for CLEC-2 signaling independent of lipid rafts.
Our observation supports the following model: CLEC-2 activation was not affected by raft disruption. We also found that disruption of lipid rafts effects Gi activation there by affecting CLEC-2 mediated platelet aggregation.
Acknowledgments
We thank Dr. Steve P Watson for providing rhodocytin and reviewed the manuscript. This work is supported by HL93231 and HL118593 from National Institutes of Health to SPK.
Footnotes
Disclosure: No relevant conflicts of interest to disclose.
Author Contributions:
Bhanu Kanth Manne - designed and performed experiments, analyzed and interpreted data, wrote manuscript
Rachit Badolia - performed experiments
Carol A Dangelmaier - analyzed interpreted data
Satya P Kunapuli - designed experiments, analyzed and interpreted data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Packham MA. Role of platelets in thrombosis and hemostasis. Can J Physiol Pharmacol. 1994;72:278–84. doi: 10.1139/y94-043. [DOI] [PubMed] [Google Scholar]
- 2.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–8. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 3.Offermanns S. The role of heterotrimeric G proteins in platelet activation. Biol Chem. 2000;381:389–96. doi: 10.1515/BC.2000.051. [DOI] [PubMed] [Google Scholar]
- 4.Watson SP, Herbert JM, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost. 8:1456–67. doi: 10.1111/j.1538-7836.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Hoylaerts MF. The P2X1 ion channel in platelet function. Platelets. 2010;21:153–66. doi: 10.3109/09537101003599549. [DOI] [PubMed] [Google Scholar]
- 6.Mahaut-Smith MP, Jones S, Evans RJ. The P2X1 receptor and platelet function. Purinergic signalling. 2011;7:341–56. doi: 10.1007/s11302-011-9224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunapuli SP. P2 receptors and platelet activation. ScientificWorldJournal. 2002;2:424–33. doi: 10.1100/tsw.2002.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, et al. CLEC-2 activates Syk through dimerization. Blood. 115:2947–55. doi: 10.1182/blood-2009-08-237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji M, Ezumi Y, Arai M, Takayama H. A novel association of Fc receptor gamma-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J Biol Chem. 1997;272:23528–31. doi: 10.1074/jbc.272.38.23528. [DOI] [PubMed] [Google Scholar]
- 10.Ozaki Y, Asazuma N, Suzuki-Inoue K, Berndt MC. Platelet GPIb-IX-V-dependent signaling. J Thromb Haemost. 2005;3:1745–51. doi: 10.1111/j.1538-7836.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki-Inoue K, Wilde JI, Andrews RK, Auger JM, Siraganian RP, Sekiya F, et al. Glycoproteins VI and Ib-IX-V stimulate tyrosine phosphorylation of tyrosine kinase Syk and phospholipase Cgamma2 at distinct sites. Biochem J. 2004;378:1023–9. doi: 10.1042/BJ20031430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May F, Hagedorn I, Pleines I, Bender M, Vogtle T, Eble J, et al. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–72. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 13.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095–9. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 14.Qi R, Ozaki Y, Asazuma N, Satoh K, Yatomi Y, Law CL, et al. FcgammaRII tyrosine phosphorylation differs between FcgammaRII cross-linking and platelet-activating anti-platelet monoclonal antibodies. Biochim Biophys Acta. 1999;1451:353–63. doi: 10.1016/s0167-4889(99)00105-6. [DOI] [PubMed] [Google Scholar]
- 15.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pohlmann S, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki Y, Suzuki-Inoue K, Inoue O. Novel interactions in platelet biology: CLEC-2/podoplanin and laminin/GPVI. J Thromb Haemost. 2009;7 (Suppl 1):191–4. doi: 10.1111/j.1538-7836.2009.03372.x. [DOI] [PubMed] [Google Scholar]
- 17.Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, Duchler M, et al. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol. 2001;31:3493–503. doi: 10.1002/1521-4141(200112)31:12<3493::aid-immu3493>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–6001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 19.Christou CM, Pearce AC, Watson AA, Mistry AR, Pollitt AY, Fenton-May AE, et al. Renal cells activate the platelet receptor CLEC-2 through podoplanin. Biochem J. 2008;411:133–40. doi: 10.1042/BJ20071216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson AA, Eble JA, O'Callaghan CA. Crystal structure of rhodocytin, a ligand for the platelet-activating receptor CLEC-2. Protein Sci. 2008;17:1611–6. doi: 10.1110/ps.035568.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spalton JC, Mori J, Pollitt AY, Hughes CE, Eble JA, Watson SP. The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets. J Thromb Haemost. 2009;7:1192–9. doi: 10.1111/j.1538-7836.2009.03451.x. [DOI] [PubMed] [Google Scholar]
- 22.Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, Naik UP, et al. Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2) J Biol Chem. 288:7717–26. doi: 10.1074/jbc.M112.424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki-Inoue K. Essential in vivo roles of the platelet activation receptor CLEC-2 in tumour metastasis, lymphangiogenesis and thrombus formation. J Biochem. 150:127–32. doi: 10.1093/jb/mvr079. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki-Inoue K, Inoue O, Ozaki Y. Novel platelet activation receptor CLEC-2: from discovery to prospects. J Thromb Haemost. 9(Suppl 1):44–55. doi: 10.1111/j.1538-7836.2011.04335.x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki-Inoue K, Inoue O, Ozaki Y. The novel platelet activation receptor CLEC-2. Platelets. 22:380–4. doi: 10.3109/09537104.2011.556274. [DOI] [PubMed] [Google Scholar]
- 26.Severin S, Pollitt AY, Navarro-Nunez L, Nash CA, Mourao-Sa D, Eble JA, et al. Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J Biol Chem. 286:4107–16. doi: 10.1074/jbc.M110.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki-Inoue K, Tulasne D, Shen Y, Bori-Sanz T, Inoue O, Jung SM, et al. Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling. J Biol Chem. 2002;277:21561–6. doi: 10.1074/jbc.M201012200. [DOI] [PubMed] [Google Scholar]
- 28.Quek LS, Pasquet JM, Hers I, Cornall R, Knight G, Barnes M, et al. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–53. [PubMed] [Google Scholar]
- 29.Bodin S, Tronchere H, Payrastre B. Lipid rafts are critical membrane domains in blood platelet activation processes. Biochim Biophys Acta. 2003;1610:247–57. doi: 10.1016/s0005-2736(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 30.Shrimpton CN, Gousset K, Tablin F, Lopez JA. Isolation and analysis of platelet lipid rafts. Methods Mol Biol. 2004;273:213–28. doi: 10.1385/1-59259-783-1:213. [DOI] [PubMed] [Google Scholar]
- 31.Shrimpton CN, Borthakur G, Larrucea S, Cruz MA, Dong JF, Lopez JA. Localization of the adhesion receptor glycoprotein Ib-IX-V complex to lipid rafts is required for platelet adhesion and activation. J Exp Med. 2002;196:1057–66. doi: 10.1084/jem.20020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinton TM, Kim S, Jin J, Kunapuli SP. Lipid rafts are required in Galpha(i) signaling downstream of the P2Y12 receptor during ADP-mediated platelet activation. J Thromb Haemost. 2005;3:1036–41. doi: 10.1111/j.1538-7836.2005.01325.x. [DOI] [PubMed] [Google Scholar]
- 33.Quinter PG, Dangelmaier CA, Quinton TM, Kunapuli SP, Daniel JL. Glycoprotein VI agonists have distinct dependences on the lipid raft environment. J Thromb Haemost. 2007;5:362–8. doi: 10.1111/j.1538-7836.2007.02309.x. [DOI] [PubMed] [Google Scholar]
- 34.Pollitt AY, Grygielska B, Leblond B, Desire L, Eble JA, Watson SP. Phosphorylation of CLEC-2 is dependent on lipid rafts, actin polymerization, secondary mediators, and Rac. Blood. 115:2938–46. doi: 10.1182/blood-2009-12-257212. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Pestina TI, Berndt MC, Steward SA, Jackson CW, Gartner TK. The roles of ADP and TXA in botrocetin/VWF-induced aggregation of washed platelets. J Thromb Haemost. 2004;2:2213–22. doi: 10.1111/j.1538-7836.2004.01023.x. [DOI] [PubMed] [Google Scholar]
- 36.Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–5. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dangelmaier C, Jin J, Smith JB, Kunapuli SP. Potentiation of thromboxane A2-induced platelet secretion by Gi signaling through the phosphoinositide-3 kinase pathway. Thromb Haemost. 2001;85:341–8. [PubMed] [Google Scholar]
- 38.Suzuki T, Obara Y, Moriya T, Nakata H, Nakahata N. Functional interaction between purinergic receptors: effect of ligands for A2A and P2Y12 receptors on P2Y1 receptor function. FEBS Lett. 585:3978–84. doi: 10.1016/j.febslet.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 39.Dorsam RT, Kim S, Jin J, Kunapuli SP. Coordinated signaling through both G12/13 and G(i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J Biol Chem. 2002;277:47588–95. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- 40.Locke D, Chen H, Liu Y, Liu C, Kahn ML. Lipid rafts orchestrate signaling by the platelet receptor glycoprotein VI. J Biol Chem. 2002;277:18801–9. doi: 10.1074/jbc.M111520200. [DOI] [PubMed] [Google Scholar]
- 41.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–4. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Mangin P, Dangelmaier C, Lillian R, Jackson SP, Daniel JL, et al. Role of phosphoinositide 3-kinase beta in glycoprotein VI-mediated Akt activation in platelets. J Biol Chem. 2009;284:33763–72. doi: 10.1074/jbc.M109.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto-Tane A, Yokosuka T, Ishihara C, Sakuma M, Kobayashi W, Saito T. T-cell receptor microclusters critical for T-cell activation are formed independently of lipid raft clustering. Mol Cell Biol. 30:3421–9. doi: 10.1128/MCB.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hundt M, Harada Y, De Giorgio L, Tanimura N, Zhang W, Altman A. Palmitoylation-dependent plasma membrane transport but lipid raft-independent signaling by linker for activation of T cells. J Immunol. 2009;183:1685–94. doi: 10.4049/jimmunol.0803921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitosugi T, Sato M, Sasaki K, Umezawa Y. Lipid raft specific knockdown of SRC family kinase activity inhibits cell adhesion and cell cycle progression of breast cancer cells. Cancer Res. 2007;67:8139–48. doi: 10.1158/0008-5472.CAN-06-4539. [DOI] [PubMed] [Google Scholar]
- 46.Hughes CE, Auger JM, McGlade J, Eble JA, Pearce AC, Watson SP. Differential roles for the adapters Gads and LAT in platelet activation by GPVI and CLEC-2. J Thromb Haemost. 2008;6:2152–9. doi: 10.1111/j.1538-7836.2008.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]