Abstract
Background
Airway secretions contain endogenous antimicrobial factors (AMFs) which contribute to the innate host defense of the respiratory tract. Antibacterial peptides as well as host-derived lipids including cholesteryl esters have been detected in maxillary lavage fluid. Sterol O-acyltransferase 1 (SOAT1) is a key enzyme in cholesteryl ester production. The purpose of this study is to determine if such intrinsic microbicidal molecules are acutely expressed within sinus tissue and to compare levels of expression between patients with and without chronic rhinosinusitis (CRS).
Methods
Sinus tissue was obtained from subjects with (24) and without (9) a history of CRS. Six CRS patients had nasal polyposis (CRSwNP). Immunofluorescence staining for human neutrophil peptide (HNP) was done as a marker for inflammation. RT-PCR following RNA extraction was used to quantify the expression of SOAT-1, the epithelial beta-defensins (HBD2,3), and the cathelicidin LL37 with ribosomal protein RPLP0 as the housekeeping gene.
Results
Immunofluorescence showed significant increase in HNP staining in CRS patients without nasal polyposis (CRSsNP) versus non-CRS specimens (p=0.010), in agreement with clinical inflammation status. SOAT1 mRNA expression was also upregulated in CRSsNP compared to non-CRS (p=0.041) and CRSwNP (p=0.005) patients; while increases for HBD2 and HBD3 were less prominent. LL37 was either absent or expressed at very low levels in all samples.
Conclusions
Increased biosynthesis of SOAT1, a key enzyme for antimicrobial cholesteryl ester production, was observed in the sinus tissue of CRSsNP but not in CRSwNP patients. This further supports the novel concept of lipid-mediated innate mucosal defense and delineates CRS with and without nasal polyposis as distinct subtypes.
Keywords: Chronic rhinosinusitis, cholesteryl esters, mucosa, defensin
Introduction
Airway secretions contain a broad spectrum of endogenous antimicrobial factors (AMFs) which contribute to the inherent host defense of the respiratory tract. Such mediators of innate immunity provide initial protection of mucosal surfaces against infection by facilitating recognition of microbial pathogens, engaging in bactericidal activity, and activating the adaptive immune response.1-3 Complement factors, surfactant proteins, antimicrobial peptides (AMPs), antibacterial polypeptides, and host-derived lipids are all integral components of this intrinsic defense system.3-8
AMPs encompass both cathelicidins and defensins.1-4 Cathelicidins are a family of propeptides consisting of a highly conserved N-terminal sequence (cathelin) and a heterogeneous cationic and antimicrobially active C-terminal domain.9 They are produced by monocytes and macrophages, but have also been detected in respiratory epithelia and thought to participate in nascent airway protection.9 Cathelicidins exhibit broad spectrum antimicrobial activity against gram-positive, gram-negative, and fungal organisms in addition to mediating a complex array of immunomodulatory responses.9 In humans, only one cathelicidin has been described thus far, human cationic antimicrobial protein (hCAP-18, 18 kDa); which undergoes extracellular cleavage to release the active C-terminal peptide, LL37 of ~ 4.5 kDa.1,2-9
Defensins are 3-5 kDa cationic peptides with a characteristic β-sheet rich framework and 6-cysteine/3-disulfide bond pattern.1-2 Like cathelicidins, defensins demonstrate a wide range of antimicrobial activity against gram-positive and negative bacteria, enveloped viruses, as well as fungi; with optimal potency at low ionic strength conditions.1 They have been classified based on their respective molecular structures into the alpha (α) and beta (β) defensins.1-2 The α-defensins include human neutrophil peptides (HNP 1-4) which are stored in the dense azurophilic granules of neutrophils, delivered to microbial invaders during phagocytosis, and are associated with inflammation.1-2 The human β-defensins (HBD 1-3) are located on mucosal barrier surfaces such as the skin, lungs, and gastrointestinal tract.1-2 HBD1 expression is mainly constitutive, with low levels detected in healthy airway epithelia.1-2 In contrast, synthesis of HBD2 and HBD3 is inducible, with production stimulated by microbial products and proinflammatory cytokines. 1-2 The presence of AMPs within airway surface fluid (ASF) and their role in the primary host defense of the respiratory tract have been well documented. 1-2, 6-7
More recently, host-derived lipids have also been recognized as novel effector molecules of the innate immune system.10-19 Cholesteryl esters (i.e. cholesteryl linoleate (CL) and cholesteryl arachidonate (CA)), are synthesized by sterol O-acyltransferase 1 (SOAT1) through the transfer of a fatty acid residue from acyl Coenzyme A to cholesterol, and have been found to express microbicidal activity against various bacteria in vitro.10-12 Antibacterial lipids within the skin, breast milk, and vernix caseosa (newborn coating) are known to provide protection for neonates against infection.13-18 Antimicrobial lipids (AMLs) and lipoproteins have also been identified in nasal and oral secretions and found to partially account for their bactericidal properties.11,19
Recent studies have indicated that AMPs and host-derived lipids both play a role in the native mucosal immunity of the paranasal sinuses. HBD1, HBD2, and HNP 1-3 have been detected in maxillary sinus lavage fluid.8,20 All major classes of lipids including polar fatty acids, nonpolar lipids (NPL), and cholesteryl esters have been demonstrated in sinus secretions.8 In addition, upregulation of antibacterial peptides and AMLs have been observed in antral washes from patients with chronic rhinosinusitis (CRS).8,20 The purpose of this study is to determine if such endogenous microbicidal molecules are acutely expressed within sinus tissue and to compare the levels of expression between patients with and without CRS.
Materials and methods
Specimen collection
Maxillary sinus mucosa was obtained during functional endoscopic sinus surgery (FESS) from 24 patients who fulfilled the diagnostic criteria for CRS, as defined by the 2007 American Academy of Otolaryngology-Head and Neck Surgery clinical practice guidelines.21 CRS subjects were classified into CRS with nasal polyposis (CRSwNP) and CRS without nasal polyposis (CRSsNP) based on clinical findings. Nasal endoscopy revealed the presence of nasal polyps in 6 of the 24 CRS patients. Computed tomography demonstrated either partial or complete opacification of the maxillary sinus from which the specimen was procured. Surgery was performed only when the patient's symptoms and radiographic findings persisted despite 4-6 weeks of treatment with oral antibiotics, saline rinses, topical corticosteroids, and systemic corticosteroids (CRSwNP). None of the CRS patients had undergone previous sinus surgery. Only one patient in the entire cohort smoked, and that individual had CRSsNP. No perioperative antibiotics were administered. All CRS patients were on topical nasal steroid sprays preoperatively. Three of the 6 CRSwNP patients also received oral steroids- prednisone 20 miligrams daily for 5 days immediately prior to surgery. Administration of preoperative oral steroids to CRSwNP patients or lack thereof was dependent on individual surgeon preference and patient willingness to take systemic steroids. Sphenoid sinus mucosa was also acquired from 9 patients without a history of CRS undergoing transsphenoidal pituitary surgery or spontaneous cerebrospinal fluid leak repair which were designated as non-CRS controls. All patients denied having any previous sinus infections, and showed no radiographic evidence of sinus pathology on magnetic resonance imaging. This study was approved by the Southern California Permanente Medical Group Institutional Review Board and informed consent obtained.
Immunofluorescence for HNP
Paraffin-embedded tissue samples were sliced into 4 μm sections, deparaffinized with xylene, and rehydrated using serial ethanol dilutions. Antigen retrieval was performed using 10 mM of sodium citrate and 100 mM of glycine buffer, pH 3.5 at 60°C. Sections were blocked with 3% Gelatin (75% Bloom Gelatin, Sigma-Aldrich, St. Louis, MO) for 1 hour at room temperature to avoid nonspecific binding. Incubation with the primary antibody, a polyclonal rabbit anti-HNP serum kindly obtained from Tomas Ganz (David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA), at a 1:400 dilution was then performed at room temperature overnight. Slides were washed and then incubated with a secondary antibody, Alexa-Fluor 488 conjugated goat anti-rabbit immunoglobulin G (Invitrogen, Carlsbad, CA), at a 1:1000 dilution for 1-2 hours in the dark, and then washed. Washes consisted of a very brief rinse in dH2O followed by three 10-min washes in TBS/0.05% Tween 20. After slides were dried, coverslips were mounted and counterstaining achieved with Permount supplemented by propidium iodide (10 μg/mL). Each tissue was divided into 3-9 equal sized areas (5 x 5mm squares) and viewed through a 40× objectives in a blinded fashion. The number of green fluorescing cells representing HNP- positive cells was enumerated and the average number per square determined. The entire tissue section was counted for each slide. All slides were coded and the person quantifying the number of HNP staining cells was blinded. Only after the counts were completed was the identity released. For imaging, optical sections of fluorescent images were analyzed using a Zeiss Axio Imager M2 microscope. The microscope was equipped with a Zeiss ApoTome 2 oscillating grating in the epifluorescence beam, which significantly reduces scattered out-of-focus stray light. Images were obtained using a Zeiss AxioCam MRm camera. Optical sections were obtained at 10 × and 63 × magnification and three slices per image were stacked using Zeiss software for analysis.
RT-PCR
RNA expression analysis was conducted with two approaches, recovery of mRNA from paraffin embedded formalin fixed tissue sections and mRNA extraction from fresh specimens. The first 18 sinus specimens (4: non-CRS, 14: CRSsNP) collected were placed in formalin and subsequently paraffin embedded. The subsequent 15 samples (5: non-CRS; 4: CRSsNP; 6: CRSwNP) obtained were processed fresh. RNA extraction from fixed tissues was initially performed due to the large number of formalin fixed tissue samples that had already been collected for purposes of immunofluorescence. All specimens were processed by the same lab using the same protocol with similar fixation times. However, due to the limited quantity and quality of RNA recovered from fixed tissues, RNA analysis of fresh specimens was subsequently conducted. It should be noted that CRSwNP samples were collected after RNA extraction had been attempted on paraffin embedded tissues from CRSsNP and control samples. At that time, all tissues were processed for RNA extraction to maximize the RNA yield and none of the fresh tissues were placed in formalin. Thus, HNP staining was not conducted for CRSwNP. When using paraffin embedded formalin tissue sections Qiagen RNeasy FFPE kit (Qiagen, Valencia, CA) was used according to the manufacturer's instructions to extract mRNA. For mRNA extraction for fresh tissues, specimens were placed into Tri-Reagent (Fisher Scientific) supplemented with glassbeads (1.0 mm diameter, BioSpec Products, Inc., Bartlesville, OK) immediately after collection and processed as recommended by the manufacturer. First-strand cDNA conversion was performed using iScript and real time PCR was conducted with SYBR Green according to manufacturers’ protocols (Bio-Rad Laboratories, Hercules, CA). The gene coding for the ribosomal protein RPLP0 was used as reference. Gene accession numbers were as follows: NM_001002.3 (RPLP0), NM_003101 (SOAT1), NM_004345 (LL37), NM_004942.2 (HBD2), and NM_0011081551.2 (HBD3). Primers had been designed by IDT (LL37 and SOAT1, Integrated DNA Technologies, Inc., San Diego, CA) or using NCBI Primer blast (RPLP0, HBD2, and HBD3) (see Table 1). All PCR primers were purchased from IDT. Amplicons had been previously sequenced (DNA Core Facility, Beckman Research Institute, City of Hope, Duarte, CA, or Retrogen Inc., San Diego, CA) and were computer matched with the known cDNA sequences of each molecule.
Table 1.
Custom designed primer sequences used in this study
| Gene target | Forward | Reverse | Product size (bp) |
|---|---|---|---|
| RPLP0 | 5′-CCAGGCG-TCCTCGTGGAAGTG-3′ | 5′-CATCACGGCGGTGCGTCAGG-3′ | 70 |
| SOAT1 | 5′ -CATCCTGTCACCAAAGCGTAA -3.′ | 5′-CTCGTGTTCTGGTCCTATGTG-3′ | 131 |
| LL37 | 5′-TGACTGCTGTGTCGTCCT-3′ | 5′ - TCCTCGGATGCTAACCTCT-3′ | 132 |
| HBD2 | 5′ GACTCAGCTC-CTGGTGAAGCTC-3′ | 5′-CACCAAAAA-CACCTGGAAGAGG-3′ | 105 |
| HBD3 | 5′ -AGCTCTGCCTTACCATTGGG-3′ | 5′-CCACGCTGAG-ACTGGATGAA-3′ | 188 |
One microliter of cDNA was used for the SYBR Green PCR assay along with 12.5 μL of SYBR Green reaction mix (Bio-Rad laboratories), 3 μL of each primer (forward, reverse, 100 μM stock), and 5.5 μL of sterile water for a total final volume of 25 μl. The PCR hot start was at 95°C for 4 minutes. Forty cycles of amplification were then performed at 95°C for 15 seconds and at 63°C for 50 seconds per cycle. A melting curve analysis of PCR products was conducted at the completion of the assays to identify peaks for target and reference genes. Specificity of PCR amplification and quality of the reactions were subsequently further assessed by agarose gel electrophoresis. All samples were tested at least in duplicate PCR reactions. Since the PCR reaction for the formalin fixed tissues showed in addition to the target sequences other bands including primer dimers, end point analysis was conducted and all PCR products subjected to ethidium bromide stained agarose gel electrophoresis, imaged with VersaDoc (BioRad), and the intensity of target bands determined with QuantityOne software. PCR products were identified based on expected bp size, and identities confirmed in selected cases by DNA sequencing.
Expression of target genes relative to RPLP0 was then calculated by first subtracting the area intensity (Volume intensity*mm2) of the no-template control from the band intensity of the sample and then dividing the target gene band intensity by the corresponding RPLP0 band intensity. For purposes of consistency, gene expression for formalin fixed and fresh tissues were both analyzed in the same manner. However, real time -PCR data for SOAT1 expression in control and diseased fresh samples was also collected for those specimens in which sufficient RNA and/or cDNA was still available for re-analysis. To accomplish this, duplexing was conducted employing assays from IDT (for SOAT1 (Soat1 + FAM, Hs.PT.49a.5041163) and RPLP0 (RPLP0 +HEX, Hs.PT.49a.849406), and Supermix (BioRad) according to the manufacturer's recommendation with the same PCR conditions as before but omitting the melt curve. Relative SOAT1 gene expression was calculated by the ΔΔCT method using BioRad CFX Manager 3.1 software. Microsoft Office Excel ® 2013 was used to calculate raw data.
Statistical Analysis
Means and SEM were determined and statistical significance of observed differences calculated using IBM SPSS Statistics version 20. For HNP staining data were not normally distributed and independent sample Mann Whitney U test was employed to test for significant differences between patient groups. Significance testing of PCR data from paraffin-embedded tissues was not included but means and sample sizes for the two groups and different variables reported. For gene expression analysis from fresh tissues, data were normally distributed and One-Way ANOVA with Bonferroni posthoc analysis was employed using disease status (non-CRS, CRSsNP, and CRSwNP) as factor and relative expression of SOAT1, LL37, HBD2, and HBD3 as dependent variables. Correlation between HNP positive cells and dependent variables was tested with two tailed Pearson analysis.
Results
Immunolocalization of HNP 1-3 demonstrates a significantly elevated number of neutrophils in the sinus tissue of CRSsNP patients
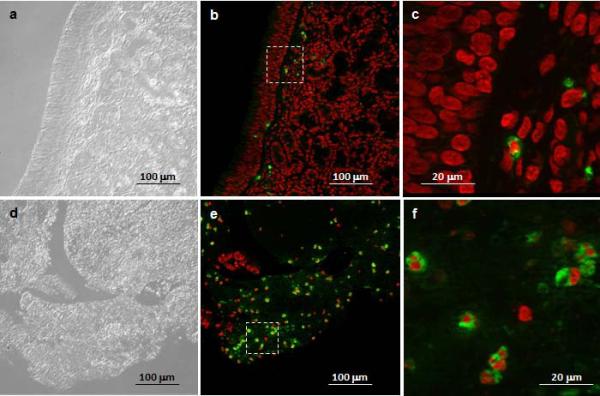
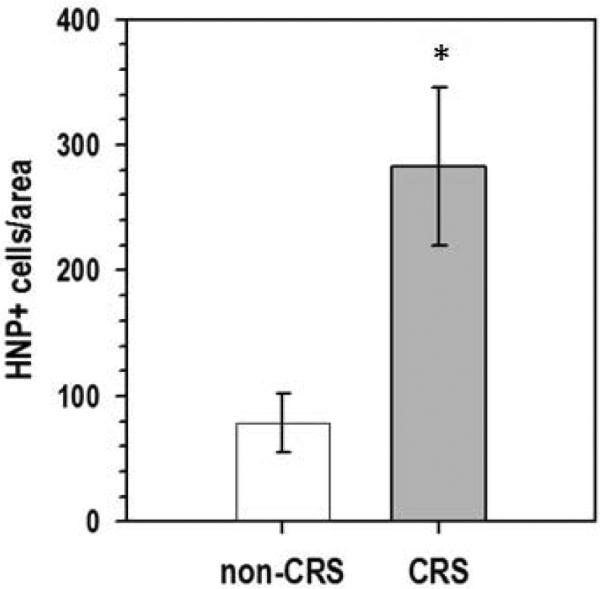
Neutrophils were detected with polyclonal antibodies against HNP 1-3 and visualized with Alexa Fluor 488 labeled secondary antibodies in the sinus tissue of patients with and without CRS (Figure 1A). None of the CRS patient specimens used for analysis had nasal polyposis. Quantification of neutrophils in tissue sections demonstrated a substantially greater number in CRSsNP (n = 13) compared to non-CRS (n = 4) subjects, with the observed difference between the 2 groups reaching statistical significance (p = 0.01, Figure 1B).
Figure 1. Quantification of neutrophils in sinus tissue from non-CRS and CRS subjects.
A. Neutrophils were detected with polyclonal antibodies against HNP1-3 and visualized with Alexa Fluor 488 labeled secondary antibodies, and tissues were counterstained with the nuclear stain propidium iodide. Alexa-Fluor 488 stain demonstrates HNP-positive neutrophils in green color, and propidium iodide highlights nuclei in red color. Shown are representative images for non-CRS (top, a-c) and CRS without nasal polyposis (bottom, d-f). a, d: phase contrast; b, c, e, f: overlay for Alexa-Fluor 488 and propidium iodide. The squares in b and e represent the area shown in c and f, respectively. B. Enumeration of neutrophils in sinus tissue sections from non-CRS and CRS subjects. Shown are means ± SEM, n = 4 for non-CRS and n = 13 for CRS. All CRS cases were without nasal polyposis. *p = 0.010 in independent samples Mann Whitney U test. HNP: human neutrophil peptide; CRS: chronic rhinosinusitis
Gene expression of SOAT1, LL37, HBD2, and HBD3 relative to RPLP0 is upregulated in the sinus tissue of CRSsNP patients but not in CRSwNP
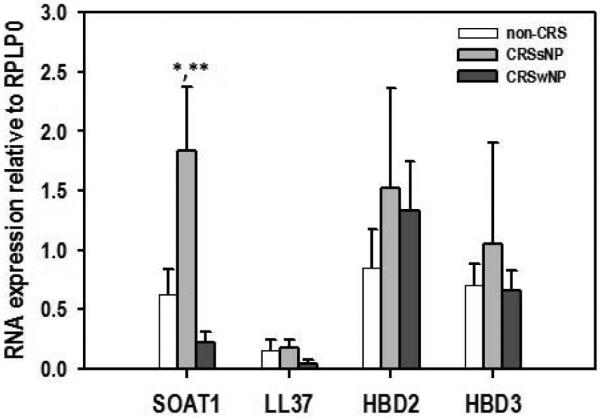
The expression of SOAT1, LL37, HBD2, and HBD3 genes in human sinus tissue of CRSsNP and non-CRS patients were investigated using RT-PCR. In formalin fixed tissues SOAT1, HBD2, and HBD3 mRNA expression were elevated in CRSsNP patients versus controls (Figure 2). A significant correlation between HNP-1 positive cells and SOAT1 gene expression was found (Pearson correlation coefficient r = 0.543 with a significance value of p = 0.024). No LL37 mRNA was found in either healthy or diseased specimens. With respect to fresh tissue, transcripts of SOAT1, LL37, HBD2, and HBD3 were detected in all patients. In addition, CRSsNP patients demonstrated increased gene expression of SOAT1, HBD2, and HBD3 compared to non-CRS controls. SOAT1 upregulation was the most prominent, reaching statistically significance (p = 0.041 when based on quantification of PCR products (Figure 3) and this was also reflected in real time gene expression analysis employing duplexing (Figure 4). In contrast, the expression of SOAT1, LL37, HBD2, and HBD3 genes in human sinus tissue of CRSwNP was not elevated compared to non-CRS and the difference between SOAT1 expression in CRSsNP and CRSwNP was significant with p = 0.005.
Figure 2. SOAT1 and antimicrobial peptide RNA expression relative to RPLP0 in formalin fixed sinus tissue.
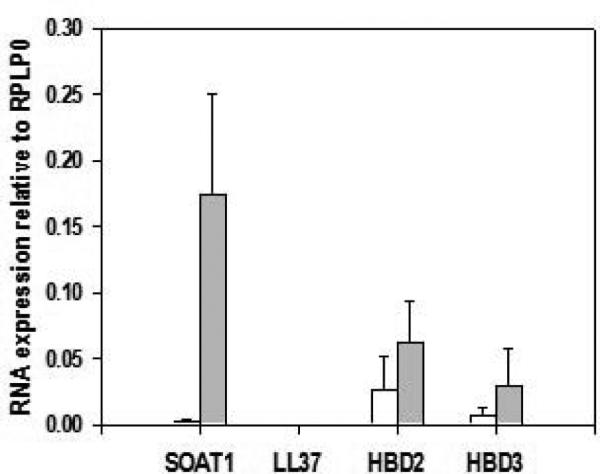
RNA was extracted from formalin fixed, paraffin embedded tissue blocks. All CRS cases were without nasal polyposis. Shown are means + SEM. For non-CRS (open bars), n = 3 but for SOAT1 analysis, where n = 4. For CRS (grey bars), n = 14. SOAT1: sterol O-acyltransferase 1; CRS: chronic rhinosinusitis; HBD: human beta defensin, RPLP0: ribosomal protein, large, P0.
Figure 3. SOAT1 and antimicrobial peptide RNA expression relative to RPLP0 in fresh sinus tissue.
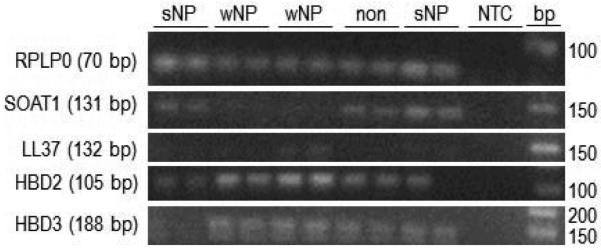
A. Representative PCR products from the various gene targets (expected product size in parentheses) resolved on ethidium bromide stained 2.5% agarose gels. B. Composite data. Shown are means + SEM, n = 5 for non-CRS, 4 for CRSsNP, and n = 6 for CRSwNP. In Oneway ANOVA with Bonferroni post hoc adjustment *p = 0.041 for CRSsNP vs. non-CRS, and ** p = 0.005 for CRSsNP vs. CRSwNP. CRS: chronic rhinosinusitis; s: sine; w: with; NP: nasal polyposis; NTC: no template control; bp: size marker; SOAT1: sterol O-acyltransferase 1; HBD: human beta defensing; RPLP0: ribosomal protein, large, P0.
Figure 4. SOAT1 RNA expression relative to RPLP0 in fresh sinus tissue.
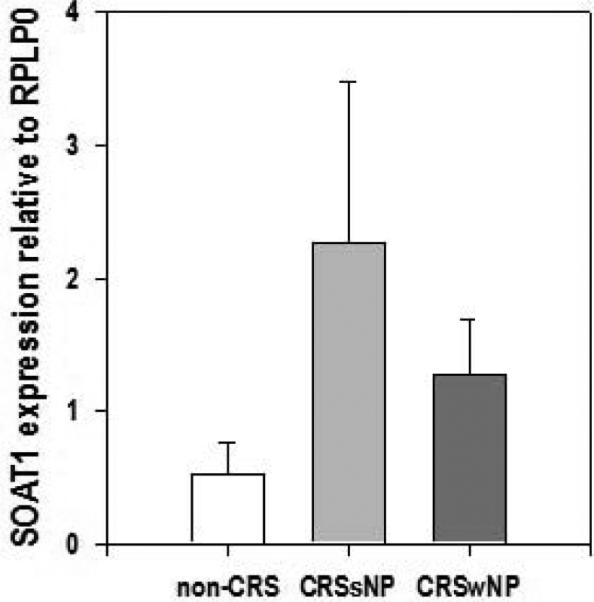
Composite real time PCR data. Shown are means + SEM, n = 2 for non-CRS (0.533 ± 0.231), 5 for CRSsNP (2.267 ± 1.215), and n = 5 for CRSwNP (1.278 ± 0.413). There was no statistically significant difference among the groups. CRS: chronic rhinosinusitis; s: sine; w: with; NP: nasal polyposis SOAT1: sterol O-acyltransferase 1; RPLP0: ribosomal protein, large, P0
Discussion
Endogenous AMLs and AMPs represent integral components of our innate immune repertoire at mucosal surfaces, providing early defense against microorganisms in barrier organs such as nasal and lower airway epithelia. AMLs have only recently been recognized as novel effector molecules in the nascent protection of the respiratory tract. 8,11 With respect to the sinuses, we previously demonstrated that maxillary sinus secretions obtained from patients with and without CRS revealed a lipid profile comparable to that of nasal fluid using high performance liquid chromatography.8 Polar fatty acids, NPL, and cholesteryl esters were all identified. However, levels of lipid composition differed dramatically between CRS versus non-CRS patients, with the former exhibiting marked elevation of AMLs in both individual and pooled specimens. At least 10-fold and 5-fold increases of NPL and CL were evident in CRS samples, respectively, when compared to healthy controls. Such upregulation suggested that antibiotic lipid production was inducible in response to inflammation. In addition, since lipid amplification was independent of neutrophil influx, this implied that AML synthesis was indigenous to the epithelia and not simply a by-product of inflammation.8
Following this tangent, in the current study we proceeded to examine sinus tissue from patients with and without CRS to determine if SOAT1, an enzyme critical to cholesteryl ester synthesis, was expressed. Through RT-PCR, SOAT1 mRNA was detected in all samples, with statistically significantly greater amounts observed in CRSsNP specimens than CRSwNP or non-CRS patients. Such augmentation was consistent with our prior findings of elevated lipid levels in CRS secretions. No previous published studies have evaluated sinonasal tissue for the presence of SOAT1. While SOAT1 levels correlated with the number of neutrophils, there are no reports on the expression of SOAT1 in neutrophils and preliminary experiments in our laboratory did not indicate a measurable gene expression of SOAT1 (unpublished data). Sinus mucosa also bears resident macrophages which have been documented to upregulate SOAT1 upon infection in the context of atherosclerosis. 22 Therefore, macrophages could contribute to the observed increase in SOAT1 expression in CRSsNP. However, the low levels of expression of LL37, another inducible gene in macrophages, and its lack of induction in CRSsNP, does not support a major contribution by macrophages. Consequently, at this time it is unclear whether increased antimicrobial cholesteryl ester content in CRS originates from neosynthesis in the epithelial cells and/or monocyte/macrophage production. Although SOAT1 mRNA upregulation in CRSsNP patients lends further credence to the notion that host-derived lipids contribute to innate sinonasal defense, additional research is needed to localize the source of this amplification. In contrast, SOAT1 expression in CRSwNP was significantly lower compared to CRSsNP (p = 0.005) and appeared to be suppressed when compared to controls; although this difference did not reach statistical significance. Further investigation is necessary to determine whether such downregulation plays a role in the pathogenesis of CRSwNP.
In addition to analyzing sinus tissue for the presence of SOAT1, we also explored gene expression of other intrinsic antibiotic peptides in CRS, including LL37. Multiple reports have implicated the cathelicidin as a key AMP in the inherent host defense of the sinonasal cavity, with nasal expression reportedly upregulated in CRS. 23-27 Prominent amplification of LL37 mRNA (by up to 17-fold) has been observed in the nasal epithelia of CRS patients with eosinophilic mucus (EM) versus healthy control and non-EMCRS patients. 26 Exposure to fungal allergens has also been shown to incite biosynthesis of LL37 in CRS patients. 27 Application of Aspergillus and Alternaria extracts to a nasal tissue explant model augmented LL37 mRNA and protein levels by 4-fold and 6-fold, respectively, in a dose-dependent manner. 27 In our study, LL37 was detected in the sinus tissue of fresh specimens obtained from healthy, CRSsNP, and CRSwNP patients. The presence of LL37 in both normal and CRS samples suggests that the cathelicidin is constitutively expressed in sinus tissue. However, we did not observe an induction in our CRS specimens. Several reasons could account for this finding. Our study specifically investigated LL37 levels in sinus tissue as opposed to nasal epithelia (i.e. turbinate mucosa or nasal polyps) which was the focus of prior investigations. In addition, none of our patients had fungal infections or EMCRS, the CRS subtype in which prominent LL37 amplification had been previously reported. In the formalin fixed specimens, no LL37 mRNA was detectable in either control or CRS patients. Given the fresh tissue results, we surmise that this outcome stemmed from technical issues yielding low quality RNA and/or degradation of the target gene during specimen processing rather than being a true representation of LL37 expression.
Similar to LL37, α and β-defensins have also been identified in respiratory mucosa and airway (nasal/bronchoalveolar) secretions, with their delivery or biosynthesis induced by local infection and inflammation.5-6, 28-29 Alpha defensins HNP 1-3 have been detected in inferior turbinate mucosa and nasal polyp tissues of CRSwNP patients.30 However, HNP 1-3 have not been found in the turbinate mucosa of normal subjects; indicating that α-defensin delivery in the nasal cavity is enhanced by inflammation.30 Likewise, in the current study, immunofluorescence demonstrated HNP 1-3 within sinus tissue, with marked increase (p = 0.01) evident in CRS samples (Figure 1). Such amplification is consistent with our previous findings, in which HNP levels were observed to be significantly higher in the maxillary sinus secretions of CRS patients than healthy subjects.8 In contrast to the α-defensins, investigations of β-defensin expression in CRS have yielded mixed results.31-33 The presence of HBD2 in the sinuses was first demonstrated by Carothers, et al., who analyzed antral lavage samples from 16 adult patients with (6) and without CRS (10).20 HBD2 was reported in only 1 of 10 control subjects versus 4 of 6 CRS patients; showing a trend towards increased defensin production in the context of inflammation.20 Subsequently, Ramanathan et al. detected HBD2 in 4/5 primary sinonasal epithelial cell cultures (SNECs) obtained from CRSwNP patients, with HBD2 mRNA levels increasing from 2.5-9 fold upon exposure to a TLR9 agonist.34 In our cohort, HBD2 and HBD3 gene expression appeared to be amplified in the sinus tissue of CRSsNP patients when compared to controls although this difference was not statistically significant and a large standard error of the means was observed. An upregulation in the setting of CRS is not unexpected given that AMPS are known to be induced by infection, with synthesis and secretion increased in response to microbial stimuli. 1,2 Neutrophil and epithelial defensin concentrations in respiratory fluid are also prominently modulated by inflammation, with chemoattractants leading to neutrophil recruitment and stimulation of β-defensin production by airway epithelial cells.1,2
However, in contrast to Carothers’ findings, Ramanathan et al. reported significant reduction of HBD2 expression in SNECs derived from CRSwNP patients.35 Such discrepancies may be due to inherent differences in the CRS subtype being explored by the respective groups. Carothers, et al. examined sinus specimens from CRSsNP patients, whereas Ramanathan et al. analyzed SNECs from CRSwNP patients.20,35 Each CRS subtype has been recognized as a distinct clinicopathologic entity with unique inflammatory mediator, cytokine, and cellular profiles.36-38 CRSsNP has been characterized by primarily neutrophilic infiltration with a Type 1 helper (Th) T cell polarization, while CRSwNP has been described as predominantly eosinophilic, with maladaptive, Th2-biased reactions.36-38 Consequently, it would not be unexpected for CRSsNP and CRSwNP patients to manifest incongruent sinonasal mucosal immune responses. In our study, no statistically significant difference was observed in HBD2 and HBD3 mRNA expression between CRSwNP patients and controls. Additional research is needed to elucidate how potential abnormalities in defensin function may be involved in the pathophysiology of the respective CRS subtypes.
The significance of amplified AMF gene expression in the sinus tissue of CRSsNP patients remains unclear at this time. Such augmentation may represent a concerted, intrinsic defense response elaborated by sinus epithelia to combat microbial invasion; with AMLs and AMPs acting synergistically to eradicate offending pathogens. Therefore, aberrancies in AMF synthesis, secretion, and regulation may disrupt local immune homoeostasis and contribute to the pathogenesis of CRS. Reduced activity of nascent AMPs and AMLs in ASF has been implicated in the development of respiratory inflammatory disease including cystic fibrosis.39-41 Diminished AMP expression has also been demonstrated in nasal polyp patients, with blunted immune function hypothesized to facilitate bacterial colonization.35 External factors such as smoking or steroid administration could have also accounted for the depressed gene expression observed. In our cohort, only one patient had a history of smoking, and that individual had CRSsNP. All CRS patients were on topical nasal steroids preoperatively, but 3 of the 6 CRSwNP also received oral steroid therapy immediately prior to surgery. Additional investigation is needed to elucidate the potential impact of smoking and systemic steroids on AMP and AML gene expression. Conversely, overactivity or hyperresponsiveness of natural AMFs to commensal organisms could trigger imbalance in the resident microbiome of the sinus cavities, predisposing to infection and chronic inflammation. Ultimately, further research is necessary to better understand the complex interplay among the various effectors of innate immunity in the paranasal sinuses and their determinant role in CRS.
Conclusion
An upregulation of multiple endogenous antimicrobial mediators including evidence for increased biosynthesis of AMLs was observed in the sinus tissue of CRSsNP patients. Whether such nascent antibiotics provide inherent protection of the sinuses from microorganism invasion or contribute to the pathogenesis of CRS is yet to be established. Additional investigation is warranted to precisely delineate the functionality of these intrinsic microbicides in the innate immunity of the sinonasal cavity and determine if different subtypes of CRS exhibit unique signatures of antimicrobial factors.
Acknowledgement
We would like to thank and acknowledge Robert Desharnais, biostatistician, for reviewing the data and statistical analyses.
Oswaldo Escobar: NIH MARC U*STAR Program through grant T34 GM008228
Edwards Eivers: NIH SC3 grant GM103699
Edith Porter: NIH SC1 grant GM096916
Footnotes
Disclosures:
Jivianne T. Lee: No funding, financial relationships, nor conflicts of interest to disclose.
Oral Presentation at the American Rhinologic Society Spring Meeting, Las Vegas NV, May 16, 2014
References
- 1.Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nature. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 3.Ooi EH, Wormald PJ, Tan LW. Innate immunity in the paranasal sinuses: A review of nasal host defenses. Am J Rhin. 2008;22:13–19. doi: 10.2500/ajr.2008.22.3127. [DOI] [PubMed] [Google Scholar]
- 4.Vandermeer J, Sha Q, Kane AP, et al. Innate immunity of the sinonasal cavity: Expression of messenger RNA for complement cascade components and toll-like receptors. Arch Otolaryngol Head Neck Surg. 2004;130:1374–1380. doi: 10.1001/archotol.130.12.1374. [DOI] [PubMed] [Google Scholar]
- 5.Singh PK, Tack BF, McCray PB, et al. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 6.Cole AM, Dewan P, Ganz Innate antimicrobial activity of nasal secretions. Infect and Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole AM, Liao J, Stuchlik O, et al. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169:6985–6991. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- 8.Lee JT, Jansen M, Yilma AN, et al. Antimicrobial Lipids: Novel Innate Defense Molecules are Elevated in Sinus Secretions of Patients with Chronic Rhinosinusitis. Am J Rhinol Allergy. 2010;24:99–104. doi: 10.2500/ajra.2010.24.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defenses and novel clinical applications. Curr Opin Hematol. 2009;16:41–7. doi: 10.1097/moh.0b013e32831ac517. [DOI] [PubMed] [Google Scholar]
- 10.Thormar H, Hilmarsson H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem Phys Lipids. 2007;150:1–11. doi: 10.1016/j.chemphyslip.2007.06.220. [DOI] [PubMed] [Google Scholar]
- 11.Do TQ, Moshkani S, Castillo P, et al. Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J of Immunol. 2008;181:4177–4187. doi: 10.4049/jimmunol.181.6.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang TY, Chang CC, Lin S, et al. Roles of acyl-coenzyme A: cholesterol acyltransferase-1 and -2. Curr Opin Lipidol. 2001;12:289–96. doi: 10.1097/00041433-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Drake DR, Brogden KA, Dawson DV, et al. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Tollin M, Bergsson G, Kai-Larsen Y, et al. Vernix caseosa as a multi-component defence system based on polypeptides, lipids, and their interactions. Cell Mol Lif Sci. 2005;62:2390–2399. doi: 10.1007/s00018-005-5260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thormar H, Isaacs CE, Brown HR, et al. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isaacs CE, Kashyap S, Heird WC, et al. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch Disease Childhood. 1990;65:861–864. doi: 10.1136/adc.65.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs CE, Litov RE, Thormar H. Antimicrobial activity of lipids added to human milk, infant formulas, and bovine milk. J Nutr Biochem. 1995;6:362–366. doi: 10.1016/0955-2863(95)80003-u. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs CE. Antimicrobial function of milk lipids. Adv Nutr Res. 2001;10:271–85. doi: 10.1007/978-1-4615-0661-4_13. [DOI] [PubMed] [Google Scholar]
- 19.Fischer CL, Walters KS, Drake DR, et al. Oral mucosal lipids are antibacterial against Porphyromonas gingivalis, induce ultrastructural damage, and alter bacterial lipid and protein compositions. Int J Oral Sci. 2013;5:130–40. doi: 10.1038/ijos.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carothers DG, Graham SM, Jia HP, et al. Production of beta defensin antimicrobial peptides by maxillary sinus mucosa. Am J Rhinol. 2001;15:175–179. doi: 10.2500/105065801779954238. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld RM. Clinical practice guideline on adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:365–377. doi: 10.1016/j.otohns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 22.He P, Mei C, Cheng B, et al. Chlamydia pneumoniae induces macrophage-derived foam cell formation by up-regulating acyl-coenzyme A: cholesterol acyltransferase 1. Microbes Infect. 2009;11:157–63. doi: 10.1016/j.micinf.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Thienhaus ML, Wohlers J, Podschun R, et al. Antimicrobial peptides in nasal secretion and mucosa with respect to Staphylococcus aureus colonization in chronic rhinosinusitis with nasal polyps. Rhinology. 2011;49:1–8. doi: 10.4193/Rhino11.072. [DOI] [PubMed] [Google Scholar]
- 24.Kim ST, Cha HE, Kim DY, et al. Antimicrobial peptide LL-37 is upregulated in chronic nasal inflammatory disease. Acta Otolaryngol. 2003;123:81–85. doi: 10.1080/0036554021000028089. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Fang S. The expression of human antimicrobial peptide LL-37 in the human nasal mucosa. Am J Rhinol. 2004;18:381–385. [PubMed] [Google Scholar]
- 26.Ooi EH, Wormald PJ, Carney AS, et al. Human cathelicidin antimicrobial peptide is upregulated in the eosinophilic mucus subgroups of chronic rhinosinusitis patients. Am J Rhinol. 2007;21:395–401. doi: 10.2500/ajr.2007.21.3048. [DOI] [PubMed] [Google Scholar]
- 27.Ooi EH, Wormald PJ, Carney AS, et al. Fungal allergens induce cathelicidin LL-37 expression in chronic rhinosinusitis patients in a nasal explant model. Am J Rhinol. 2007;21:367–372. doi: 10.2500/ajr.2007.21.3025. [DOI] [PubMed] [Google Scholar]
- 28.Singh PK, Jia HP, Wiles K, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe S, Wang J, Matsukura S, et al. Expression of antiviral molecular genes in nasal polyp-derived cultured epithelial cells. Acta Oto-Laryngologica. 2009;129:101–104. doi: 10.1080/00016480902912001. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Kim JE, Lim HH, et al. Antimicrobial defensin peptides of the human nasal mucosa. Ann Otol Rhinol Laryngol. 2002;111:135–141. doi: 10.1177/000348940211100205. [DOI] [PubMed] [Google Scholar]
- 31.Claeys S, de Belder T, Holtappels G, et al. Human β defensins and toll-like receptors in the upper airway. Allergy. 2003;58:748–753. doi: 10.1034/j.1398-9995.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamin M, Holbrook E, Gray ST, et al. Cigarette smoke combined with Toll-like receptor 3 signaling triggers exaggerated epithelial regulated upon activation, normal T-cell expressed and secreted /CCL5 expression in chronic rhinosinusitis. J Allergy Clin Immunol. 2008;122:1145–1153. doi: 10.1016/j.jaci.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Passariello A, Di Costanzo M, Terrin G, et al. Crenotherapy modulates the expression of proinflammatory cytokines and immunoregulatory peptides in nasal secretions of children with chronic rhinosinusitis. Am J Rhinol Allergy. 2012;26:e15–e19. doi: 10.2500/ajra.2012.26.3733. [DOI] [PubMed] [Google Scholar]
- 34.Ramanathan M, Lee WK, Dubin MG, et al. Sinonasal epithelial cell expression of toll-like receptor 9 is decreased in chronic rhinosinusitis with polyps. Am J Rhinol. 2007;21:110–116. doi: 10.2500/ajr.2007.21.2997. [DOI] [PubMed] [Google Scholar]
- 35.Ramanathan M, Lee W, Spannhake EW, et al. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008;22:115–121. doi: 10.2500/ajr.2008.22.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang N, Van Zele T, Perez-Novo C, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–968. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Keswani A, Chustz RT, Suh L, et al. Differential expression of interleukin-32 in chronic rhinosinusitis with and without nasal polyps. Allergy. 2012;67:25–32. doi: 10.1111/j.1398-9995.2011.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;23:1–298. [PubMed] [Google Scholar]
- 39.Freedman SD, Blanco PG, Zaman MM, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Eng J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 40.Strandvik B, Gronowitz E, Enlund G, et al. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J Pediatr. 2001;139:650–655. doi: 10.1067/mpd.2001.118890. [DOI] [PubMed] [Google Scholar]
- 41.Claeys S, Van Hoecke H, Holtappels G, et al. Nasal polyps in patients with and without cystic fibrosis: a differentiation by innate markers and inflammatory mediators. Clin Exp Allergy. 2005;35:467–472. doi: 10.1111/j.1365-2222.2005.02215.x. [DOI] [PubMed] [Google Scholar]