Abstract
Purpose
Infertility is a frequent consequence of cancer therapy and is often associated with psychological distress. Although adult survivors prioritize fertility and parenthood, this issue remains unexplored among adolescent males. This study examined future fertility as a priority (relative to other life goals) at time of diagnosis for at-risk adolescents and their parents.
Methods
Newly diagnosed adolescent males (n=96; age=13.0-21.9 years) at increased risk for infertility secondary to cancer treatment prioritized eight life goals: to have school/work success, children, friends, wealth, health, a nice home, faith, and a romantic relationship. Patients' parents (fathers, n=30; mothers, n=61) rank-ordered the same priorities for their children.
Results
“Having children” was ranked as a “top 3” life goal among 43.8% of adolescents, 36.7% of fathers, and 21.3% of mothers. Fertility ranked 3rd among adolescents, 4th among fathers, and 5th among mothers. Future health was ranked the top priority across groups, distinct from all other goals (ps<.001), and fertility ranked higher than home ownership and wealth for all groups (ps<.001). For adolescents, low/moderate fertility risk perception was associated with higher fertility rankings than no/high risk perceptions (p=.01).
Conclusions
Good health is the most important life goal among adolescents newly diagnosed with cancer and their parents. In this relatively small sample, adolescents prioritized fertility as a top goal, parents also rated fertility as being more important than home ownership and financial wealth. Health care providers should communicate fertility risk and preservation options at diagnosis and facilitate timely discussion among families, who may differ in prioritization of future fertility.
Keywords: Fertility, Sperm Banking, Cancer, Oncology, Adolescents, Parents
Introduction
Infertility is a recognized late effect of childhood cancer therapy, affecting between 15% and 48% of male survivors [1, 2]. Loss of fertility is associated with psychological distress, particularly among patients who are childless at diagnosis [3-6]. Currently, the majority of adolescent cancer patients are living into adulthood secondary to advancements in therapy, with approximately 80% surviving five or more years post treatment completion [7-9]. Adults diagnosed with cancer value fertility and parenthood [5, 6], with some patients choosing less effective treatments in efforts to decrease infertility risk [10]. Most adolescent females with cancer desire future motherhood [11] and rank having children as a prioritized life goal relative to financial success, home ownership, or traveling [12]. However, prioritization of future parenthood has not been investigated among adolescent males newly diagnosed with cancer.
Adolescents diagnosed with cancer, as well as their parents, share a concern for future fertility post treatment [13]. Health care providers report that some parents encourage their sons to cryopreserve (or “bank”) sperm so that the option of biological parenthood is maintained, along with their own desires of having grandchildren in the future [14]. Parental wishes can be exceptionally influential relative to procreation. In fact, it is fairly typical for parents and sons to make fertility-preservation decisions collaboratively, with 58.3% of adolescent cancer patients and 79.5% of parents reporting the decision to bank (or not bank) sperm was made conjointly [15]. The role of parents also extends to logistical and practical issues associated with fertility preservation, including paying for sperm banking, communicating with their son's oncologist/medical team regarding preservation coordination and outcome, and arranging transportation to and from the sperm banking clinic [16]. Therefore, the current study examines both adolescent and parent priority for patients' future fertility.
At diagnosis, males with adult-onset cancers have reported primary concerns associated with adjustment to disease and treatment, survival, and maintaining normalcy [14]. Health care providers may not perceive fertility to be a priority for these patients at this initial point in the disease process; however, when queried, 77% of childless males at diagnosis desire children, and prefer biological children when possible [6, 17]. Furthermore, male survivors (16-44 years of age) report their cancer experience increased their desire for fatherhood and strengthened the value placed on family, parenting, and the joy of having/raising children [17]. Over 80% of these survivors report their cancer experience enhanced their skills as parents, noting cancer taught them how to persevere through life's challenges [5]. Similarly, long-term survivors of childhood cancer also report a desire for children as well [18]. Yet at the time of diagnosis, adolescents and their parents often feel overwhelmed and may have difficulty conceptualizing issues such as parenthood or grand parenting [13, 19]. However, adolescents diagnosed with cancer remain distressed about the adverse effects that cancer treatment may have on fertility, even when treatment poses no increased risk for infertility [12].
Psychologically speaking, compromised fertility is associated with increased distress and impaired quality of life [6, 20, 21]. Among adult survivors and female survivors of childhood cancer, infertility is associated with unresolved grief, life dissatisfaction, depression, anxiety, and other undesirable quality of life outcomes [18, 21-24]. Qualitative retrospective reports from adolescent survivors indicate frustration, anger, and regret due to unawareness of their fertility status, lack of discussion about preservation options prior to treatment, and lack of control regarding their fertility outcome [19, 25]. Based on these insights, it is not surprising that sperm banking has been associated with lower anxiety at diagnosis and increased psychological health post-treatment among both adolescent and adult survivors [26, 27]. Over 88% of adolescents and their parents report feeling either neutral or comfortable with the sperm banking process when surveyed afterwards, and 100% of parents and patients who attempted to bank sperm felt they had made the right decision, regardless of whether the attempt was successful or not [15]. Furthermore, the majority of adult patients who banked sperm also indicated they would recommend the procedure to others [27].
Despite the known adverse effects of specific cancer treatments on fertility, only 18% to 26% of at-risk adolescent males cryopreserve their sperm prior to treatment in the United States and Canada [28, 29]. Sperm cryopreservation has been utilized to preserve men's fertility prior to damaging cancer treatment for over 50 years and is now accepted as the standard of care. The American Society of Clinical Oncology (ASCO) guidelines recommend providers discuss options for fertility preservation among those of reproductive age [30]; however, it is unclear why sperm banking is underutilized in light of the high priority that survivors of childhood cancer place on fertility and the high psychological distress associated with fertility loss. Finally, it has been demonstrated that desire for future children and prioritizing future fatherhood is associated with increased rates of sperm cryopreservation [6, 14].
Much remains unknown regarding how adolescent males prioritize fertility and whether parents prioritize fertility similarly for their sons. Therefore, the aim of the current study is to investigate the priority that adolescent males and their parents place on future fertility relative to other life goals at time of cancer diagnosis. The study also considers correlates of ranking fertility as a “top” life goal and describes these differences between adolescents and their parents.
Methods
Participants
Adolescent males newly diagnosed with cancer were consecutively recruited from 9 collaborating institutions across the United States and Canada. Eligible participants were: 1) newly diagnosed with a first malignancy, 2) between 13.0 and 21.9 years of age, 3) at increased risk for infertility based on cancer treatment (per oncologist's rating), 4) at Tanner Stage III or higher, 5) English or Spanish speaking, and 6) cognitively intact. Parents of assenting/consenting adolescents were also recruited for participation. Among adolescents eligible for the study, 86% participated. Of the 247 completed questionnaires, 75.7% (n = 187) correctly completed the life priorities ranking scale and were included in the analyses. Of the 33 parents (26.4% of total parents) and 23 adolescents (19.3% of total adolescents) who did not correctly complete this scale, the most common error (n = 29) was ranking each of the life goals in a Likert-type fashion (1 = Extremely Important to 8 = Extremely Unimportant, for example) as opposed to utilizing ranking as was directed. Other errors made included leaving the scale blank (n = 16) or not completing the scale in its entirety (n = 7). Follow-up comparisons of adolescent participants who did/did not correctly complete the scale revealed no significant differences in demographic variables. Of the parents who completed the scale correctly, mothers were more likely to report a higher education level and fathers were more likely to report a higher income relative to mothers/fathers who did not complete the survey correctly.
Adolescents
Adolescents (n = 96) were between the ages of 13.00 and 21.99 years (Mage = 16.4 years, SD = 2.1 years), primarily White (67.7%), Christian (78.1%), and had at least some relationship/dating experience in their lifetime (70.9%). Adolescents were diagnosed predominantly with leukemia/lymphoma (n = 51, 53.1%) or solid tumor (n = 38, 39.6%), with a minority diagnosed with brain tumor (n = 7, 7.3%). See Table 1 for adolescent sociodemographic data.
Table 1. Sociodemographic and medical characteristics for adolescent males newly diagnosed with cancer (n = 96).
| Frequency (%) | |
|---|---|
| Race/Ethnicity | |
| White | 66 (68.8) |
| Non-White | 30 (31.2) |
| Age group | |
| 13-15 years | 36 (37.5) |
| 16-17 years | 28 (29.2) |
| 18-21 years | 32 (33.3) |
| Diagnosis | |
| Leukemia/Lymphoma | 51 (53.1) |
| Brain Tumors | 7 (7.3) |
| Solid Tumors | 38 (39.6) |
| Language of Questionnaire | |
| English | 95 (99.0) |
| Spanish | 1 (1.0) |
| Education | |
| Less than H.S. Diploma | 73 (76.0) |
| H.S. Diploma/GED | 6 (6.3) |
| More than H.S. Diploma | 14 (14.6) |
| Missing | 3 (3.1) |
| Relationship Status | |
| Single, never dated | 27 (28.1) |
| Dating Experience | 45 (46.9) |
| Committed Relationship | 23 (24.0) |
| Missing | 1 (1.0) |
| Religion | |
| Christian | 75 (78.1) |
| Non-Christian | 15 (15.6) |
| Missing | 6 (6.3) |
| Patient's Perceived Fertility Risk | |
| None | 9 (9.4) |
| Low | 41 (42.7) |
| Moderate | 38 (39.5) |
| High | 4 (4.2) |
| Missing | 4 (4.2) |
Parents
Mothers (n = 61) were between the ages of 32 and 53 years (Mage = 44.2 years, SD = 5.6 years), mostly White (77.0%), Christian (91.8%), and married (72.1%). Fathers (n = 30) were between the ages of 35 and 55 years (Mage = 46.7 years, SD = 4.9 years), and were also mostly White (70.0%), Christian (90.0%), and married (83.3%). See Table 2 for sociodemographic data for both mothers and fathers.
Table 2. Sociodemographic and medical characteristics for mothers (n = 61) and fathers (n = 30) of adolescent males newly diagnosed with cancer.
| Mothers | Fathers | |
|---|---|---|
| Frequency (%) | Frequency (%) | |
| Race/Ethnicity | ||
| White | 47 (77.1) | 21 (70.0) |
| Non-White | 13 (21.3) | 9 (30.0) |
| Missing | 1 (1.6) | 0 (0.0) |
| Age group | ||
| 30-39 years | 17 (27.9) | 2 (6.7) |
| 40-44 years | 13 (21.3) | 7 (23.3) |
| 45-49 years | 16 (26.2) | 11 (36.7) |
| 50-59 years | 15 (24.6) | 10 (33.3) |
| Language of Questionnaire | ||
| English | 60 (98.3) | 29 (96.7) |
| Spanish | 1 (1.7) | 1 (3.3) |
| Education | ||
| Less than Bachelor Degree | 32 (52.5) | 15 (50.0) |
| Bachelor Degree or More | 28 (45.9) | 14 (46.7) |
| Missing | 1 (1.6) | 1 (3.3) |
| Marital Status | ||
| Married/Living as Married | 44 (72.1) | 25 (83.3) |
| Separated/Divorced/Widowed | 14 (23.0) | 3 (10.0) |
| Single, never married | 3 (4.9) | 1 (3.3) |
| Missing | 0 (0.0) | 1 (3.3) |
| Religion | ||
| Christian | 56 (91.8) | 27 (90.0) |
| Non-Christian | 5 (8.2) | 2 (6.7) |
| Missing | 0 (0.0) | 1 (3.3) |
| Household Income | ||
| Less than $20,000 | 6 (9.8) | 1 (3.3) |
| $20,000 - $59,999 | 23 (37.7) | 9 (30.0) |
| $60,000 and above | 26 (42.6) | 18 (60.0) |
| Missing | 6 (9.8) | 2 (6.7) |
| Son's Age group | ||
| 13-15 years | 23 (37.7) | 17 (56.7) |
| 16-17 years | 20 (32.8) | 8 (26.7) |
| 18-21 years | 17 (27.9) | 5 (16.7) |
| Missing | 1 (1.6) | 0 (0.0) |
| Son's Diagnosis | ||
| Leukemia/Lymphoma | 32 (52.5) | 15 (50.0) |
| Brain Tumors | 7 (11.5) | 3 (10.0) |
| Solid Tumors | 22 (36.1) | 12 (40.0) |
| Perceived Fertility Risk for Son | ||
| None | 5 (8.2) | 3 (10.0) |
| Low | 18 (29.5) | 8 (26.7) |
| Moderate | 27 (44.3) | 15 (50.0) |
| High | 6 (9.8) | 3 (10.0) |
| Missing | 5 (8.2) | 1 (3.3) |
Procedure
Research staff at participating pediatric oncology centers reviewed clinic lists daily for adolescent males newly diagnosed with cancer. Once potential participants met the initial screening criteria, attending oncologists were emailed and asked to assign a fertility risk estimate for their patients based on the proposed cancer therapy (0 = no risk, 1 = low risk, 2 = moderate risk, 3 = high risk). Adolescents rated by their oncologist as being at an increased risk for infertility due to cancer treatment (scores ≥ 1) were then considered eligible for the study.
Eligible participants were approached for enrollment between days 2 and 8 of cancer therapy (between days 2-15 for patients from the Canadian site) in efforts to capture “real time” perspectives from families just after the narrow window in which sperm banking typically takes place. Regardless of their son's age, parents of enrolled adolescents were also invited to participate in this study given their influential role in the decision-making process. Consent/assent was obtained consistent with Institutional Review Board (IRB) guidelines.
Measures
Adolescents and parents who agreed to participate completed a questionnaire assessing, in part, factors associated with sperm banking outcome. Questionnaires were available in paper-and-pencil form and online in English and in Spanish. As with the Burns et al. (2006) study [12], adolescents (rating their own life goals) and parents (rating the life goals they desired for their son) were asked to rank the following life goals in order of priority from 1 (most important) to 8 (least important), using each number only once: have children, be in good health, have a romantic relationship, do well at work or school, grow in dedication to faith, make a lot of money, own a nice home, and have a lot of close friends. Additionally, perceived fertility risk (none, low, moderate, or high), cancer diagnosis (leukemia/lymphoma, solid tumor, or brain tumor) and either dating experience for the adolescent (single and never dated, current or past dating experience, or current committed relationship) or relationship status for the parent (married, single and never married, or separated/divorced/widowed) were also considered as possible correlates of ranking fertility as a top life priority (defined below).
Analytic Plan
Chi-square tests were performed to compare the proportion of each participant group (adolescents, mothers, and fathers) that ranked fertility 1 through 3 versus 4 through 8. Therefore, any reference to a “top priority” translates to the ranking of a life goal 1 through 3. Three rank-order analyses were utilized to examine how each of the three participant groups (adolescents, mothers, and fathers) prioritized the 8 life goals. Friedman tests were utilized as omnibus tests to detect if there were significant differences in the rankings of the life goals. In the event that significant differences were found, follow-up Wilcoxon Signed-Rank Tests were utilized to identify specific differences between rankings. To determine correlates of fertility rankings for each of the three participant groups, independent-sample median tests were utilized for categorical and ordinal variables (cancer diagnosis, ethnicity, relationship/marital status, income, and perceived fertility risk), and Spearman's rho was utilized for continuous age variables.
Results
Fertility as a Top Priority
Nearly half of newly diagnosed adolescent males (n = 42, 43.8%), less than one quarter of mothers (n = 13, 21.3%), and just over one third of fathers (n = 11, 36.7%) reported that “having children” was a top priority for the patient's future. A Chi-square test comparing the proportion of respondents in each group that ranked fertility as a top priority (1-3 vs. 4-8) was significant (χ2 = 8.25, p = .02). A follow-up univariate Chi-square test indicated that a significantly greater proportion of adolescents (relative to mothers) ranked fertility as a top life goal (χ2 = 8.25, p < .01). Paternal-son and paternal-maternal comparisons were not significantly different (χ2 = 0.47, p = .49 and χ2 = 2.44, p = .12, respectively).
Adolescents
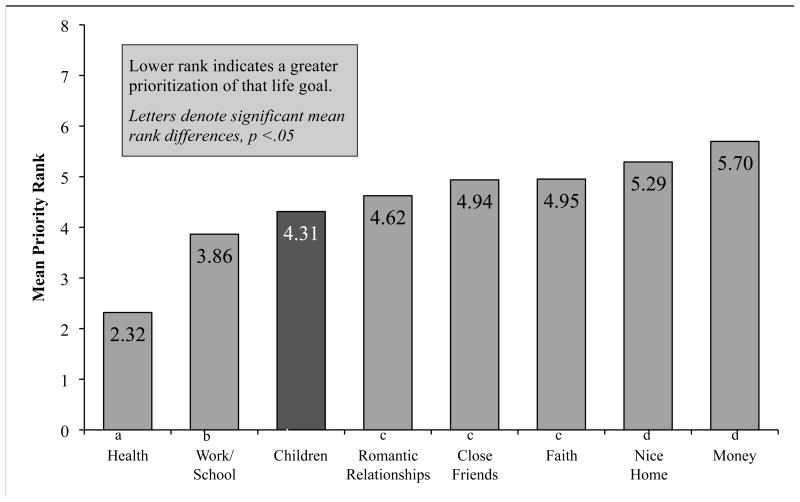
Adolescents ranked “having children” 3rd out of 8 life goals (Mdn = 4, mode = 2, range = 1-8), and the Friedman test of the overall rankings was significant (χ2 = 122.82, p < .001). See Figure 1. Follow-up Wilcoxon Signed-Rank Test for selected a priori comparisons found that having “good health” was significantly more important than all other life goals (ps < .001). Having children was ranked significantly higher than owning a nice home (p < .01) and financial wealth (p < .001). Adolescents ranked work/school success as second as a life goal, and significantly higher than faith (p < .01), close friendships (p < .01), and romantic relationships (p = .04), but not significantly higher than having children.
Figure 1.
Mean rank of adolescent life goals within one week of initiating cancer therapy.
Independent samples median tests were conducted to examine whether any demographic factors were correlated with adolescent rankings of “having children” as a life goal. Adolescents' perceived fertility risk was significantly associated with ranking fertility as a top life goal (p = .01), with adolescents who perceived they were at no risk (n = 9, Mdn = 6.0) or high risk (n = 4, Mdn = 5.5) for infertility placing less priority on “having children” as compared to those adolescents who perceived a low (nlow = 41, Mdnlow = 3.0) or moderate (nmod = 38, Mdn mod =4.5) risk of infertility. A Spearman's rho correlation revealed a trend toward significance for adolescents with increased age being related to increased rank of “having children” as a life goal (ρ = -.17, p = .09). Race/ethnicity, relationship status, and cancer diagnosis were not significant correlates of fertility rankings.
Mothers
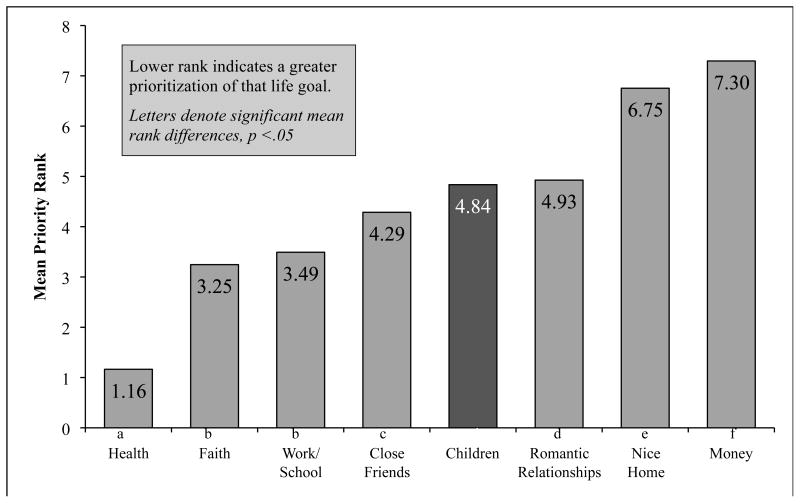
Overall, mothers ranked “having children” as 5th of 8 life goals for their sons (Mdn = 5, mode = 4, range = 2-8), and the Friedman test of the overall rankings was significant (χ2 = 274.49, p < .001). See Figure 2. Follow-up Wilcoxon Signed-Rank Test for selected a priori comparisons found that their sons' having “good health” in the future was significantly more important than all other goals (ps < .001). Having children was ranked significantly higher than home ownership and financial wealth (ps < .001). Their sons' dedication to faith and work/school success was ranked higher than having future children among mothers (ps < .001). Independent samples median tests and Spearman's rho correlations failed to find that maternal rank of their sons' “having children” as a life goal was significantly related to any demographic variables (i.e., race/ethnicity, marital status, maternal age, son's age, and household income), maternal perceptions of their son's infertility risk, or their son's diagnosis.
Figure 2.
Mean rank of maternal life goals for their son within one week of initiating cancer therapy.
Fathers
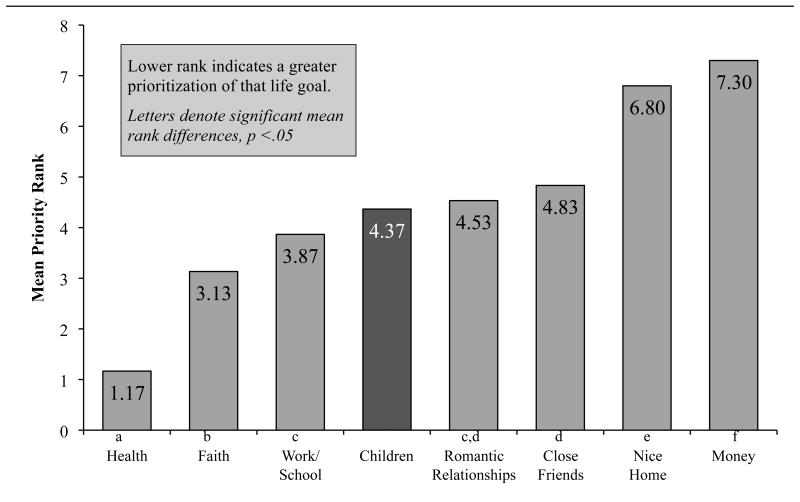
Overall, fathers ranked “having children” as 4th of 8 life goals for their sons (Mdn = 4, mode = 4, range = 2-8), and the Friedman test of overall rankings were significant (χ2 = 133.20, p < .001). See Figure 3. Follow-up Wilcoxon Signed-Rank Tests for selected a priori comparisons found that having sons in “good health” was more important than all other life goals (ps < .001), followed by faith and school/work success. Faith was ranked higher than having children and work/school success (ps = .04), though having work/school success did not significantly differ in rank than having children. For fathers, their sons' “having children” in the future was ranked significantly more important than home ownership and financial wealth (ps < .001).
Figure 3.
Mean rank of paternal life goals for their son within one week of initiating cancer therapy.
Independent samples median tests and Spearman's rho correlations revealed that paternal rankings of having children as a top life goal for their son was not associated with of any considered demographic factors (i.e., race/ethnicity, marital status, maternal age, son's age, and household income), paternal perceptions of their son's infertility risk, or their son's diagnosis.
Discussion
Nearly half of adolescents reported having children as a top life priority, which suggests that adolescent males newly diagnosed with cancer value biological fatherhood. Although similar findings have been found in the adult and female childhood cancer literature [5, 6, 12], these data are the first to examine this prioritization among adolescent males and their families. Both adolescents and their parents rated having children as significantly more important than material goals such as financial success or owning a nice home. However, patient health was uniformly the top priority for families, with 100% of parents and 76.0% of adolescents placing health in their top three life goals. Physical health was ranked as being more important than any other life goal, which is not surprising, given the life-threatening nature of cancer. Outside of these findings, sons, mothers, and fathers had somewhat different priorities for top life goals.
Whereas 43% of adolescents prioritized parenthood, only 26.4% of parents endorsed it as a top goal for their sons. Interestingly, mothers were less likely to rank their son's future fertility as a top priority (21.3%) compared to fathers (36.7%). Mothers tended to emphasize the importance of faith (ranked 2nd of 8 life priorities) and school/work success (ranked 3rd of 8 life priorities) as top goals for their sons at diagnosis. Perhaps mothers are focusing on more proximal goals during this difficult time and are biased toward mechanisms that could aid in their son's cancer recovery (faith and close friendships) or daily normalcy (school/work success). In contrast, more distal, future-oriented goals (i.e., grand-parenting, son's future partnerships) were ranked as being lower priorities.
In addition to prioritizing their son's overall health status, fathers also differentiate faith as a desired life goal (2nd of 8 life priorities). For fathers, faith was more important than fertility and, to a lesser extent, valued more than school/work success as well. Interestingly, adolescent males were more likely than their parents, particularly their mothers, to rate fertility as a top priority. Of note, differences in fertility rankings were also found within the adolescent sample as well. Specifically, those adolescents who perceive themselves to be at low to moderate risk of infertility were significantly more likely to rank having children as a top priority compared to those perceiving either no or high risk. While there is no literature known to us upon which to compare this finding, it may be that adolescents who perceive no risk of infertility are not actively considering whether they want to have children in the future, as their perceived ability to father a child should remain intact. Alternately, those who perceive themselves as being at high risk of infertility may have given up hope of having children or have greater concerns regarding their prognosis. Overall, this finding suggests that perceptions of fertility risk may contribute to the prioritization of future parenting, and in turn, affect decision-making specific to fertility preservation. It is important to note that adolescent and parent perceptions of fertility risk are frequently lower than the risk assignment reported by the treating oncologist [30, 31]. As perceptions of fertility risk significantly affect banking decisions, future efforts should be focused on improving provider communication of fertility risk as a mechanism to improve familial accuracy of risk, which in turn, should increase rates of sperm banking.
Almost 60% of survivors of childhood cancer do not know whether their treatment had any effect on their fertility, and consistent with the high prioritization of parenting found among adolescents in this study, most desire fatherhood [18]. Only half of childhood cancer survivors recall their health care provider discussing fertility issues with them at diagnosis [18], and the lack of awareness of fertility status is a cause of psychological distress for many survivors [6, 18]. Childhood cancer survivors report they do not want their fertility risk status kept from them [18], and the majority of parents whose children survived childhood cancer report being dissatisfied with the information they received from health care providers regarding their son's fertility risk [13]. Future studies should quantify the prioritization of fatherhood as it directly relates to preservation discussions and sperm banking outcomes among newly diagnosed adolescents. The 2013 American Society of Clinical Oncology (ASCO) guidelines on fertility preservation state that if a patient is at an increased risk for infertility secondary to cancer treatment, fertility preservation discussions should occur directly with patients of “reproductive age” or with parents of children [32]. Should a relationship between prioritization of fatherhood and sperm banking be identified, this construct could then be targeted in discussions as a potential mechanism for increasing rates of banking.
As our data suggest, providers should be aware that parents and adolescents might have discrepant priorities regarding future fertility and fertility preservation. This discrepancy has previously been found among female adolescents with cancer. For example, one study found the majority of adolescent females desired future parenthood and were concerned about their fertility, whereas their mothers underestimated their daughter's worries and thought survivorship would be satisfying enough [11]. Due to these discrepancies, discussions should not only be conducted with both parent/guardians and adolescent patients, but parents should also be aware that their son's fertility priorities may be discrepant from their own. Furthermore, these discussions need to allow for a timely referral to a fertility specialist should the patient have reproductive potential along with a desire to bank. For females, menarche provides a marker of reproductive maturation and cues providers for inclusive fertility discussions; however, reproductive maturation is less obvious among males, and the ASCO guidelines do not recommend a specific age at which fertility discussions should begin. Our results underscore the importance of future fertility and offering preservation options to qualified males as young as 13 years of age. Pediatric oncologists and other healthcare professionals should be aware that adolescent patients desire the option of having children in their future, and the best possible way to protect their fertility is to include them in discussions, thus promoting informed decision-making regarding their reproductive futures. Physician recommendation for fertility preservation is one of the most influential factors when sperm banking decisions are made [6, 33], and choosing to bank could result in multiple favorable outcomes, from improved psychological functioning to eventual fatherhood.
In terms of timing, it is necessary that these conversations occur as a process. Discussions should begin immediately following diagnosis, ensuring all involved parties understand the provided information. The delivery and content of messages should continue to evolve throughout treatment and the survivorship continuum. Clinically speaking, discussions surrounding fertility risk should take place with health care providers regardless of the adolescent's fertility risk. Adolescents whose proposed treatment will not confer an increased risk should also be informed of their status to reduce infertility-related distress, promote safe sexual practices, and allow for appropriate family planning in the future [13]. For those at increased risk for infertility, early and tailored discussions are important so the patient and family have time to process the information, ask questions, and make decisions about sperm banking prior to the start of cancer treatment. While health care providers must consider the patient's developmental level, cognitive functioning, and emotional maturity in determining how to best facilitate these discussions, all adolescents and families should be informed of the patient's fertility risk (or lack of) and the options for preservation. Once fertility discussions have been initiated, medical teams must then be prepared to work with families to overcome potential barriers (information-based, financial, logistic, or otherwise), make appropriate referrals, and facilitate the process should the family decide to bank.
These presented results should be interpreted within the context of study limitations. It is important to note that adolescents and parents rated “having children” as a life priority, and as such, may have considered the broader construct of parenthood, as opposed to biological parenthood, in their responding. Furthermore, the scale measured priority of parenthood/fertility relative to other specific life goals, but may be less informative regarding the value of fertility across all life circumstances. Regardless, fertility is consistently given a high priority among adult cancer survivors, and discussions of fertility preservation are imperative, not only for promoting parenthood, but for decisional satisfaction in families surviving childhood cancer as well.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (HD-061296, Klosky – PI), and National Cancer Institute (CA-21765, Gilbertson - PI), with support provided to St. Jude Children's Research Hospital by the American Lebanese Syrian Associated Charities (ALSAC). As the Principal Investigator on the sponsoring R21, Dr. Klosky has full control of the data reported upon in this manuscript.
Footnotes
No conflicts of interests were reported by any of the authors.
References
- 1.Schrader M, Müller M, Straub B, et al. The impact of chemotherapy on male fertility: A survey of the biologic basis and clinical aspects. Reprod Toxicol. 2001;15:611–617. doi: 10.1016/S0890-6238(01)00182-4. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AB, Campbell AJ, Irvin DS, et al. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: A case-control study. Lancet. 2002;360:361–367. doi: 10.1016/S0140-6736(02)09606-X. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann JT, Albrecht C, Schmoll HJ, et al. Long-term effects on sexual function and fertility after treatment for testicular cancer. Br J Cancer. 1999;80:801–807. doi: 10.1038/sj.bjc.6690424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schover LR, Rybicki LA, Martin BA, et al. Having children after cancer: A pilot survey of survivors' attitudes and experiences. Cancer. 1999;86:697–709. doi: 10.1002/(SICI)1097-0142(19990815)86:4<697::AID-CNCR20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Schover LR. Psychosocial aspects of infertility and decisions about reproduction in young cancer survivors: A review. Med Pediatr Oncol. 1999;33:53–59. doi: 10.1002/(SICI)1096-911X(199907)33:1<53::AID-MPO10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Schover LR, Brey K, Lichtin A, et al. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20:1880–1889. doi: 10.1200/JCO.2002.07.175. [DOI] [PubMed] [Google Scholar]
- 7.Bleyer A, O'Leary M, Barr R, et al., editors. NIH Pub. No. 06-5767. National Cancer Institute; Bethesda, MD: 2006. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. [Google Scholar]
- 8.Mariotto AB, Rowland JH, Yarbroff KR, et al. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2009;18:1033–1040. doi: 10.1158/1055-9965.EPI-08-0988. [DOI] [PubMed] [Google Scholar]
- 9.Reis LA, Melbert D, Krapcho M, et al., editors. National Cancer Institute; [Accessed November 27, 2012]. SEER cancer statistics review, 1975-2004. http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 10.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 11.Quinn GP, Knapp C, Murphy D, Sawczyn K, Sender L. Congruence of reproductive concerns among adolescents with cancer and parents: pilot testing an adapted instrument. Pediatrics. 2012;129(4):e930–6. doi: 10.1542/peds.2011-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns KC, Boudreau C, Panepinto JA. Attitudes regarding fertility preservation in female adolescent cancer patients. J Pediatr Hematol Oncol. 2006;28:350–354. doi: 10.1097/00043426-200606000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Oosterhuis EB, Goodwin T, Kiernan M, et al. Concerns about infertility risks among pediatric oncology patient and their parents. Pediatr Blood Cancer. 2008;50:85–89. doi: 10.1002/pbc.21261. [DOI] [PubMed] [Google Scholar]
- 14.Achille MA, Rosberger Z, Robitaille R, et al. Facilitators and obstacles to sperm banking in young men receiving gonadotoxic chemotherapy for cancer: The perspective of survivors and health care professionals. Hum Reprod. 2006;21:3206–3216. doi: 10.1093/humrep/del307. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg JP, Ogle SK, Tuchman LK, et al. Sperm banking for adolescent and young adult cancer patients: Sperm quality, patient, and parent perspectives. Pediatr Blood Cancer. 2008;50:594–598. doi: 10.1002/pbc.21257. [DOI] [PubMed] [Google Scholar]
- 16.Murphy D, Klosky JL, Termuhlen A, et al. The need for reproductive and sexual health discussions with adolescent and young adult cancer patients. Contraception. 2013;88:215–220. doi: 10.1200/JCO.2012.43.5511. [DOI] [PubMed] [Google Scholar]
- 17.Reinmuth S, Liebeskind AK, Wickmann L, et al. Having children after surviving cancer in childhood or adolescence: Results of a Berlin survey. Klin Padiatr. 2008;220:159–165. doi: 10.1055/s-2008-1073143. [DOI] [PubMed] [Google Scholar]
- 18.Zebrack BJ, Casillas J, Nohr L, et al. Fertility issues for young adult survivors of childhood cancer. Psychooncology. 2004;13:689–699. doi: 10.1002/pon.784. [DOI] [PubMed] [Google Scholar]
- 19.Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: a qualitative investigation. Psychooncology. 2003;12:141–52. doi: 10.1002/pon.622. [DOI] [PubMed] [Google Scholar]
- 20.Canada AL, Schover LR. The psychosocial impact of interrupted childbearing in long-term female cancer survivors. Psychooncology. 2012;21:134–43. doi: 10.1002/pon.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond C, Abrams JR, Syrjala KL. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J Clin Oncol. 2007;25:3511–3517. doi: 10.1200/JCO.2007.10.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawshaw MA, Glaser AW, Hale JP, et al. Male and female experiences of having fertility matters raised alongside a cancer diagnosis during the teenage and young adult years. Eur J Cancer Care. 2009;28:381–390. doi: 10.1111/j.1365-2354.2008.01003.x. [DOI] [PubMed] [Google Scholar]
- 23.Gorman JR, Malcarne VL, Roesch SC, et al. Depressive symptoms among young breast cancer survivors: The importance of reproductive concerns. Breast Cancer Res Treatment. 2010;123:477–485. doi: 10.1007/s10549-010-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen A, Rodriguez-Wallberg KA, Rosenzweig L. Psychosocial distress in young cancer survivors. Semin Oncol Nurs. 2009;25:268–77. doi: 10.1016/j.soncn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Gorman JR, Bailey S, Pirece JP, et al. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv. 2012;6:200–209. doi: 10.1007/s11764-011-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edge B, Holmes D, Makin G. Sperm banking in adolescent cancer patients. Arch Dis Child. 2006;91:149–152. doi: 10.1136/adc.2005.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito K, Suzuki K, Iwasaki A, et al. Sperm cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer. 2005;104:521–524. doi: 10.1002/cncr.21185. [DOI] [PubMed] [Google Scholar]
- 28.Klosky JL, Randolph ME, Navid F, et al. Sperm cryopreservation practices among adolescent cancer patients at risk for infertility. J Pediatr Hematol Oncol. 2009;26:252–260. doi: 10.1080/08880010902901294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neal MS, Nagel K, Duckworth J, et al. Effectiveness of sperm banking in adolescents and young adults with cancer: A regional experience. Cancer. 2007;110:1125–1129. doi: 10.1002/cncr.22889. [DOI] [PubMed] [Google Scholar]
- 30.Simmons JL, Russell KM, Canavera KE, et al. Discrepancies in fertility risk perceptions and communication among providers and adolescents newly diagnosed with cancer. Presented at The Society of Pediatric Psychology's National Conference on Child Health Psychology; April 11-13, 2013; New Orleans, LA. [Google Scholar]
- 31.Canavera KE, Russell KM, Simmons JL, et al. Underestimation of fertility risk among families newly diagnosed with adolescent cancer. Presented at the American Psychological Association Annual Convention; July 31-August 4, 2013; Honolulu, HI. [Google Scholar]
- 32.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation in patients with cancer: American Society of Clinical Oncology Guideline update. J Clin Oncol. 2013 doi: 10.1200/JCO.2013.49.2678. published online ahead of print May 28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee S, Fuller-Thomson E, Dwyer C, et al. Just what the doctor ordered”: Factors associated with oncology patients' decision to bank sperm. Can Urol Assoc J. 2012;6:E174–178. doi: 10.5489/cuaj.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]