Abstract
Methamphetamine abuse is common among individuals infected by human immunodeficiency virus (HIV). Neurocognitive outcomes tend to be worse in methamphetamine users with HIV. However, it is unclear whether discrete cognitive domains are susceptible to impairment after combined HIV infection and methamphetamine abuse. The expression of HIV/gp120 protein induces neuropathology in mice similar to HIV-induced pathology in humans. We investigated the separate and combined effects of methamphetamine exposure and gp120 expression on cognitive function in transgenic (gp120-tg) and control mice. The mice underwent an escalating methamphetamine binge regimen and were tested in novel object/location recognition, object-in-place recognition, and Barnes maze tests. gp120 expression disrupted performance in the object-in-place test (i.e., similar time spent with all objects, regardless of location), indicating deficits in associative recognition memory. gp120 expression also altered reversal learning in the Barnes maze, suggesting impairments in executive function. Methamphetamine exposure impaired spatial strategy in the Barnes maze, indicating deficits in spatial learning. Methamphetamine-exposed gp120-tg mice had the lowest spatial strategy scores in the final acquisition trials in the Barnes maze, suggesting greater deficits in spatial learning than all of the other groups. Although HIV infection involves interactions between multiple proteins and processes, in addition to gp120, our findings in gp120-tg mice suggest that humans with the dual insult of HIV infection and methamphetamine abuse may exhibit a broader spectrum of cognitive deficits than those with either factor alone. Depending on the cognitive domain, the combination of both insults may exacerbate deficits in cognitive performance compared with each individual insult.
Keywords: spatial learning, recognition memory, behavior, transgenic
1. Introduction
Methamphetamine is one of the most commonly abused drugs among individuals infected with human immunodeficiency virus (HIV; (Marquez et al., 2009). Mesocorticolimbic brain regions, including the basal ganglia and cerebral cortex, are particularly sensitive to neuropathology induced by HIV (Gorry et al., 2003) and methamphetamine (Chang et al., 2005; Berman et al., 2008). Consistent with the observed neuropathology, frontostriatal- and corticolimbic-mediated patterns of cognitive deficits, including impairments in memory, executive function, and motor performance, are commonly associated with both HIV infection (Heaton et al., 1995; Lindl et al., 2010) and methamphetamine abuse (Nordahl et al., 2003; Scott et al., 2007). Considering the significant overlap between the brain regions commonly damaged by HIV infection and methamphetamine, HIV-associated neurocognitive impairments may be exacerbated in individuals with methamphetamine abuse.
The combined effects of methamphetamine and HIV on cognitive function have been difficult to investigate in clinical populations because of polydrug abuse (i.e., the concomitant abuse of other drugs in addition to methamphetamine) and noncompliance with medications in drug-abusing subjects (Nath, 2010). The rate of global neurocognitive impairment tends to be greater in HIV-infected methamphetamine-dependent patients than in individuals who are either HIV-infected or methamphetamine-dependent only (Rippeth et al., 2004). Furthermore, greater cortical interneuron loss has been observed after combined HIV infection and methamphetamine dependence (Langford et al., 2003; Chana et al., 2006). Markers of neuronal injury, including decreased levels of N-acetylaspartate and increased levels of myo-inositol, are also exacerbated in HIV-infected methamphetamine-dependent patients (Chang et al., 2005). Animal models of HIV-related pathology can be used to understand the effect of the interaction between HIV infection and methamphetamine abuse on cognitive function without the aforementioned confounds.
Although HIV does not typically infect neurons (Lindl et al., 2010), HIV-induced neuropathology involves the indirect neurotoxic effects of HIV viral proteins, such as the envelope glycoprotein gp120. Transgenic (tg) mice that produce gp120 in the brain (gp120-tg) at levels similar to those found in HIV patients (Toneatto et al., 1999) can be used to model the indirect effects of HIV viral products. The gp120-tg mouse exhibits a similar profile of neuropathology to that observed in HIV-infected humans and therefore represents a valid model for HIV-induced pathology. For example, widespread reactive astrocytosis, suggestive of neurological injury (Eddleston and Mucke, 1993), has been observed in the brains of gp120-tg mice (Toggas et al., 1994) and postmortem HIV-infected human brains (Navia et al., 1986; Gray et al., 1992; Weis et al., 1993; Vitkovic and daCunha, 1995). Moreover, gp120 expression in mice results in the loss of large cortical pyramidal neurons (Toggas et al., 1994), consistent with pyramidal neuron deficits found in HIV-infected humans (Masliah et al., 1997; Achim et al., 2009).
Limited studies have begun to explore the combined effects of gp120 expression and methamphetamine exposure on behavior. For example, gp120-tg mice are more sensitive to methamphetamine reward and aversion (Kesby et al., 2012) and show subtle alterations in methamphetamine-induced stereotyped behavior after acute methamphetamine administration (Roberts et al., 2010). Moreover, alterations in behavioral disinhibition have been observed in gp120-tg mice after exposure to a methamphetamine binge regimen (Henry et al., 2013). However, little is still known about the consequences of combined pathology induced by HIV-related proteins, such as gp-120, and methamphetamine exposure on cognitive function.
Thus, the aim of the present study was to investigate the effects of the interactions of gp120 expression in the brain and exposure to a chronic methamphetamine binge on various cognitive domains. Novel object, place, and object-in-place recognition tests were used to assess discrimination learning/memory. The Barnes maze test was used to assess spatial learning, spatial memory, and spatial reversal learning. Both the discrimination learning and spatial learning and memory are known to be disrupted by methamphetamine (Belcher et al., 2008; Chen et al., 2012; Reichel et al., 2012). To the best of our knowledge, only one study demonstrated spatial memory deficits in aged gp120 transgenic mice in the Morris water maze (D’Hooge et al., 1999). The general hypothesis for these studies was that methamphetamine exposure would augment gp120-induced deficits in cognitive function. In addition, anxiety-like behavior was assessed using the light-dark box test at an illumination level similar to that used in the Barnes maze test to account for any motivational differences in spatial learning.
2. Materials and methods
2.1. Animals
The present study used a total of 55 male transgenic mice (gp120-tg; 4–5 months old; n = 13–14 per group) on a C57BL/6 × DBA genetic background that expressed the gp120 protein under the regulatory control of modified murine glial fibrillary acidic protein (GFAP). The mice were generated as previously described (Toggas et al., 1994) and provided by the Neuroscience and Animal Models Core of the Translational Methamphetamine AIDS Research Center (TMARC; University of California San Diego, La Jolla, CA). The mice were group-housed with 2–4 mice per cage in a humidity- and temperature-controlled animal facility on a 12 h/12 h reverse light/dark cycle (lights off at 7:00 AM) with ad libitum access to food and water. Behavioral testing was conducted during the dark phase of the light/dark cycle. All of the experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the University of California San Diego Institutional Animal Care and Use Committee.
2.2. Methamphetamine binge exposure
Methamphetamine hydrochloride (Sigma, St. Louis, MO, USA) was dissolved in saline (0.9%) and administered subcutaneously with a 5 ml/kg injection volume. Control mice received saline. Stock solutions of methamphetamine were prepared weekly and diluted as needed during the drug regimen. The mice were administered a methamphetamine binge regimen featuring an escalating dose phase followed by a subsequent binge phase designed to mimic the pharmacokinetic pattern of methamphetamine use observed in human abusers (Kuczenski et al., 2007). During the escalating methamphetamine regimen, gp120-tg and non-tg mice were injected three times per day (9:00 AM, 12:15 PM, 4:30 PM) for 14 days with saline or escalating doses of methamphetamine starting with 0.1 mg/kg and increasing to 4.0 mg/kg, with a step-wise increase of 0.1 mg/kg per injection. After this 14-day period, the animals were exposed to an 11-day “binge” period and administered four daily injections of 6.0 mg/kg methamphetamine or vehicle at 2-h intervals (10:00 AM, 12:00 PM, 2:00 PM, 4:00 PM). After a 1 week washout period, behavioral testing began in the following order: light-dark box test, object/place/object-in-place recognition tests, and Barnes maze test. After testing each mouse, the equipment was cleaned using disinfectant (AirX 44 HDQ; Airex Laboratories, PA, USA).
2.3. Light-dark box test
Three chambers were used for the light-dark box test (San Diego Instruments, San Diego, CA, USA). Each chamber consisted of two compartments: a dark compartment (16 × 21 × 33 cm) and a light compartment (26 × 21 × 33 cm) that were separated by a divider that left a 5 cm horizontal gap for the mouse to move from one compartment to the other. The light intensity in the middle of the light compartment was approximately 900 lux (as used in the Barnes maze). In the dark compartment, the level of illumination was ~4 lux. During the 5 min test, the mice were placed in the dark compartment with their head facing away from the light compartment. The total time spent in the light compartment and latency to the first transition to the light compartment (± 2.5 s) were recorded.
2.4. Object and place recognition tests
Behavior was sequentially assessed in the novel object, novel place, and object-in-place recognition tests using a 5-day protocol. The mice were habituated to a white acrylic open field arena (27 × 29 × 40 cm) for 15 min on day 1 and day 2. Subsequently, the mice were tested in the novel object recognition test (day 3), novel place recognition test (day 4), and object-in-place recognition test (day 5). The novel object test consisted of a 5 min habituation session, 10 min familiarization session with two identical objects, and 10 min test session in which one object was replaced with a novel object. The novel place recognition test consisted of a 5 min habituation session, 10 min familiarization session with two identical objects, and 10 min test session in which one object was moved to a novel location within the arena. The object-in-place recognition test consisted of a 5 min habituation session, 10 min familiarization session with four different objects, and 10 min test session in which the positions of two of the objects were switched. The side (left or right) of object and location changes were alternated for each task (i.e., for a specific mouse the novel object was presented on the left side, followed by the new novel place position occurring on the right side and finally the object-in-place positional switch occurring on the left side) and counterbalanced between groups. The mice were returned to their home cages for 5 min between each session and the objects/open field were cleaned using disinfectant to eliminate scent cues on the objects and open field. The objects consisted of geometric shapes of varying colors made from plastic (Learning Resources Inc., Vernon Hills, IL, USA). There were two copies of each object and all of the objects had consistent height and volume. Each object was placed in the corner of the arena approximately 6 cm from each side so that the mice could circumvent the object while maintaining a sufficiently large distance to accurately determine object interaction. A scored interaction involved the nose oriented toward the object (no further than 1 cm). Climbing onto the objects and using the objects to rear were not scored as interactions. The data are expressed as the following discrimination ratio of the duration of object interaction: (Novel - Sample)/Total. A positive discrimination ratio represents a greater level of interaction with the non-familiar object or place. Mice were excluded from the analysis if they failed to interact with any of the objects during the familiarization session or interacted for less than a total of 5 s in the familiarization or test session. In total, six mice were excluded from the novel object test, four mice were excluded from the novel place test and one mouse was excluded from the object-in-place test.
2.5. Barnes maze test
The Barnes maze is a spatial learning task that is sensitive to hippocampal dysfunction (Paylor et al., 2001). The maze consisted of a white, acrylic, circular disc (90 cm diameter) that was elevated 90 cm above the floor, with 20 equally spaced holes (San Diego Instruments) with a black acrylic escape box (20 × 5 × 6 cm) placed under one of the holes. The maze was surrounded by four spatial cues at the height of the maze. Illumination in the center of the maze was approximately 900 lux. The maze was rotated 90 degrees each day to avoid the use of local cues on the maze by the mice.
2.5.1. Acquisition trials
Each mouse underwent 10 acquisition trials over 5 days, tested once in the morning and once in the afternoon. Immediately prior to the first trial, all of the mice were individually placed into the escape tunnel for 1 min to avoid any neophobic responses. During testing, the mice were placed into a starting cylinder (10 cm diameter) in the center of the maze for 30 s. The cylinder was then removed, and the mouse was allowed to explore the arena to find the escape tunnel. The trial ended when the mouse entered the escape tunnel (i.e., when all four paws left the maze). When the mouse entered the escape tunnel, the entry was blocked, and the mouse was left in the tunnel for 1 min. If the mouse did not find or enter the escape tunnel within 5 min, then it was manually placed into the escape tunnel.
2.5.2. Probe trial
The probe trial was conducted on day 6 and identical to the acquisition trials, with the exception that the escape tunnel was removed. Therefore, each mouse was tested for a total of 5 min before being returned to the home cage.
2.5.3. Memory retention
Two weeks after the probe test, the mice were tested for memory retention using a single two-trial test that was identical to the acquisition trials.
2.5.4. Reversal learning
During testing for reversal learning, there were four reversal trials over 2 days that were identical to the acquisition trials, but the location of the escape tunnel for each mouse was shifted 180°.
2.5.5. Behavioral measures
All behaviors were scored from video files by an experimenter who was blind to the experimental conditions. The measures assessed were the latency to find the target hole, number of reference errors, and number of working memory errors. Reference errors were defined as any incorrect hole inspection. Working memory errors were defined as searching the same hole twice within a trial when the revisit to the same hole occurred after the inspection of other holes. In the probe trial, the time spent by each mouse in the quadrant of the maze that contained the target hole was calculated.
Search strategy was also assessed in the acquisition, retention, and reversal trials. The search strategy was defined as one of four categories, similar to those described previously (Barnes, 1979), with the following scores; 1 = random, 2 = mixed, 3 = serial, and 4 = spatial. A spatial strategy was defined as finding the target hole directly or after inspecting one of the adjacent holes first (thus having a maximum of one prior error). Random (≤ 25%), mixed (26–74%), and serial (≥ 75%) strategy scores were all defined based on the percentage of errors that were made in a serial fashion. For an error to be defined as serial, this error had to be part of a minimum of three consecutive errors made in either direction around the maze without skipping a hole or changing direction. Thus, a greater search strategy score represents the use of a more efficient search strategy to locate the target hole.
2.6. Statistical analyses
All of the analyses were performed with SPSS Statistics 19 (Chicago, IL, USA). All of the data were analyzed using analysis of variance (ANOVA), with Genotype and Methamphetamine as the between-subjects factors. Repeated-measures ANOVAs were used when additional within-subjects factors were present. When appropriate, post hoc comparisons were performed using Least Significant Difference (LSD) analyses. Confidence intervals (95%) were used to determine significant differences from zero in novel object, novel place and object-in-place recognition tests. All of the results are expressed as mean ± SEM. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Body weights
As reported previously (Kesby et al., 2012; Henry et al., 2013), gp120-tg mice weighed less than non-tg mice at all stages of testing (F1,51 = 24.8, p < 0.001). Therefore, in subsequent analyses of the effects of methamphetamine/saline exposure, body weights were normalized to a percentage of baseline to account for the weight difference between genotypes (Fig. 1). A significant main effect of Day (F8,408 = 56.3, p < 0.001) was observed, with all mice weighing less than baseline throughout the procedure, regardless of methamphetamine or saline exposure. Methamphetamine treatment led to significantly lower overall weight (F1,51 = 28.5, p < 0.001). Furthermore, a significant Methamphetamine × Day interaction (F8,408 = 15.3, p < 0.001) was detected because methamphetamine exposure led to a greater decrease in weight on days 7–32 (p < 0.001) than saline exposure. A significant Genotype × Day interaction (F8,408 = 4.4, p < 0.01) was also found, with gp120-tg mice showing a greater reduction of weight on days 11 and 14 (p < 0.05) compared with non-tg mice. By day 65, the effects of methamphetamine exposure on body weight were no longer evident.
Fig. 1.
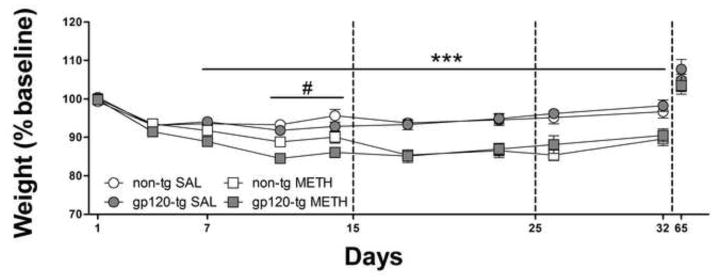
Effects of an escalating methamphetamine (METH) binge on fluctuations in the percentage of baseline weight. Methamphetamine-exposed mice lost significantly more weight than saline (SAL)-exposed mice from day 7–32 of the regimen. Furthermore, gp120-tg mice lost more weight than non-tg mice on days 11 and 14 of the regimen, regardless of methamphetamine or saline exposure. The data are expressed as mean ± SEM. ***p < 0.001, significant difference between saline- and methamphetamine-exposed mice; #p < 0.05, significant difference between non-tg and gp120-tg mice.
3.2. Light-dark box test
All of the mice spent significantly less time in the light compartment than in the dark compartment (F1,51 = 579.9, p < 0.001). The duration of time spent in the light compartment did not differ between groups (Fig. S1 in Supplementary Materials). However, a significant main effect of Methamphetamine (F1,51 = 4.9, p < 0.05) on the latency to enter the light compartment was found, with methamphetamine-exposed mice taking longer on average to enter the light compartment compared with saline-exposed mice (saline: 74.0 ± 13.7 s; methamphetamine: 123.1 ± 17.9 s). No significant effects of genotype were found.
3.3. Object and place recognition tests
No significant effects of gp120 expression or methamphetamine exposure on the discrimination index in the novel object (Fig. 2A) or novel place (Fig. 2B) test were found. In the object-in-place recognition test (Fig. 2C), a main effect of Genotype (F1,50 = 5.1, p < 0.001) was detected, with gp120-tg mice showing a significantly lower discrimination score compared with non-tg mice. Saline-exposed, non-tg mice successfully discriminated the novel object/place/side in all tests (Fig. S2 in Supplementary Materials). No significant effects of gp120 expression were detected on the total interaction time in the novel object, novel place or object-in-place tests (Table S1 in Supplementary Materials). However, a significant main effect of Methamphetamine (F1,50 = 5.6, p < 0.05) was revealed in the familiarization phase of the object-in-place test, with methamphetamine-exposed mice showing higher interaction levels than saline-exposed mice (Table S1 in Supplementary Materials).
Fig. 2.
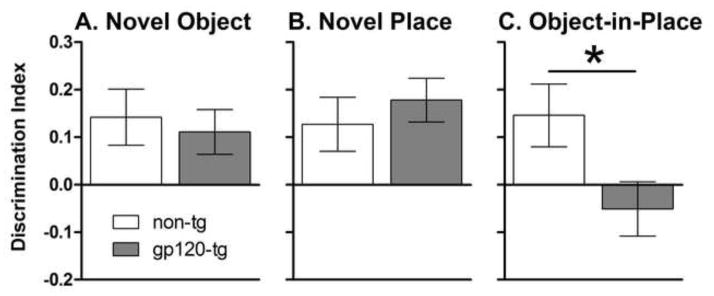
Novel object (A), novel place (B), and object-in-place (C) recognition tasks in non-tg and gp120-tg mice. The expression of gp120 led to a significantly reduced discrimination index ([novel - familiar]/total) in the object-in-place recognition task compared with non-tg mice. No significant effects of methamphetamine exposure were evident; therefore, the data were collapsed for the purpose of representing genotype effects. The data are expressed as mean ± SEM. *p < 0.05.
3.4. Barnes maze test
3.4.1. Latency
Significant effects of Day on the acquisition (F4,200 = 26.0, p < 0.001) and reversal (F1,50 = 25.4, p < 0.001) stages were observed, with all mice showing a reduction in the latency to find the target hole across the days of testing. No significant effects of gp120 expression or methamphetamine exposure on latency during the acquisition, retention, or reversal test were observed (data not shown).
3.4.2. Reference errors
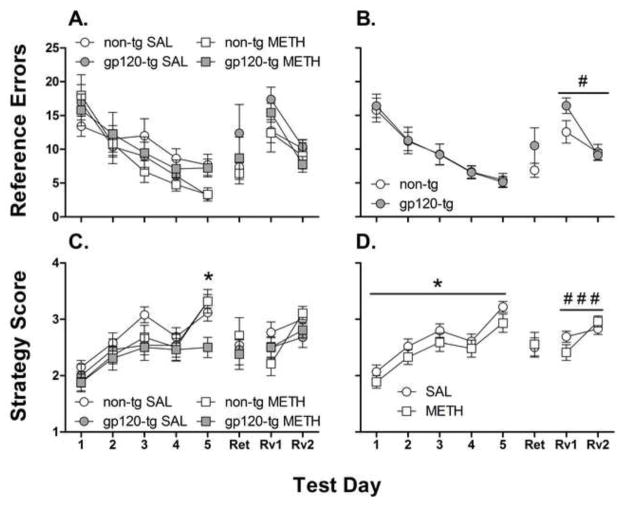
Significant effects of Day on the acquisition (F4,200 = 18.6, p < 0.001) and reversal (F1,50 = 26.6, p < 0.001) stages were observed, with all mice exhibiting a reduction of the number of errors prior to finding the target hole across the days of testing (Fig. 3A). A significant Genotype × Day interaction (F1,50 = 4.7, p < 0.05) was observed during the reversal trial (Fig. 3B). A trend toward gp120-tg mice making more errors on reversal day 1 compared with non-tg mice was observed (p < 0.1). This analysis revealed that gp120-tg mice showed a greater decrease in the number of errors from day 1 to day 2 in reversal testing compared with non-tg mice (gp120-tg: 44% decrease, p < 0.001; non-tg: 24% decrease, p < 0.05). No significant effects of gp120 expression or methamphetamine exposure on reference errors were found during the memory retention test.
Fig. 3.
Average reference errors made (A, B) and strategy scores (C, D) in the Barnes maze during acquisition (days 1–5), retention (Ret), and reversal learning on days 1 and 2 (Rv1 and Rv2, respectively). gp120-tg mice tended to make more errors on the first day of reversal learning (B) compared with non-tg mice. Methamphetamine (METH)-exposed gp120-tg mice had significantly worse strategy scores on day 5 of acquisition (C) than all of the other groups, suggesting that gp120 expression further impaired methamphetamine-induced spatial strategy deficits during acquisition (D). Methamphetamine-exposed mice used a worse average strategy on reversal learning day 1 vs. day 2 (D), whereas saline (SAL)-exposed mice did not improve from day 1 to day 2 of reversal learning. The data are expressed as mean ± SEM. (B) #p < 0.05, significant Genotype × Day interaction; (C) *p < 0.05, methamphetamine-exposed gp120-tg mice significantly different from all other groups; (D) *p < 0.05, significant main effect of Methamphetamine; ###p < 0.001, significant difference between day 1 and day 2 in methamphetamine-exposed mice.
3.4.3. Working memory errors
Significant effects of Day on the acquisition (F4,200 = 20.0, p < 0.001) and reversal (F1,50 = 33.1, p < 0.001) stages were observed, with all mice exhibiting a reduction of the number of errors prior to finding the target hole across the days of testing. No significant effects of gp120 expression or methamphetamine exposure on working memory errors were found during the acquisition, retention, or reversal test (data not shown).
3.4.4. Strategy scores
Significant effects of Day on the acquisition (F4,200 = 20.2, p < 0.001) and reversal (F1,50 = 19.9, p < 0.001) stages were observed, with all mice showing an increase in strategy scores across the days of testing (Fig. 3C). A significant main effect of Methamphetamine (F1,50 = 4.8, p < 0.05) was found. During the acquisition stage, methamphetamine exposure resulted in significantly lower average strategy scores compared with saline exposure (Fig. 3D). A significant Methamphetamine × Trial interaction (F1,50 = 4.8, p < 0.05) was also detected, with saline-exposed mice showing a greater strategy score in trial 2 compared with trial 1, whereas methamphetamine-exposed mice showed no significant difference between trials 1 and 2 (Fig. 4; p < 0.01). During the acquisition stage, a significant three-way Genotype × Methamphetamine × Day interaction (F4,200 = 2.4, p < 0.05) was detected, with methamphetamine-exposed gp120-tg mice showing significantly decreased strategy scores on day 5 compared with all of the other groups (Fig. 3C; p < 0.05). This outcome was further examined with an analysis of simple effects followed by trend analysis. A significant linear trend across the days of testing was observed in saline-exposed non-tg mice (F1,12 = 17.4, p < 0.01), saline-exposed gp120-tg mice (F1,13 = 30.6, p < 0.001), and methamphetamine-exposed non-tg mice (F1,13 = 39.5, p < 0.001) but not in methamphetamine-exposed gp120-tg mice (F1,12 = 2.7, p > 0.05). This pattern of results suggests that improvements in the spatial strategies of methamphetamine-exposed gp120-tg mice began to plateau toward the final days of testing.
Fig. 4.
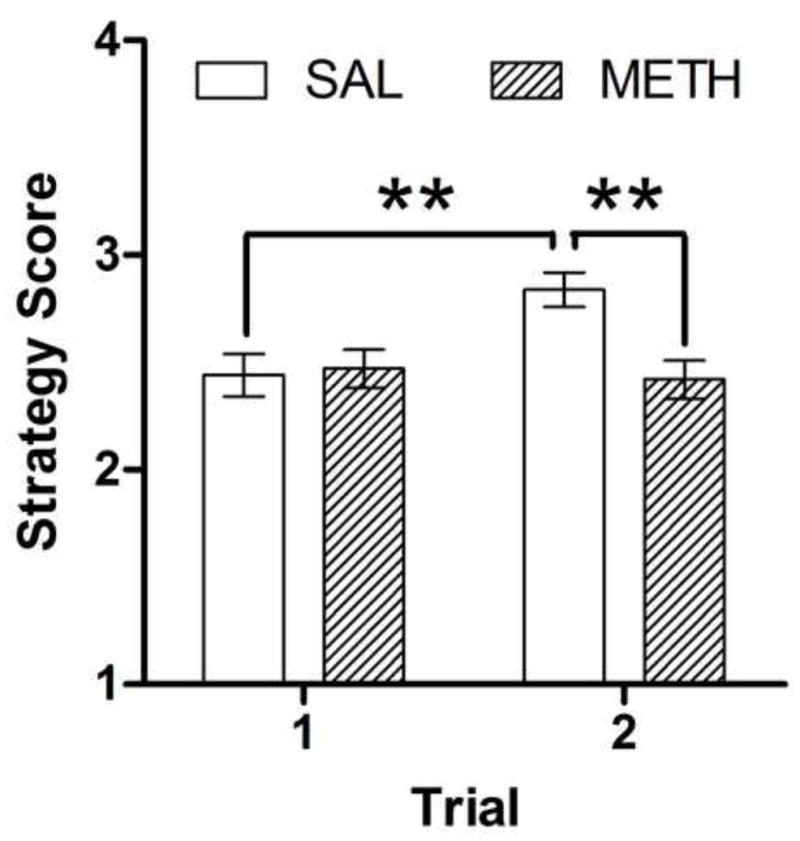
Comparison of strategy scores between saline (SAL)- and methamphetamine (METH)-exposed mice in the first and second trials of each day of acquisition in the Barnes maze. Saline-exposed mice showed a significantly improved strategy score in trial 2 compared with trial 1, whereas methamphetamine-exposed mice did not. No significant effects of genotype were detected; therefore, the data were collapsed for the purpose of representing methamphetamine effects. The data are expressed as mean ± SEM. **p < 0.01.
During the reversal stage, a significant Methamphetamine × Day interaction (F1,50 = 4.8, p < 0.05) was detected, with methamphetamine-exposed mice showing a higher strategy score on day 2 compared with day 1 (Fig. 3D; p < 0.001), whereas saline-exposed mice did not. No significant effects of gp120 expression or methamphetamine exposure were observed in the retention test.
3.4.5. Probe test
No significant effects of gp120 expression or methamphetamine exposure on the duration of time spent in the target quadrant were found (Fig. S3 in Supplementary Materials).
4. Discussion
The main findings of the present study indicate that methamphetamine exposure and gp120 expression produced both factor-specific and additive effects on aspects of cognitive function. Mice that expressed gp120 exhibited an impairment in object-in-place associative memory and tended to make more reference errors on the first day of reversal learning in the Barnes maze compared with non-tg mice. Methamphetamine-exposed mice took longer to enter the light compartment in the light-dark test and maintained a consistently lower strategy score during the acquisition trials in the Barnes maze but tended to more quickly acquire advantageous strategies during the reversal trials than saline-exposed mice. Importantly, the combination of methamphetamine exposure and gp120 expression potentiated the impairments in the strategies used to find the target hole in the Barnes maze task during the last day of acquisition. Methamphetamine-exposed gp120-tg mice showed less-advantageous strategy choices than all of the other groups, suggesting that gp120 expression further impaired the already deficient spatial strategy in methamphetamine-exposed mice. Altogether, both gp120 expression and methamphetamine exposure led to specific deficits in cognitive function.
The expression of gp120 led to selective impairment in object-in-place performance but not novel object or novel place performance. The lack of gp120-induced impairments in novel object and novel place performance suggests that both discrete spatial and recognition memory are intact. Therefore, the impairment in object-in-place performance is specific to associative recognition memory that involves complex interactions between several key brain regions (see below). In contrast, methamphetamine exposure did not alter performance on any of the recognition tasks. Methamphetamine binges have been shown to impair novel object and object-in-place performance in rats (Belcher et al., 2008; Reichel et al., 2012). However, rats exposed to escalating binge regimens as used in the current study, rather than single-day dosing regimens, did not exhibit novel object recognition deficits (Clark et al., 2007; Belcher et al., 2008), highlighting the importance of the particular methamphetamine binge regimen used. The key brain regions implicated in object-in-place associative memory include the medial prefrontal cortex (mPFC), perirhinal cortex (PRH), and hippocampus (Barker and Warburton, 2011). Barker and colleagues (Barker et al., 2007; Barker and Warburton, 2011) demonstrated that object-in-place performance depends on the functional integrity of each of these regions, both independently and in connection with each other. Novel object and novel place performance, however, depends on discrete regions within the hippocampus-mPFC-PRH circuit. For example, only lesions of the PRH impair novel object performance, whereas only lesions of the hippocampus impair novel place performance. Thus, selective impairment in object-in-place performance, such as that observed in gp120-expressing mice, would suggest either an impairment in mPFC function or compromised connectivity within the hippocampus-mPFC-PRH circuit. The PFC is commonly implicated in HIV-induced neuropathology. Specifically, functional deficits (Melrose et al., 2008), reduced morphological volume (Jernigan et al., 2005), and increased pathology, including the loss of large neurons and dendritic damage (Masliah et al., 1992), have all been observed in the frontal cortex in HIV-infected individuals. Moreover, multiple studies have shown that patients infected with HIV show widespread decreases in white matter tracts (Stubbe-Drager et al., 2012; Zhu et al., 2013), suggesting that compromised regional connectivity may contribute to cognitive deficits. In humans, associative recognition memory is important for contextual awareness of changes within the environment making it essential for normal everyday living (Barker and Warburton, 2013). Our findings suggest that the expression of gp120 protein in the brain may contribute to compromised mPFC function or compromised functional connectivity of the hippocampus-mPFC-PRH circuit in HIV-infected humans leading to deficits in associative recognition memory.
Overall, the mice were able to learn the Barnes maze task, suggesting no gross hippocampal dysfunction after either gp120 expression or methamphetamine exposure. Nevertheless, deficits in the spatial strategies used by methamphetamine-exposed mice suggest impairment in hippocampal function. Specifically, compared with saline exposure, methamphetamine exposure led to consistently lower strategy scores throughout the acquisition trials. This consistent and subtle decrease in optimal strategy preference may be the result of reduced within-day, trial-by-trial improvements. That is, saline-exposed mice tended to choose more efficient strategies during the second trial each day compared with the first trial, whereas methamphetamine-exposed mice did not. In C57BL/6 mice, spatial learning deficits have been observed after daily exposure to high-dose methamphetamine for 7 days (Chen et al., 2012) but not after exposure to a single-day neurotoxic binge (Grace et al., 2010). Together with our findings, these studies suggest that spatial learning may be more susceptible to impairment from prolonged methamphetamine exposure, rather than acute methamphetamine-induced neurotoxicity. Thus, methamphetamine exposure appears to impair spatial learning, but the effects are subtle and depend on the methamphetamine regimen used.
Observed deficits in spatial learning in the Barnes maze may be attributed to altered anxiety-like behavior in methamphetamine-exposed mice. In the light-dark box, methamphetamine exposure increased the latency to enter the light compartment suggesting a possible increase in anxiety-like behavior. However, there was no effect of methamphetamine on the total time spent in the light compartment, which is considered a more accurate measure of anxiety-like behavior than the latency to enter the light compartment (Chaouloff et al., 1997; Bourin and Hascoet, 2003). Moreover, there was no effect of methamphetamine on the latency to find the target hole in the Barnes maze. Therefore, it seems unlikely, that cognitive deficits detected in the Barnes maze in the present studies may be attributed to alterations in anxiety-like behavior.
The lack of an observed impairment in spatial learning in the Barnes maze after gp120 expression complements the absence of a deficit in novel place performance because both tasks are hippocampus-dependent (Paylor et al., 2001; Barker and Warburton, 2011). Spatial memory deficits in gp120-expressing mice have been observed previously in the Morris water maze (D’Hooge et al., 1999), but these deficits were age-dependent. Nevertheless, gp120 expression led to an exacerbation of the methamphetamine-induced spatial strategy deficit during the acquisition stage. Rather than a simple additive effect throughout the entire acquisition stage, gp120 expression in methamphetamine-exposed mice led to further impairment only toward the later stages of learning and therefore may represent a ceiling effect. The possibility of a ceiling effect is suggested by the fact that all of the groups, with the exception of the methamphetamine-exposed gp120-tg group, were still improving their spatial strategy in a linear fashion. Thus, to detect greater strategy deficits and deficits in other less sensitive measures such as reference errors, more training trials may be required. Deficits in both long- and short-term potentiation have been observed in the hippocampus in gp120-expressing mice (Krucker et al., 1998), suggesting that hippocampal function is compromised. Although the present study demonstrated that gp120 expression alone is not sufficient to impair spatial learning, gp120 expression may exacerbate methamphetamine-induced pathology to further compromise hippocampal function. Learning appears to be particularly sensitive to combined methamphetamine dependence and HIV infection in human subjects (Rippeth et al., 2004). Thus, the observation of augmented spatial learning deficits (i.e., deficits in spatial strategy) observed in methamphetamine-exposed gp120-tg mice may reflect a general deficit in learning rather than a deficit in a particular learning construct (e.g., spatial memory).
Interestingly, both methamphetamine exposure and gp120 expression led to alterations in reversal learning in the Barnes maze which were both subtle and difficult to interpret. That is, decreased spatial strategy scores in methamphetamine-exposed mice on Day 1 of reversal learning may be a carry-over effect of the lower strategy scores observed throughout the acquisition trials. Reversal learning deficits have been noted after methamphetamine treatment in rats (Izquierdo et al., 2010) but have not been previously assessed in gp120-expressing mice. Because both methamphetamine exposure (Berman et al., 2008) and gp120 expression (Toggas et al., 1994) can lead to cortical pathology, this deficit may represent altered cognitive flexibility. Further studies that assess more complex executive functions are warranted.
As demonstrated previously, the combined effects of gp120 expression and methamphetamine exposure on cognition appear to be both subtle and complex (Henry et al., 2013). The combination of gp120 expression and methamphetamine exposure led to further deficits in spatial learning compared with either factor alone. However, these deficits were only observed after extensive training, suggesting a possible ceiling effect in performance (see above). Thus, specific testing conditions that require high cognitive demands (e.g., rule learning in operant-based cognitive tasks) may be needed to reveal the additive effects of gp120 and methamphetamine on cognitive performance. These results are similar to those reported in the clinical literature. For example, the rate of global neuropsychological impairment is greatest when HIV-infected patients are also methamphetamine-dependent (Rippeth et al., 2004) and particularly so when these ndividuals are immunosuppressed (CD4 < 200; (Carey et al., 2006)).
In conclusion, our work extends upon previous findings after gp120 expression by demonstrating gp120-specific deficits in associative recognition memory and cognitive flexibility. In addition, we have demonstrated long-lasting cognitive impairments during protracted abstinence from chronic methamphetamine exposure that mimics human abuse patterns. The combination of gp120 expression with methamphetamine exposure resulted in additive effects that were subtle and may represent an impairment in maximal ability rather than a generalized baseline deficit. This pattern of results suggests that hippocampal-cortical networks may be particularly affected, and tasks that assess more complex executive functions are required to completely understand the consequences of methamphetamine exposure and gp120 expression on cognitive function. The gp120-expressing mouse represents a useful model for studying the combined effects of HIV infection and methamphetamine dependence and may help identify specific aspects of cognitive function that are particularly sensitive to this comorbidity.
Supplementary Material
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute (SBMRI). The TMARC is comprised of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Steven Paul Woods, Psy.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Clinical Assessment and Laboratory (CAL) Core: Scott L. Letendre, M.D. (Core Director), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric (NP) Core: Robert K. Heaton, Ph.D. (Core Director), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D.; Neuroimaging (NI) Core: Gregory Brown, Ph.D. (Core Director), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D., Virawudh Soontornniyomkij, M.D.; Administrative Coordinating Core (ACC) – Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC – Statistics Unit: Ian Abramson, Ph.D. (Unit Chief), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC – Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D., Jared Young, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (Project Director), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (Project Director), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James P. Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director). The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States government.
Role of Funding Source
Funding for this study was provided by the Translational Methamphetamine AIDS Research Center NIDA grant (TMARC P50 DA26306) and Interdisciplinary Research Fellowship in NeuroAIDS (IRFN, MH81482 to JPK). The TMARC and IRFN had no further role in the study design; collection, analysis, and interpretation of the data; writing of the report; or decision to submit the paper for publication.
Footnotes
Contributors
JPK, SS, and AM were responsible for the study concept and design. JPK contributed to the acquisition of the animal data. JPK and SS completed the data analysis and interpretation of findings. JPK drafted the manuscript. SS and AM provided critical revision of the manuscript for important intellectual content. All of the authors critically reviewed the content and approved the final version for publication.
Conflicts of interest
AM has received contract research support from Bristol-Myers Squibb, Forest Laboratories, and Astra-Zeneca and honoraria/consulting fees from AbbVie during the past 3 years. The remaining authors report no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharm. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: a critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefronal cortices. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht245. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Addict Rev 2008. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10:185–190. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Durand M, Mormede P. Anxiety- and activity-related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behav Brain Res. 1997;85:27–35. doi: 10.1016/s0166-4328(96)00160-x. [DOI] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Liu YL, Zhong Q, Yu YF, Su HL, Toque HA, Dang YH, Chen F, Xu M, Chen T. Tetrahydropalmatine protects against methamphetamine-induced spatial learning and memory impairment in mice. Neurosci Bull. 2012;28:222–232. doi: 10.1007/s12264-012-1236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Kuczenski R, Segal DS. Escalating dose, multiple binge methamphetamine regimen does not impair recognition memory in rats. Synapse. 2007;61:515–522. doi: 10.1002/syn.20397. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Mucke L. Molecular profile of reactive astrocytes - implications for their role in neurological disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Wesselingh SL, Purcell DFJ. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, Williams MT, Vorhees CV. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol. 2010;32:346–355. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Lescs MC, Keohane C, Paraire F, Marc B, Durigon M, Gherardi R. Early brain changes in HIV-infection - neuropathological study of 11 HIV seropositive, non-AIDS cases. J Neuropathol Exp Neurol. 1992;51:177–185. doi: 10.1097/00005072-199203000-00007. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, Wolfson T, Velin R, Marcotte TD, Hesselink JR, Jernigan TL, Chandler J, Wallace M, Abramson I. The HNRC 500--neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell M, Perry W, Young JW, Minassian A. Behavioral effects of chronic methamphetamine treatment in HIV-1 gp120 transgenic mice. Behav Brain Res. 2013;236:210–220. doi: 10.1016/j.bbr.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TL, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kesby JP, Hubbard DT, Markou A, Semenova S. Expression of HIV gp120 protein increases sensitivity to the rewarding properties of methamphetamine in mice. Addict Biol. 2012;19:593–605. doi: 10.1111/adb.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Toggas SM, Mucke L, Siggins GR. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Mashliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Exp Neurol. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharm. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009;21:575–582. doi: 10.1080/09540120802385579. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol. 1992;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I, Chandler JL, Wallace MR, Spector SA, Jernigan T, Hesselink J, Hansen L, Abramson I, Masys D. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JMB, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188:337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus-associated neurocognitive disorder pathophysiology in relation to drug addiction. In: Uhl GR, editor. Addiction Reviews. Vol. 2. Hoboken: Wiley; 2010. pp. 122–128. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: 2. Neuropathology Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Paylor R, Zhao YG, Libbey M, Westphal H, Crawley JN. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol Behav. 2001;73:781–792. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Maung R, Sejbuk NE, Ake C, Kaul M. Alteration of methamphetamine-induced stereotypic behaviour in transgenic mice expressing HIV-1 envelope protein gp120. J Neurosci Methods. 2010;186:222–225. doi: 10.1016/j.jneumeth.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Stubbe-Drager B, Deppe M, Mohammadi S, Keller SS, Kugel H, Gregor N, Evers S, Young P, Ringelstein EB, Arendt G, Knecht S, Husstedt IW. Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurology. 2012;12:23. doi: 10.1186/1471-2377-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central-nervous-system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Toneatto S, Finco O, van der Putten H, Abrignani S, Annunziata P. Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS. 1999;13:2343–2348. doi: 10.1097/00002030-199912030-00005. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, daCunha A. Role for astrocytosis in HIV-1-associated dementia. In: Oldstone MB, Vitkovic L, editors. HIV and Dementia. Berlin: Springer-Verlag; 1995. pp. 105–116. [DOI] [PubMed] [Google Scholar]
- Weis S, Haug H, Budka H. Astroglial changes in the cerebral-cortex of AIDS brains - a morphometric and immunohistochemical investigation. Neuropathol Appl Neurobiol. 1993;19:329–335. doi: 10.1111/j.1365-2990.1993.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Zhu T, Zhong JH, Hu R, Tivarus M, Ekholm S, Harezlak J, Ombao H, Navia B, Cohen R, Schifitto G. Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. J Neurovirol. 2013;19:10–23. doi: 10.1007/s13365-012-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.