Abstract
Limonene is a lipophilic monoterpene found in high levels in citrus peel. Limonene demonstrates anti-cancer properties in preclinical models with effects on multiple cellular targets at varying potency. While of interest as a cancer chemopreventive, the biological activity of limonene in humans is poorly understood. We conducted metabolite profiling in 39 paired (pre/post-intervention) plasma samples from early-stage breast cancer patients receiving limonene treatment (2 g QD) before surgical resection of their tumor. Metabolite profiling was conducted using ultra-performance liquid chromatography (UPLC) coupled to a linear trap quadrupole (LTQ) system and gas chromatography mass spectrometry (GC-MS). Metabolites were identified by comparison of ion features in samples to a standard reference library. Pathway-based interpretation was conducted using the human metabolome database (HMDB) and the MetaCyc database. Of the 397 named metabolites identified, 72 changed significantly with limonene intervention. Class-based changes included significant decreases in adrenal steroids (P’s<0.01), and significant increases in bile acids (P’s≤0.05) and multiple collagen breakdown products (P’s<0.001). The pattern of changes also suggested alterations in glucose metabolism. There were 47 metabolites whose change with intervention was significantly correlated to a decrease in cyclin D1, a cell cycle regulatory protein, in patient tumor tissues (P’s≤0.05). Here, oral administration of limonene resulted in significant changes in several metabolic pathways. Further, pathway-based changes were related to the change in tissue level cyclin D1 expression. Future controlled clinical trials with limonene are necessary to determine the potential role and mechanisms of limonene in the breast cancer prevention setting.
Keywords: Limonene, metabolomics, bioactivity, breast cancer, clinical trial
Introduction
Limonene is a monocyclic monoterpene and the major component in the essential oils of citrus fruits. Extensive preclinical evidence supports a number of anti-cancer properties of limonene with the most consistent being prevention in experimental models of mammary carcinogenesis. In carcinogen-induced rat mammary tumor models, limonene fed during the promotion/progression stage inhibits the development of tumors induced by 7,12-dimethylbenz(α)anthracene (DMBA) as well as tumors induced by N-methyl-N-nitrosourea (NMU) [1, 2]. Dietary feeding of limonene also inhibits the development of ras oncogene-induced mammary carcinomas in rats [3]. In addition to demonstrating activity to inhibit tumor development, limonene has been investigated for chemotherapeutic activity. Oral feeding of limonene has been shown to induce a dose-dependent regression of DMBA- and NMU-induced mammary tumors without observable systemic toxicity [4, 5].
In rodent models of different solid tumors, limonene has been reported to exhibit effects on a number of the cancer hallmarks (i.e., proliferation, apoptosis, inflammation) with the exact mechanism of action unknown [6]. Dietary feeding of limonene has also been shown to modulate carcinogen-metabolizing enzymes in rats, affecting the detoxification of chemical carcinogens [7]. The ability of limonene to inhibit proliferation has been attributed to effects on isoprenylation of small proteins in the molecular weight range of 21,000–26,000 Da that includes members of the Ras family of geranylpyrophosphate binding proteins that regulate cell growth and are commonly deregulated in human cancers. Further, several studies have reported changes in gene and protein expression in monoterpene-treated tumors undergoing regression [8]. Of these, induction of mannose-6-phosphate/insulin-like growth factor II receptor (M6P/IGF II receptor) and activation of transforming growth factor beta (TGFβ) signaling pathway are among the best described [9]. More recently, Yoon et al., demonstrated that limonene inhibits prostaglandin E2 (PGE2) production in macrophages [10] while d’Alesso showed that limonene exerts anti-inflammatory properties in preclinical models and in humans [11]. Other preclinical evidence indicates that limonene may enhance immune response and act generally as an immune modulator [12].
Clinical development of monoterpenes has focused on the limonene analogue, perillyl alcohol in advanced cancer patients [13–17]. The underwhelming results of these trials reduced enthusiasm for limonene development. Unlike perillyl alcohol, limonene distributes favorably to adipose tissue in rodents [18, 19] and humans [20] resulting in tissue levels comparable to the active concentrations in preclinical models. Favorable distribution of limonene to adipose tissue suggests that evaluation of limonene’s potential clinical activity deserves further attention, particularly within the context of cancers arising from organs with high adiposity such as breast.
We have recently completed a pre-surgical study of limonene in women with newly diagnosed operable breast cancer to determine the breast tissue disposition of limonene and its associated bioactivity [21]. In the completed trial, 2 g of oral limonene daily for 2–6 weeks resulted in low micromolar limonene concentrations in the breast tissue that was associated with a significant 22% reduction in cyclin D1 expression in tumor tissue [21]. Overexpression of cyclin D1 promotes the transition of cells out of the G1 and into the cell cycle [22] and is commonly overexpressed and deregulated early in breast tumorigenesis in humans [23, 24]. To gain further insights into the in vivo activity of limonene and to identify blood correlates of limonene effect at the tissue level, we conducted an analysis of plasma metabolites using samples collected from our recently completed trial and correlated our results with cyclin D1 tissue level changes.
Materials and Methods
Clinical study
Details of the clinical study and main study findings are published elsewhere [21]. Briefly, we accrued forty-three women with newly diagnosed operable breast cancer to take 2 grams of limonene daily for two to six weeks before voluntary surgical excision of their tumor. Forty women completed the intervention. Blood and breast tissue were collected to determine limonene and metabolite concentrations and limonene-induced changes in systemic and tissue biomarkers of breast cancer risk or carcinogenesis. Expression of the cell-cycle regulator (cyclin D1) in diagnostic and surgical tissue sections was assessed using immunohistochemistry (IHC). Positively stained nuclei were quantified using Aperio Spectrum (Aperio Technologies) [25], and software performance was validated by a trained pathologist. After completion of the aims of the original trial cited above, thirty-nine pairs of plasma samples collected before and after limonene intervention were available for metabolomic profiling.
Metabolomic profiling
Metabolomic studies were conducted at Metabolon Inc. on non-targeted platforms that enable relative quantitative analysis of a broad spectrum of molecules with a high degree of confidence [26]. All samples were shipped on dry ice to Metabolon, Inc. (Durham, NC, USA), were assigned unique identifiers to track samples during processing, and were stored at −80°C until processed. For sample preparation, proteins were precipitated from the plasma with methanol that contained standards in order to report extraction efficiency. The resulting supernatant was split into equal parts for analysis on the three platforms. Detailed descriptions of the instrumentation configurations and conditions, data acquisition, and software approaches for data handling, were previously described [26, 27]. Briefly, samples destined for gas chromatography (GC) mass spectrometry (MS) analysis were dried under vacuum desiccation for a minimum of 24 h and then derivatized under nitrogen using bistrimethyl-silyl-triflouroacetamide (BSTFA). Samples were analyzed on a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) using electron impact ionization. Ultra performance liquid chromatography (UPLC)/MS/MS2 was carried out using a Waters Acquity UPLC (Waters Corporation, Milford, MA, USA) coupled to a linear trap quadrupole (LTQ) MS (Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with an electrospray ionization source. Two separate UPLC/MS/MS2 injections were performed on each sample: one optimized for positive ions and one for negative ions. Metabolites were identified by automated comparison of the ion features in the experimental samples to an in-house reference library composed of more than 2,400 authentic chemical standard entries that included retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as their associated MS/MS2 spectra. This library allowed the rapid identification of metabolites in the experiment with high confidence.
Instrument variability was determined by calculating the median relative standard deviation (RSD) for the internal standards that were added to each sample prior to injection into the mass spectrometers and was found to be 6%. A homogenous pool containing a small amount of all study samples was included in the analysis as technical replicate samples. Overall process variability was determined by calculating the median RSD for all endogenous metabolites (i.e., non-instrument standards) present in the technical replicates and was found to be 12%.
Statistical analysis
Missing values for a given metabolite were imputed with the observed minimum detection value based on the assumption that they were below the limits of instrument detection sensitivity. All comparisons were performed using log-transformed data. All statistical analyses were performed using the “R” program v2.14.2 (R Foundation, http://cran.r-project.org/) [28]. Paired two sample t-tests were used for all comparisons unless otherwise noted. The false discovery rate (FDR) method was used to correct for multiple comparisons; these estimated Q-values are reported [29]. For convenience of data visualization, raw area counts for each biochemical were re-scaled by dividing the value for a specific biochemical in each sample by the median value for that specific biochemical. Correlations changes in metabolites, represented as the post-treatment/pre-treatment ratio, were compared to post-treatment/pre-treatment changes in cyclin D1 using Spearman’s correlation. Pathway based interpretation of biochemicals were constructed using MetaCyc [30] and the human metabolome database (HMDB) [31].
Results
Individual metabolite changes
Global plasma metabolomic profiling was conducted using a combination of high-throughput LC- and GC-based MS on a total of 39 paired plasma samples collected from women on a short term oral limonene intervention prior to surgery. Patient, tumor and treatment characteristics have been previously reported [21]. A total of 397 named biochemicals were detected using a standard reference library. Table 1 summarizes metabolites that changed with limonene intervention. Because this is an effort to generate hypotheses for limonene’s systemic activity, a Q ≤ 0.10 after FDR correction was considered significant. All metabolites, however, with P’s ≤ 0.05 are listed along with the fold-change in order to capture pathway-based change. Of the identified biochemicals, plasma levels of 72 changed with P’s ≤ 0.05 (42 rose and 30 fell); of these, 39 survived correction for false discovery given a tolerance of Q ≤ 0.10 (19 rose and 20 fell). Of the metabolites that changed with limonene treatment, several could be grouped as specific classes of metabolites or along metabolic pathways as described below (Q’s ≤ 0.10).
Table 1.
Metabolites that changed after 2–6 weeks daily limonene intervention (72 metabolites P≤0.05; 39 metabolites Q≤0.10).
| Adrenal steroids | Fold-Change | P-value | Q-value |
|---|---|---|---|
| androsterone-S | 0.75 | <0.001 | <0.001 |
| epiandosterone-S | 0.79 | <0.001 | <0.001 |
| pregnendiol disulfate | 0.82 | <0.001 | 0.01 |
| 4-androsten-3β,17β diol disulfate 2 | 0.90 | 0.01 | 0.06 |
| dehydroisoandosterone sulfate (DHEA-S) | 0.93 | 0.02 | 0.12 |
| Secondary Bile Acids | |||
| glycodeoxycholate | 2.30 | <0.01 | 0.02 |
| glycoursodeoxycholate | 3.60 | <0.01 | 0.03 |
| taurodeoxycholate | 3.50 | <0.01 | 0.03 |
| taurocholate | 3.40 | <0.01 | 0.03 |
| taurocholenate sulfate | 0.90 | 0.01 | 0.06 |
| glychochenodeoxycholate | 2.50 | 0.01 | 0.92 |
| glycocholate | 2.60 | 0.02 | 0.10 |
| taurochenodeoxycholate | 2.00 | 0.02 | 0.12 |
| deoxycholate | 1.70 | 0.05 | 0.18 |
| Glucose/Energy Metabolism | |||
| glucuronate | 1.83 | <0.01 | 0.03 |
| pyruvate | 1.66 | <0.01 | 0.03 |
| acetylcarnitine | 0.89 | <0.01 | 0.03 |
| 3-hydroxybutyrate (BHBA) | 1.09 | 0.01 | 0.06 |
| fructose | 1.56 | 0.02 | 0.10 |
| mannitol | 4.87 | 0.03 | 0.14 |
| citrate | 0.94 | 0.04 | 0.17 |
| malate | 1.38 | 0.04 | 0.17 |
| Collagen Breakdown Products | |||
| glycine | 1.38 | 0.01 | 0.01 |
| proline | 1.20 | <0.001 | 0.01 |
| trans-4-hydroxyproline | 1.70 | <0.001 | 0.01 |
| pro-hydroxy-pro | 1.40 | 0.02 | 0.11 |
| Amino Acids and Metabolites | |||
| indolepropionate | 2.60 | <0.001 | <0.001 |
| alanine | 1.21 | <0.001 | 0.02 |
| 3-phenylpropionate (hydrocinnamate) | 1.95 | <0.001 | 0.01 |
| 3-(4-hydroxyphenyl)lactate | 0.87 | <0.01 | 0.03 |
| kynurenate | 0.87 | <0.01 | 0.04 |
| phenylacetate | 2.08 | 0.01 | 0.06 |
| gamma-glutamylisoleucine | 0.91 | 0.01 | 0.07 |
| α-hydroxybutyrate (AHB) | 0.90 | 0.01 | 0.08 |
| phenylalanyltryptophan | 0.92 | 0.01 | 0.08 |
| aspartate | 0.93 | 0.02 | 0.12 |
| N-acetylglycine | 1.41 | 0.03 | 0.13 |
| gamma-glutamylalanine | 1.15 | 0.03 | 0.13 |
| alpha-hydroxyisovalerate | 0.93 | 0.03 | 0.13 |
| aspartylphenylalanine | 1.09 | 0.03 | 0.13 |
| isoleucylvaline | 1.47 | 0.03 | 0.15 |
| phenylalanine | 0.95 | 0.04 | 0.13 |
| isobutyrylcarnitine | 1.48 | 0.05 | 0.18 |
| Fatty Acids | |||
| tetradecanedioate | 0.84 | <0.001 | 0.01 |
| hexadecanedioate | 0.82 | <0.001 | 0.02 |
| tetradecanedioate | 0.84 | <0.001 | 0.01 |
| 10-undecenoate (11:1n1) | 0.86 | <0.01 | 0.03 |
| 5-dodecenoate (12:1n7) | 0.91 | <0.01 | 0.04 |
| myristate (14:0) | 0.94 | 0.01 | 0.09 |
| myristoleate (14:1n5) | 1.21 | 0.01 | 0.09 |
| 3-hydroxydecanoate | 0.93 | 0.01 | 0.09 |
| pentadecanoate (15:0) | 0.94 | 0.02 | 0.10 |
| octadecanedioate | 0.94 | 0.02 | 0.11 |
| eicosenoate (20:1n9 or 11) | 1.06 | 0.04 | 0.17 |
| palmitoleate (16:1n7) | 1.13 | 0.04 | 0.17 |
| eicosapentaenoate (EPA; 20:5n3) | 0.97 | 0.05 | 0.18 |
| Lysolipids | |||
| 2-myristoylglycerophosphocholine | 2.30 | 0.01 | 0.06 |
| 1-oleoylglycerophosphoethanolamine | 1.63 | 0.02 | 0.11 |
| 1-heptadecanoylglycerophosphocholine | 3.63 | 0.02 | 0.12 |
| 2-stearoylglycerophosphocholine | 2.80 | 0.03 | 0.14 |
| 1-eicosadienoylglycerophosphocholine | 2.68 | 0.04 | 0.17 |
| 2-palmitoylglycerophosphocholine | 3.78 | 0.05 | 0.18 |
| Other | |||
| homostachydrine | 1.50 | <0.01 | 0.04 |
| trigonelline (N′-methylnicotinate) | 1.20 | 0.01 | 0.05 |
| N4-acetylcytidine | 0.89 | 0.01 | 0.06 |
| 1-methylxanthine | 1.61 | 0.01 | 0.09 |
| hypoxanthine | 0.91 | 0.01 | 0.10 |
| 5-acetyl-2-pyridinecarboxylic acid | 0.92 | 0.02 | 0.10 |
| Ascorbate | 15.23 | 0.02 | 0.12 |
| D-Histidyl-D-tryptophyl-D-α-glutamyl-L-seryl-L-alanyl-L-serylleucylleucine (HWESASXX) | 0.94 | 0.02 | 0.12 |
| L-urobilin | 1.02 | 0.02 | 0.12 |
| inositol 1-phosphate (I1P) | 1.44 | 0.04 | 0.17 |
Adrenal/gonadal steroids: A number of the sulfated steroids produced by the adrenal gland and gonads and sulfated by liver sulfotransferases were affected by oral administration of limonene. Specifically, there were significant decreases in androsterone sulfate, epiandrosterone sulfate, 4-androsten-3β,17β-diol disulfate 2, and pregnendiol disulfate.
Bile acids: Multiple bile acids that were identified by this analysis increased with limonene intervention. Significant increases were observed for glycodeoxycholate, glycoursodeoxycholate, taurodeoxycholate, taurocholate, and deoxycholate. Conversely, there was a significant decrease in taurocholenate sulfate.
Collagen breakdown products: The collagen components glycine, proline, and 4-hydroxyproline were all significantly increased in plasma post-limonene intervention.
-
Glucose/energy metabolism:
Elevations of the terminal product of the glycolysis pathway, pyruvate, fructose of the sorbitol pathway, and glucuronate were observed post-limonene intervention.
Ketones are formed using acetyl-CoA generated by fatty acid oxidation; there was a small but statistically significant increase in the ketone, β-hydroxybutyrate.
Acetylcarnitine, derived from acetyl-CoA, was significantly decreased.
Amino acids and oxidative products: Following the limonene intervention, statistically significant increases in glycine, indolpropionate, alanine, 3-phenylpropionate, and phenylacetate were observed whereas 3-(4-hydroxyphenyl)lactate, kynurenene, gamma-glutamylisoleucine, alpha-hydroxybutyrate, and phenylalanyltryptophan, were significantly decreased.
Fatty acids: There was also general decrease in plasma short- and medium-chain dicarboxylic fatty acids; significant decreases were observed in tetradecanedioate, hexadecanedioate, 10-undecenoate, 5-dodecenoate, myristate, 3-hydroxydecanoate, and pentadecanoate. Conversely, there was a slight but significant increase in myristoleate,
Lysolipids: There was a general increase in all detected lysoglycerophosphorylcholines (lysoGPCs) with limonene treatment. However, only 2-myristoylglycerophosphocholine was significantly changed after FDR correction.
Metabolite changes correlate with tissue-level cyclin D1 changes
Because an important need for prevention studies is to have biomarkers of intervention effect that can be obtained non-invasively, we investigated those metabolites whose change correlated with the change in tissue level expression of cyclin D1 (as expressed by percent positively stained nuclei). Table 2 presents the 47 metabolites whose change from pre- to post-intervention was correlated with the change in cyclin D1, of these 23 were significant after FDR correction. As a class, lysoglycerophosphocholines and acylcarnitines increased with the decrease cyclin D1 expression, with the exception of acetylcarnitine which decreased (R2’s > 0.4). Changes in amino acids as well as fatty acids were also significantly correlated with changes in cyclin D1 expression.
Table 2.
Metabolites whose change is significantly correlated to the change in tissue Cyclin D1 expression (53 metabolites P≤0.05; 23 metabolites Q≤0.10).
| Lysolipids | P-value | Q-value | R2 |
|---|---|---|---|
| 1-palmitoylglycerophosphocholine | <0.001 | 0.01 | 0.62 |
| 1-palmitoleoylglycerophosphocholine | <0.001 | 0.02 | 0.59 |
| 1-stearoylglycerophosphocholine | <0.001 | 0.01 | 0.61 |
| 2-stearoylglycerophosphocholine | <0.001 | 0.01 | 0.61 |
| 1-oleoylglycerophosphocholine | <0.001 | 0.01 | 0.62 |
| 1-eicosadienoylglycerophosphocholine | <0.001 | 0.01 | 0.63 |
| 2-oleoylglycerophosphocholine | <0.01 | 0.05 | 0.55 |
| 2-linoleoylglycerophosphocholine | 0.01 | 0.09 | 0.51 |
| 1-eicosatrienoylglycerophosphocholine | 0.01 | 0.09 | 0.50 |
| 1-heptadecanoylglycerophosphocholine | 0.01 | 0.09 | 0.49 |
| 1-palmitoylplasmenylethanolamine | 0.02 | 0.14 | 0.42 |
| Acylcarnitines | |||
| palmitoylcarnitine | <0.001 | 0.01 | 0.63 |
| oleoylcarnitine | <0.001 | 0.02 | 0.59 |
| decanoylcarnitine | 0.01 | 0.10 | 0.47 |
| butyrylcarnitine | 0.02 | 0.14 | 0.43 |
| acetylcarnitine | 0.02 | 0.14 | 0.43 |
| Amino Acids and Metabolites | |||
| indolelactate | 0.01 | 0.09 | 0.49 |
| kynurenine | 0.01 | 0.10 | 0.47 |
| alpha-hydroxyisocaproate | 0.01 | 0.10 | 0.47 |
| phenylalanylserine | 0.01 | 0.10 | 0.47 |
| serine | 0.01 | 0.11 | 0.46 |
| phenol sulfate | 0.02 | 0.12 | 0.45 |
| 3-indoxyl sulfate | 0.02 | 0.14 | 0.43 |
| alanine | 0.02 | 0.14 | 0.43 |
| asparagine | 0.03 | 0.16 | 0.41 |
| aspartate | 0.04 | 0.17 | 0.39 |
| gamma-glutamylvaline | 0.04 | 0.17 | 0.39 |
| symmetric and asymmetric dimethylarginine (SDMA + ADMA) | 0.04 | 0.18 | 0.39 |
| Glucose/Energy Metabolism | |||
| 1,5-anhydroglucitol (1,5-AG) | 0.01 | 0.09 | 0.50 |
| Fatty Acids | |||
| 3-carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF) | 0.01 | 0.03 | 0.57 |
| 15-methylpalmitate | 0.01 | 0.10 | 0.48 |
| cis-vaccenate (18:1n7) | 0.02 | 0.12 | 0.45 |
| palmitoyl sphingomyelin | 0.02 | 0.14 | 0.42 |
| adrenate (22:4n6) | 0.03 | 0.16 | 0.41 |
| 17-methylstearate | 0.03 | 0.17 | 0.40 |
| palmitate, methyl ester | 0.04 | 0.17 | 0.39 |
| oleate (18:1n9) | 0.04 | 0.19 | 0.38 |
| Other | |||
| bilirubin (E,E) | <0.001 | 0.01 | 0.65 |
| N1-methyladenosine | <0.01 | 0.05 | 0.53 |
| quinate | 0.01 | 0.09 | 0.49 |
| 2-ethylhexanoate | 0.02 | 0.14 | 0.43 |
| pregnendiol disulfate | 0.02 | 0.15 | 0.42 |
| erythritol | 0.03 | 0.16 | 0.41 |
| bilirubin (Z,Z) | 0.03 | 0.17 | 0.40 |
| urate | 0.04 | 0.17 | 0.39 |
| cholesterol | 0.04 | 0.19 | 0.38 |
| alpha-tocopherol | 0.04 | 0.19 | 0.38 |
Discussion
This study is the first to apply metabolomics to a pre-surgical trial of the bioactive food component limonene. In addition, this is one of the first to correlate plasma metabolomics to a putative breast tumor drug response biomarker. Among the individual metabolites that changed with limonene intervention, there were several consistent biochemical pathway-based alterations. The most striking included a general decrease in sulfated adrenal/gonadal steroids, increases in bile acids and collagen breakdown products, and changes in energy metabolism.
The ability of limonene to reduce circulating adrenal steroid levels could be an important contribution to its overall chemopreventive and oncostatic properties. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S) are released into the circulation as inactive hormone precursors and taken up by specific tissues, such as breast, for the local conversion into androgen and estrogen sex steroids where they have a proliferative effect [32]; DHEA-S was non-significantly reduced in our study. In two nested case-control clinical trials, women in the highest vs lowest quintiles of plasma levels of DHEA-S and androgens had greater risk of developing breast cancer [33, 34].
Conversely, almost all detected glycine- and taurine-conjugated secondary bile acids were elevated post-limonene intervention as was non-conjugated deoxycholate. Taurocholenate sulfate, however, was an exception and was significantly decreased, possibly due to non-specific effects of limonene on sulfation since all detected sulfated adrenal steroids were decreased post-intervention. Bile acids act as emulsifying agents to aid the absorption of dietary fats as well as help eliminate hepatic wastes and cholesterol. They also act as signaling molecules and metabolic regulators through the farnesoid X receptor (FXR) [35]. The FXR is expressed in breast ductal epithelial cells, human breast cancer cell lines [36] and normal breast tissue [37]. Therefore, while the changes observed here most likely reflect liver metabolism, systemic changes in the bile acid profile could also have an effect on breast tissue.
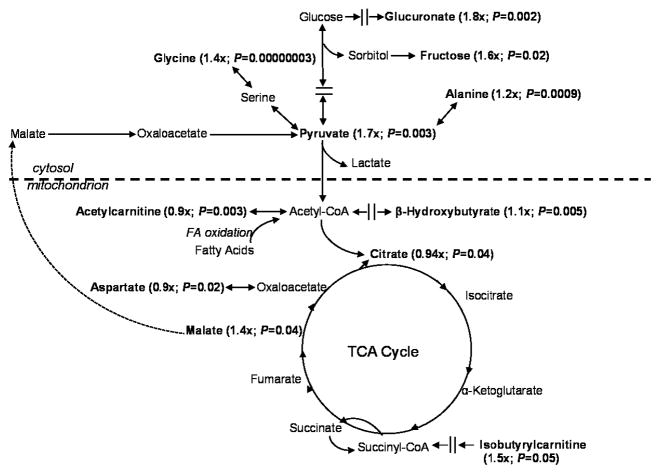
Based on an overall pattern of changes, it appeared that transfer of glucose-derived carbons to the mitochondria was restricted by limonene and/or that carbons were removed from the tricarboxylic acid (TCA) cycle, in the form of malate, to be converted into glucose via the gluconeogenesis pathway (modeled in Figure 1). Pyruvate, a terminal product of glycolysis, was significantly elevated in plasma following limonene intervention, as was fructose of the sorbitol pathway, suggesting restricted entry or usage of glucose-derived carbons by the TCA cycle in the mitochondria. A shift in energy metabolism to preferential use of fatty acid incorporation into the TCA cycle is also supported by a small but significant increase in β-hydroxybutyrate which is increased during oxidation of fatty acids [38]. Acetylcarnitine is used in the transport of fatty acids into the mitochondria [39], and was slightly but significantly decreased. Additionally, isobutyrylcarnitine, which is a product of the acyl-CoA dehydrogenases (a group of mitochondrial enzymes involved in the metabolism of fatty acids or branched-chain amino acids) [40] was increased 1.5-fold. Acetyl-CoA represents a major entry point of carbons derived from the oxidation of glucose, several amino acids, and fatty acids into the TCA cycle and is also a the precursor for cytosolic fatty acid synthesis [39]. Amino acids and peptides also incorporate into energetic pathways at several entry-points [41], specific changes are indicated in the figure.
Figure 1.
Metabolites that changed significantly from pre to post-intervention are in bold with their fold-change and P-value indicated. The pattern of changes supports restricted entry or usage of glucose-derived carbons with preferential incorporation of fatty acids into the TCA cycle.
After oral limonene intervention, there were also elevations in markers indicative of collagen remodeling or degradation, glycine, proline, hydroxyproline, and proline-hydroxyproline [42]. Collagen is a major component of the extracellular matrix that serves as connective tissue that fills the interstitial space between cells [43]. Breast cancer tissues have been reported to have a significant decrease in collagen and an accompanying increase in collagen degradative enzyme activity [44]. Conversely, percent breast density is the strongest known, non-familial breast cancer risk factor, with the dense portion being primarily composed of collagen [45]. It has also been recently proposed that proline acts as a “stress substrate” with increased levels indicating peroxisome proliferator-activated receptor gamma (PPAR-γ) up-regulation and anti-cancer activity linked to increased apoptosis [46]. Therefore, it will be important to determine the tissue source(s) of the collagen breakdown in this study in order to determine whether it represents a protective effect of limonene.
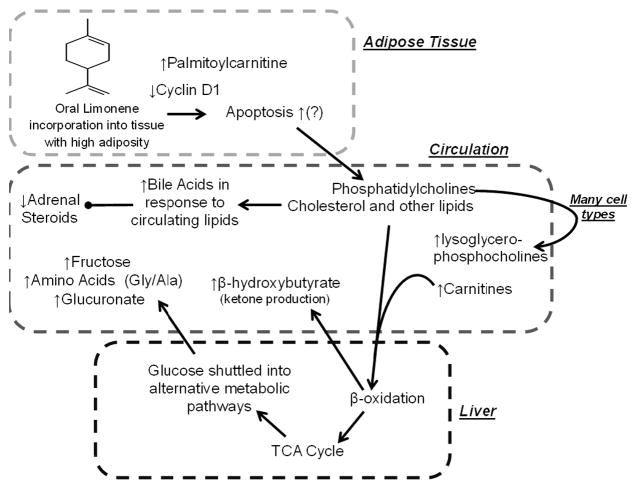
Preclinical studies have shown that limonene causes apoptosis in multiple cell types through indirect mechanisms [47, 48]. While several mechanisms may explain limonene’s concerted effect, increased apoptosis in multiple cell types may potentially explain many of the observed metabolic changes in conjunction with the observed decrease in cyclin D1 in the breast tissue [49]. The hypothesized effect is modeled in Figure 2. Limonene has also been shown to inhibit proliferation through a cyclin D1 dependent mechanism in breast cancer cell lines [50]. We have previously demonstrated that oral limonene deposits in breast tissue [21] and in other tissues with high adiposity [20]. Apoptosis of adipocytes and other cell types would release the main component of cellular membranes, phosphatidylcholines [51] which are metabolized to glycerophosphocholines (lysoGPC) [52] which were significantly increased with limonene intervention. Additionally palmitoylcarnitine increases with apoptosis [53] consistent with our observed effect. Further, changes in plasma levels of eleven lysoGPCs were highly negatively correlated with the change in cyclin D1 expression in tumor tissue (R2 values ranging from 0.42 to 0.63).
Figure 2.
The up and down arrows indicate observed changes with limonene intervention. Bold arrows indicate the hypothesized chain of events; limonene causes apoptosis in multiple cells types and is known to deposit in adipose tissue (top box), this leads to the hypothesized altered metabolism in the liver (bottom box) and may explain the observed changes in metabolites in circulation (middle box). Gly: glycine; Ala: alanine.
Apoptosis of adipocytes (or lipolysis, another limonene effect [54]), would also increase circulating lipids and cholesterol. The observed increase in bile acids could be a response to the increased lipids, given that this is their normal physiological role [55]. An increase in available lipids increases fatty acid oxidation for energy over glucose uptake [56], which is consistent with the observation that post-intervention metabolite profiles indicate glucose shuttling into alternative pathways. Long-chain acylcarnitines, which are intermediates of fatty acid import into the mitochrondria via the carnitine-palmitoyltransferase system [57] and can also be an indication of mitochondrial fatty acid oxidation, were positively correlated with the change of cyclin D1 levels.
While the primary endpoint of the completed clinical trial was disposition of limonene to the breast tissue and plasma [21], here we used metabolomic profiling to generate hypotheses about limonene’s systemic anti-cancer effects. A limitation of this study is that our sample size was too small to relate changes in the metabolome to breast tumor pathology. Another limitation is that assessment of cyclin D1 by IHC is semi-quantitative. While an automated count of positive nuclei via Aperio is considered more quantitative than a pathologist score, the automation is limited by the quality of staining. Also, because this study was not originally designed with metabolomics as a primary endpoint, fasting status and timing of blood draw and surgery were not controlled. Thus, we are unable to relate changes in metabolites to blood or tissue levels of limonene. Further, when the statistical analysis was controlled for fasting status, the observed pre to post-intervention changes remained statistically significant. The concerted change of multiple biochemicals associated with each of these pathways strengthens the confidence in the results. In this study, the limonene treatment duration ranged from 2 to 6 weeks, governed by the surgery schedule. Analysis of the data showed that duration of limonene treatment did not affect the observed metabolite changes (data not shown).
This study supports the hypothesis that limonene’s activity is likely through a general systemic effect rather than through a specific target. The finding that changes in expression of cyclin D1 in the tumor tissue was significantly inversely related to several acylcarnitines, lysoglycerophosphocholines and amino acids suggests that surrogate markers of limonene effect are detectable in plasma. The overall pattern of energy changes in addition to independent markers following limonene treatment are consistent with the range of anti-cancer effects in preclinical models and support further research with limonene in the breast cancer prevention setting that are designed with metabolomic analyses as a primary outcome.
Acknowledgments
Financial Support
J. Miller: National Cancer Institute 2R25 CA078447-11
H. Chow: National Cancer Institute R21CA123033 and Arizona Cancer Center Support Grant P30CA023074
The authors would like to acknowledge Valerie Butler, Bonita Weible, Samantha Castro, Donna Vining, Kathy McDaniel, and Katherine Smith for their excellent assistance in the performance of the clinical study and endpoint assays.
Grant Support
This work was supported by grants from the National Cancer Institute (R21CA123033 and 2R25 CA078447-11) and Arizona Cancer Center Support Grant (5P30CA023074-34).
The abbreviations used
- BTSFA
Bistrimethyl-silyl-triflouroacetamide
- DHEA
Dehydroepiandrosterone
- DHEA-S
Dehydroepiandrosterone sulfate
- DMBA
7,12-dimethylbenz(α)anthracene
- FDR
False discovery rate
- GC
Gas chromatography
- GCP
Glycerophosphocholine
- HMDB
Human metabolome database
- LC
Liquid chromatography
- LTQ
Linear trap quadrupole
- M6P/IGF II
mannose-6-phosphate/insulin-like growth factor II
- MS
Mass spectrometry
- NMU
N-methyl-N-nitrosourea
- PGE2
prostaglandin E2
- PPAR-γ
Peroxisome proliferator-activated receptor gamma
- RSD
relative standard deviation
- TCA
Tricarboxylic acid
- TGFβ
transforming growth factor beta
- UPLC
Ultra performance liquid chromatography
Footnotes
Potential Conflicts of Interest Disclosure: No authors have any potential conflicts of interest to disclose.
References
- 1.Elson CE, Maltzman TH, Boston JL, Tanner MA, Gould MN. Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis. 1988;9:331–332. doi: 10.1093/carcin/9.2.331. [DOI] [PubMed] [Google Scholar]
- 2.Maltzman TH, Hurt LM, Elson CE, Tanner MA, Gould MN. The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis. 1989;10:781–783. doi: 10.1093/carcin/10.4.781. [DOI] [PubMed] [Google Scholar]
- 3.Gould MN, Moore CJ, Zhang R, Wang B, Kennan WS, Haag JD. Limonene chemoprevention of mammary carcinoma induction following direct in situ transfer of v-Ha-ras. Cancer Research. 1994;54:3540–3543. [PubMed] [Google Scholar]
- 4.Elegbede JA, Elson CE, Tanner MA, Qureshi A, Gould MN. Regression of rat primary mammary tumors following dietary d-limonene. Journal of the National Cancer Institute. 1986;76:323–325. [PubMed] [Google Scholar]
- 5.Haag JD, Lindstrom MJ, Gould MN. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992;52:4021–4026. [PubMed] [Google Scholar]
- 6.Miller JA, Thompson PA, Hakim IA, Chow H-HS, Thomson CA. D-Limonene: A Bioactive Food Component in the Mediterranean Diet and Evidence for a Potential Role in Breast Cancer Prevention. Oncology Reviews. 2011;5:31–42. [Google Scholar]
- 7.Maltzman TH, Christou M, Gould MN, Jefcoate CR. Effects of monoterpenoids on in vivo DMBA-DNA adduct formation and on phase I hepatic metabolizing enzymes. Carcinogenesis. 1991;12:2081–2087. doi: 10.1093/carcin/12.11.2081. [DOI] [PubMed] [Google Scholar]
- 8.Ariazi EA, Gould MN. Identifying differential gene expression in monoterpene-treated mammary carcinomas using subtractive display. The Journal of biological chemistry. 1996;271:29286–29294. doi: 10.1074/jbc.271.46.29286. [DOI] [PubMed] [Google Scholar]
- 9.Jirtle RL, Haag JD, Ariazi EA, Gould MN. Increased mannose 6-phosphate/insulin-like growth factor II receptor and transforming growth factor beta 1 levels during monoterpene-induced regression of mammary tumors. Cancer Research. 1993;53:3849–3852. [PubMed] [Google Scholar]
- 10.Yoon WJ, Lee NH, Hyun CG. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J Oleo Sci. 59:415–421. doi: 10.5650/jos.59.415. [DOI] [PubMed] [Google Scholar]
- 11.d’Alessio PA, Ostan R, Bisson JF, Schulzke JD, Ursini MV, Bene MC. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life sciences. 2013;92:1151–1156. doi: 10.1016/j.lfs.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Raphael TJ, Kuttan G. Immunomodulatory activity of naturally occurring monoterpenes carvone, limonene, and perillic acid. Immunopharmacology and immunotoxicology. 2003;25:285–294. doi: 10.1081/iph-120020476. [DOI] [PubMed] [Google Scholar]
- 13.Azzoli CG, Miller VA, Ng KK, Krug LM, Spriggs DR, Tong WP, et al. A phase I trial of perillyl alcohol in patients with advanced solid tumors. Cancer chemotherapy and pharmacology. 2003;51:493–498. doi: 10.1007/s00280-003-0599-7. [DOI] [PubMed] [Google Scholar]
- 14.Hudes GR, Szarka CE, Adams A, Ranganathan S, McCauley RA, Weiner LM, et al. Phase I pharmacokinetic trial of perillyl alcohol (NSC 641066) in patients with refractory solid malignancies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6:3071–3080. [PubMed] [Google Scholar]
- 15.Morgan-Meadows S, Dubey S, Gould M, Tutsch K, Marnocha R, Arzoomanin R, et al. Phase I trial of perillyl alcohol administered four times daily continuously. Cancer chemotherapy and pharmacology. 2003;52:361–366. doi: 10.1007/s00280-003-0684-y. [DOI] [PubMed] [Google Scholar]
- 16.Ripple GH, Gould MN, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, et al. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6:390–396. [PubMed] [Google Scholar]
- 17.Bailey HH, Wilding G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, et al. A phase I trial of perillyl alcohol administered four times daily for 14 days out of 28 days. Cancer chemotherapy and pharmacology. 2004;54:368–376. doi: 10.1007/s00280-004-0788-z. [DOI] [PubMed] [Google Scholar]
- 18.Crowell PL, Kennan WS, Haag JD, Ahmad S, Vedejs E, Gould MN. Chemoprevention of mammary carcinogenesis by hydroxylated derivatives of d-limonene. Carcinogenesis. 1992;13:1261–1264. doi: 10.1093/carcin/13.7.1261. [DOI] [PubMed] [Google Scholar]
- 19.Miller JA, Thompson PA, Hakim IA, Lopez AM, Thomson CA, Chew W, et al. Safety and Feasibility of Topical Application of Limonene as a Massage Oil to the Breast. Journal of cancer therapy. 2012:3. doi: 10.4236/jct.2012.325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JA, Hakim IA, Chew W, Thompson P, Thomson CA, Chow HH. Adipose tissue accumulation of d-limonene with the consumption of a lemonade preparation rich in d-limonene content. Nutrition and cancer. 2010;62:783–788. doi: 10.1080/01635581003693066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JA, Lang JE, Ley M, Nagle R, Hsu CH, Thompson PA, et al. Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev Res (Phila) 2013;6:577–584. doi: 10.1158/1940-6207.CAPR-12-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musgrove EA, Lee CS, Buckley MF, Sutherland RL. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci U S A. 1994;91:8022–8026. doi: 10.1073/pnas.91.17.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alle KM, Henshall SM, Field AS, Sutherland RL. Cyclin D1 protein is overexpressed in hyperplasia and intraductal carcinoma of the breast. Clin Cancer Res. 1998;4:847–854. [PubMed] [Google Scholar]
- 24.Gillett CE, Lee AH, Millis RR, Barnes DM. Cyclin D1 and associated proteins in mammary ductal carcinoma in situ and atypical ductal hyperplasia. J Pathol. 1998;184:396–400. doi: 10.1002/(SICI)1096-9896(199804)184:4<396::AID-PATH1259>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Cardiff RD, Hubbard NE, Engelberg JA, Munn RJ, Miller CH, Walls JE, et al. Quantitation of fixative-induced morphologic and antigenic variation in mouse and human breast cancers. Laboratory investigation; a journal of technical methods and pathology. 2013;93:480–497. doi: 10.1038/labinvest.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical chemistry. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 27.Nieman DC, Shanely RA, Gillitt ND, Pappan KL, Lila MA. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. Journal of proteome research. 2013;12:4577–4584. doi: 10.1021/pr400717j. [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org. [Google Scholar]
- 29.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caspi R, Altman T, Dreher K, Fulcher CA, Subhraveti P, Keseler IM, et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2012;40:D742–753. doi: 10.1093/nar/gkr1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, et al. HMDB 3. 0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Frontiers in neuroendocrinology. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 33.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 34.Tworoger SS, Rosner BA, Willett WC, Hankinson SE. The combined influence of multiple sex and growth hormones on risk of postmenopausal breast cancer: a nested case-control study. Breast Cancer Res. 2011;13:R99. doi: 10.1186/bcr3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer research. 2006;66:10120–10126. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- 37.Journe F, Durbecq V, Chaboteaux C, Rouas G, Laurent G, Nonclercq D, et al. Association between farnesoid X receptor expression and cell proliferation in estrogen receptor-positive luminal-like breast cancer from postmenopausal patients. Breast cancer research and treatment. 2009;115:523–535. doi: 10.1007/s10549-008-0094-2. [DOI] [PubMed] [Google Scholar]
- 38.Bartlett K, Eaton S. Mitochondrial beta-oxidation. European journal of biochemistry/FEBS. 2004;271:462–469. doi: 10.1046/j.1432-1033.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- 39.Marcovina SM, Sirtori C, Peracino A, Gheorghiade M, Borum P, Remuzzi G, et al. Translating the basic knowledge of mitochondrial functions to metabolic therapy: role of L-carnitine. Translational research: the journal of laboratory and clinical medicine. 2013;161:73–84. doi: 10.1016/j.trsl.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mels CM, Jansen van Rensburg P, van der Westhuizen FH, Pretorius PJ, Erasmus E. Increased excretion of c4-carnitine species after a therapeutic acetylsalicylic acid dose: evidence for an inhibitory effect on short-chain fatty acid metabolism. ISRN pharmacology. 2011:851870. doi: 10.5402/2011/851870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reeds PJ, Berthold HK, Boza JJ, Burrin DG, Jahoor F, Jaksic T, et al. Integration of amino acid and carbon intermediary metabolism: studies with uniformly labeled tracers and mass isotopomer analysis. Eur J Pediatr. 1997;156 (Suppl 1):S50–58. doi: 10.1007/pl00014272. [DOI] [PubMed] [Google Scholar]
- 42.Okuyama K. Revisiting the molecular structure of collagen. Connective tissue research. 2008;49:299–310. doi: 10.1080/03008200802325110. [DOI] [PubMed] [Google Scholar]
- 43.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. Journal of cell science. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cechowska-Pasko M, Palka J, Wojtukiewicz MZ. Enhanced prolidase activity and decreased collagen content in breast cancer tissue. Int J Exp Pathol. 2006;87:289–296. doi: 10.1111/j.1365-2613.2006.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 46.Phang JM, Donald SP, Pandhare J, Liu Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino acids. 2008;35:681–690. doi: 10.1007/s00726-008-0063-4. [DOI] [PubMed] [Google Scholar]
- 47.Jia SS, Xi GP, Zhang M, Chen YB, Lei B, Dong XS, et al. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncology reports. 2013;29:349–354. doi: 10.3892/or.2012.2093. [DOI] [PubMed] [Google Scholar]
- 48.Rabi T, Bishayee A. d -Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. Journal of carcinogenesis. 2009;8:9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roue G, Pichereau V, Lincet H, Colomer D, Sola B. Cyclin D1 mediates resistance to apoptosis through upregulation of molecular chaperones and consequent redistribution of cell death regulators. Oncogene. 2008;27:4909–4920. doi: 10.1038/onc.2008.126. [DOI] [PubMed] [Google Scholar]
- 50.Bardon S, Picard K, Martel P. Monoterpenes inhibit cell growth, cell cycle progression, and cyclin D1 gene expression in human breast cancer cell lines. Nutr Cancer. 1998;32:1–7. doi: 10.1080/01635589809514708. [DOI] [PubMed] [Google Scholar]
- 51.Cole LK, Vance JE, Vance DE. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochimica et biophysica acta. 2012;1821:754–761. doi: 10.1016/j.bbalip.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Morash SC, Cook HW, Spence MW. Lysophosphatidylcholine as an intermediate in phosphatidylcholine metabolism and glycerophosphocholine synthesis in cultured cells: an evaluation of the roles of 1-acyl- and 2-acyl-lysophosphatidylcholine. Biochimica et biophysica acta. 1989;1004:221–229. doi: 10.1016/0005-2760(89)90271-3. [DOI] [PubMed] [Google Scholar]
- 53.Wenzel U, Nickel A, Daniel H. Increased mitochondrial palmitoylcarnitine/carnitine countertransport by flavone causes oxidative stress and apoptosis in colon cancer cells. Cellular and molecular life sciences: CMLS. 2005;62:3100–3105. doi: 10.1007/s00018-005-5378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi HS. Lipolytic effects of citrus peel oils and their components. Journal of agricultural and food chemistry. 2006;54:3254–3258. doi: 10.1021/jf052409j. [DOI] [PubMed] [Google Scholar]
- 55.Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes care. 2009;32 (Suppl 2):S237–245. doi: 10.2337/dc09-S355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfe RR. Metabolic interactions between glucose and fatty acids in humans. The American journal of clinical nutrition. 1998;67:519S–526S. doi: 10.1093/ajcn/67.3.519S. [DOI] [PubMed] [Google Scholar]
- 57.Eaton S. Control of mitochondrial beta-oxidation flux. Prog Lipid Res. 2002;41:197–239. doi: 10.1016/s0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]