Abstract
Electroconvulsive therapy (ECT) and magnetic seizure therapy (MST) are conventionally applied with a fixed stimulus current amplitude, which may result in differences in the neural stimulation strength and focality across patients due to interindividual anatomical variability. The objective of this study is to quantify the effect of head anatomical variability associated with age, sex, and individual differences on the induced electric field characteristics in ECT and MST. Six stimulation modalities were modeled including bilateral and right unilateral ECT, focal electrically administered seizure therapy (FEAST), and MST with circular, cap, and double-cone coils. The electric field was computed using the finite element method in a parameterized spherical head model representing the variability in the general population. Head tissue layer thicknesses and conductivities were varied to examine the impact of interindividual anatomical differences on the stimulation strength, depth, and focality. Skull conductivity most strongly affects the ECT electric field, whereas the MST electric field is independent of tissue conductivity variation in this model but is markedly affected by differences in head diameter. Focal ECT electrode configurations such as FEAST is more sensitive to anatomical variability than that of less focal paradigms such as BL ECT. In MST, anatomical variability has stronger influence on the electric field of the cap and circular coils compared to the double-cone coil, possibly due to the more superficial field of the former. The variability of the ECT and MST electric field due to anatomical differences should be considered in the interpretation of existing studies and in efforts to improve dosing approaches for better control of stimulation strength and focality across patients, such as individualization of the current amplitude. The conventional approach to individualizing dosage by titrating the number of pulses cannot compensate for differences in the spatial extent of stimulation that result from anatomical variability.
Keywords: electroconvulsive therapy, magnetic seizure therapy, electric field, strength, focality, FEM, anatomical variability
I. Introduction
Electroconvulsive therapy (ECT), in which seizures are electrically induced under anesthesia, is the most effective treatment for severe major depression [1]. Seizure threshold, defined as the minimum electrical dose necessary to induce an adequate seizure, is used clinically to guide the dosing of ECT. Across ECT studies, there is a marked variability among patients in seizure threshold, with a range of 40-fold in terms of stimulus charge [2]. Factors contributing to this large variability include variations in patient demographics such as sex and age, as well as treatment factors, such as electrode placement, anesthesia type, threshold titration methods, and stimulus parameters including current waveform, pulse width, and frequency [3], [4]. Seizure threshold is conventionally determined by incrementally increasing the duration and/or frequency of the pulse train. During seizure threshold titration as well as during the administration of the treatments, the stimulus pulse trains are administered at a fixed current amplitude (typically 800 or 900 mA). Thus, interindividual anatomical differences in head geometry can lead to different induced electric field distributions in the brain for the same applied pulse amplitude. This could, in turn, result in variations in clinical outcome across patients, even if the stimulus train duration and/or frequency are individualized.
Magnetic seizure therapy (MST), in which seizures are induced using high dose repetitive transcranial magnetic stimulation, offers greater control of the seizure initiation site and a superior side effect profile compared to conventional ECT [5]–[7]. Previously, we showed that the MST electric field is up to 6 times weaker, and up to 60 times more focal compared to conventional ECT [8]. While the stimulus strength in MST is weaker than in ECT, like ECT, the MST stimulus pulse trains are administered with a fixed current amplitude, which could result in variations of the induced electric field strength and focality across patients as a results of anatomical differences.
Some aspects of the effect of anatomical variability on the electric field or seizure threshold have been explored; however a systematic study and comparison of clinically salient ECT and MST paradigms is lacking. The effect of varying cerebrospinal fluid (CSF) thickness and tissue conductivity ratios on the current density distribution in a three-layer spherical model was explored by Stecker, who also provided a closed-form, general solution to the Laplace equation in the three-shell sphere [9]. Stecker concluded that the induced field distribution was only slightly dependent on the conductivity and thickness of the CSF. Yet, Stecker’s spherical model was limited to three-layers: the skull, CSF, and brain. Without a scalp layer the model did not account for current shunting in the scalp. Rath performed parameterizations of tissue layer thicknesses in a four-layer spherical model and arrived at a similar conclusion, namely, the CSF layer thickness has little impact on the induced field distribution [10]. This parameterization was only done for the constant-voltage bilateral ECT configuration, whereas modern ECT uses constant-current devices and is often administered with unilateral or bifrontal electrode configurations. Indeed, a imaging study showed that higher CSF volume predicts higher seizure threshold in both BL and RUL ECT [11]. However, since the seizure threshold was titrated with constant stimulus amplitude, it is unclear to what extent the electric field varied among the patients. Finally, we studied how stimulus current amplitude adjustment can be used to compensate for anatomical variability in BL ECT, RUL ECT, double cone coil MST, and an MST-matched ECT configuration [12]. However, that study did not report electric field variation for conventional stimulation with fixed current amplitude, and did not explore other forms of ECT and MST.
Addressing these questions, in this study we examine how the electric field induced by ECT and MST is affected by variability in head diameter, scalp and skull thickness and conductivity, as well as brain volume. We provide average, lower, and upper estimates for the induced stimulation strength, depth, focality, and scalp shunting in the adult population. These results can help explain seizure threshold variation across convulsive therapy studies, and can also help guide the development of dosing paradigms that are less sensitive to anatomical variation or that compensate for it via individualization procedures with the ultimate goal of leading to better and more consistent clinical outcomes.
II. Methods
We simulated the electric field induced by ECT and MST in a spherical head model using the finite element method packages ElecNet and MagNet 7 (Infolytica Corp., Montreal, Canada), as described in detail in our previous work [8].
A. Parametric Head Model
The human head was modeled as a sphere consisting of five concentric shells: scalp, skull, CSF, gray matter, and white matter (Fig. 1(g)). The spherical model is particularly advantageous for parameterization and perturbation of anatomical features to represent variability in a large population that is difficult to capture in a limited collection of realistic models.
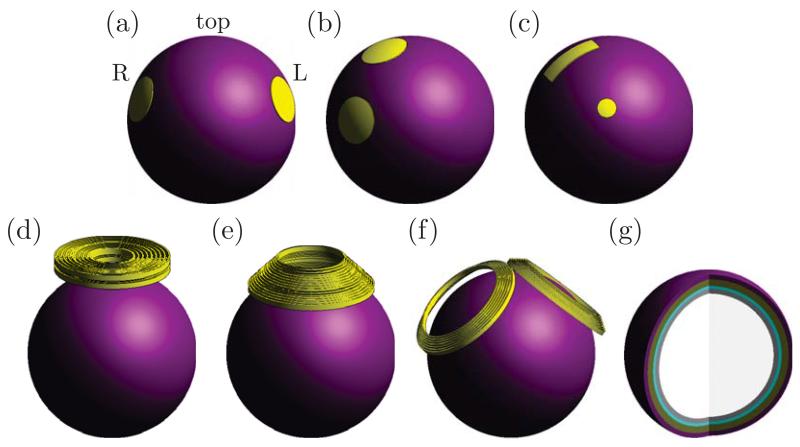
Fig. 1.
Simulation models of ECT electrode and MST coil configurations: (a) bilateral (BL), (b) right unilateral (RUL), and (c) focal electrically administered seizure therapy (FEAST) ECT; and (d) circular coil (CIRC), (e) cap coil (CAP), and (f) double cone coil (DCONE) MST. (g) Interior of five-layer spherical head model. Tissue layers from outer to inner shell: scalp, skull, cerebrospinal fluid, gray matter, and white matter.
Since about 70% of ECT patients are women [13], and depression, the leading reason for referral to ECT, is twice as common in women than men, we used the average adult female head parameters listed in Table I for the nominal head model. The range of head diameter, scalp and skull thickness are based on morphometric studies [14]–[18]. The tissue layers were assigned nominal isotropic conductivities also given in Table I [19]–[24]. From the nominal female head model, we perturbed each tissue layer’s thickness and conductivity to explore how the electric field characteristics are affected by anatomical differences representative of the adult population.
TABLE I.
Nominal head model parameters
| Anatomical parameter | Human |
|
|---|---|---|
| Female | Male | |
| Head diameter (cm) | 17.3 | 17.5 |
| Scalp thickness (mm) | 5.60 | 5.53 |
| Skull thickness (mm) | 7.08 | 6.50 |
| CSF thickness (mm) | 3.00 | 3.00 |
| Gray matter thickness ( mm) | 3.00 | 3.00 |
| White matter thickness (mm) | 67.8 | 69.6 |
| Brain volume (cm3) | 1486.6 | 1602.9 |
| Scalp conductivity (S m−1) | 0.33 | 0.33 |
| Skull conductivity (S m−1) | 0.0083 | 0.0083 |
| CSF conductivity (S m−1) | 1.79 | 1.79 |
| Gray matter conductivity (S m−1) | 0.33 | 0.33 |
| White matter conductivity (S m−1) | 0.14 | 0.14 |
1) Head diameter
The diameter of the nominal head model was based on the weighted mean of adult measurements for head circumference [14], [15]. The upper and lower limits of the perturbation corresponded to two standard deviations above and below the mean head circumference measurements presented in Örmeei et al. [14] and Manjunath et al. [15], respectively. As the head diameter was varied, the white matter diameter was adjusted accordingly, while the thicknesses of all other tissue layers were held constant.
2) Scalp thickness
The scalp thicknesses of the nominal head model was based on the weighted mean of adult measurements [16], [17]. The upper and lower limits of the perturbation were taken from the maximum measurement in Lupin and Gardiner [17] and minimum measurement in Hori et al. [16], respectively.
3) Skull thickness
While some morphometric studies suggest no significant sex difference in cranium thickness [25]–[27], a study by Li et al. based on computed tomography head scans of a large population of living subjects showed women having significantly thicker skulls than men [18]. The skull thicknesses of our nominal head model was based on averaging the frontal, parietal and occipital bone thickness measurements reported in Li et al. [18]. The upper and lower limits of the perturbed model corresponded to the 10th and 90th percentile measurements in that study.
4) Brain volume
Structural MRI estimates of peripheral CSF and brain volume show significantly greater cortical atrophy in elderly men compared to women [28]. The decrease in volume at the frontal, temporal and parieto-occipital lobes can be as large as 15% in elderly men compared to 4% in women [28]. In this study, we examined the effect of shrinking the total brain volume by 5% and by 15%. The skull shell diameter was kept constant and the CSF volume was increased to fill the vacated space between the cranium and the brain.
5) Skull conductivity
The most commonly used gray-matter-to-skull conductivity ratio in the neural source localization literature is 80 [19]. A series of in vivo measurements suggested that this conductivity ratio should be lower [29], [30]. There is no evidence of sex difference in skull conductivity, although the data are too sparse to draw any firm conclusions [29], [31], [32]. In this study, gray-matter-to-skull conductivity ratio of 40 is used for the nominal model [33]. The upper limit of the conductivity ratio is taken to be 80 [19], and the lower limit is taken to be 18.7 [30].
6) Scalp conductivity
The available literature on sex difference in scalp conductivity is even more limited than that for the cranium. Baysal and Haueisen estimated the scalp resistivity in four adult male and five adult female subjects [32]; the small sample showed no systematic difference between men and women. Scalp conductivity would be expected to depend on the composition of its constituent layers. The hypodermis, which makes up about 50% of the scalp cross section, is thicker in women than in men [16]. Since this fat-storing layer has higher impedance than skin and muscle [34], it is conceivable that women may have lower overall scalp conductivity compared to men. To examine the effect of potentially lower scalp conductivity, we simulated a 25% and 50% decrease in scalp conductivity.
B. ECT Electrode and MST Coil Configurations
We simulated three ECT electrode configurations: bilateral (BL), right unilateral (RUL), and focal electrically administered seizure therapy (FEAST); and three MST coil configurations: circular (CIRC), cap (CAP), and double-cone (DCONE). These are illustrated in Fig. 1(a)–(f). BL and RUL ECT are standard electrode placements; FEAST is an investigational electrode configuration designed to initiate focal prefrontal seizures prior to secondary generalization [35]. In our previous study, we showed that in the spherical model the bifrontal electrode placement resulted in similar electric field characteristics as RUL ECT [8]; therefore, the bifrontal configuration is not included in this study. The three MST coil configurations have been used in prior MST studies [5], [36]. Details of electrode and coil geometry, placement, and intensities are presented in our previous study [8].
C. Electric Field Characterization
We quantified electric field penetration by the half-strength depth, d1/2, defined as the radial distance from the cortical surface to the deepest point where the electric field strength E is half of its maximum value on the cortical surface, Emax [37]. To account for different brain radii, Rbrain, the half-strength depth is expressed as a fraction of the brain radius, d1/2/Rbrain. Analogously, we quantified the intrinsic focality of each electrode or coil configuration by the half-strength volume, V1/2, defined as the volume of the brain sphere (gray and white matter) that is exposed to electric field stronger than half of the maximum electric field [8], [37]. To account for different brain volumes, Vbrain, the half-strength volume is expressed as a fraction of the total volume, V1/2/Vbrain. We also characterized the ratio of current induced in the brain to current induced in the scalp, Ibrain/Iscalp. The d1/2/Rbrain, V1/2/Vbrain, and Ibrain/Iscalp metrics are independent of the stimulus current parameters (pulse amplitude, shape, and width) and characterize solely the spatial targeting properties of the electrode or coil configuration.
For a fixed electrode or coil configuration, the stimulation strength and the directly stimulated brain volume are determined by the pulse waveform characteristics, such as pulse amplitude, shape, and width [38]. To assess the degree of direct neural stimulation, the electric field strength could be compared to an approximate neural activation threshold, Eth, which was estimated to be 0.35 V cm−1 for ECT (0.3 ms pulse width), and 0.88 V cm−1, 0.90 V cm−1, and 1.0 V cm−1 for CIRC, CAP, and DCONE MST, respectively, using methods described in our previous study [8]. We characterized the maximum induced electric field in the brain relative to neural activation threshold, Emax/Eth. We also quantified the radial distance from the cortical surface to the deepest point where the electric field strength is still above the neural activation threshold, dA. It is expressed as a fraction of the brain radius, dA/Rbrain. Finally, we quantified the portion of the brain directly activated by ECT and MST by the brain volume that is exposed to suprathreshold electric field, VA [8]. It is expressed as a percentage of the total brain volume, VA/Vbrain.
III. Results
A. Nominal Head Model
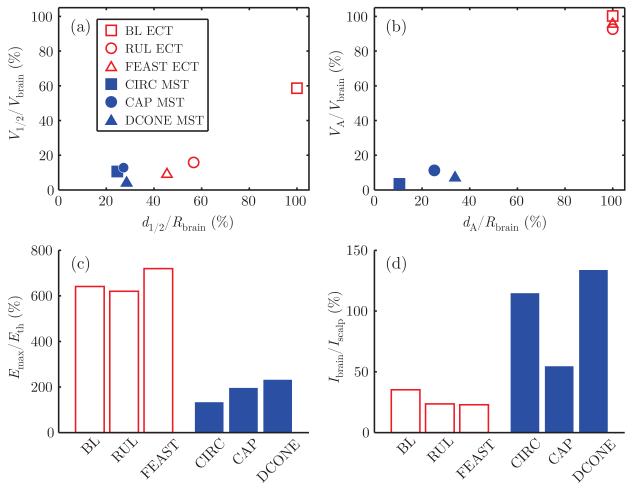
Fig. 2 characterizes the electric field induced by ECT and MST in the nominal female head model. Fig. 2(a) shows the intrinsic focality assessed by the half-strength volume, V1/2/Vbrain, as a function of the half-strength depth, d1/2/Rbrain, for the six stimulation configurations. For the MST configurations, d1/2/Rbrain ranges from 25% to 29% corresponding to more superficial electric field compared to ECT, in which d1/2/Rbrain ranges from 45% to 100%. Of all configurations, BL ECT has the strongest electric field in depth relative to the brain surface. Among the six stimulation modalities, DCONE MST has the most focal electric field (lowest V1/2/Vbrain), whereas BL ECT is the least focal.
Fig. 2.
Electric field characteristics of ECT (empty red markers) and MST (filled blue markers) in the nominal head model: (a) electric field half-strength volume, V1/2/Vbrain, as a function of half-strength depth, d1/2/Rbrain; (b) directly activated brain volume, VA/Vbrain, as a function of depth of activation, dA/Rbrain; (c) maximum electric field relative to neural activation threshold, Emax/Eth; (d) ratio of current induced in the brain to current induced in the scalp, Ibrain/Iscalp. The ECT electrode current is 800 mA and the peak MST coil voltage is 1.65 kV. The assumed Eth is 0.35 V cm−1 (0.3 ms pulse width) for ECT and 0.88 V cm−1, 0.90 V cm−1, and 1.0 V cm−1 for CIRC, CAP, and DCONE MST, respectively.
Fig. 2(b) shows the percentage of brain volume stimulated above threshold, VA/Vbrain, as a function of the stimulation depth, dA/Rbrain. At maximum stimulator output, MST stimulates at depths of 11% to 34% of the brain radius, whereas at 800 mA of electrode current, ECT stimulates at depths reaching the center of the brain (dA/Rbrain = 100%) for all electrode placements. VA/Vbrain is also much higher with ECT than with MST (up to 100% for ECT, and up to only 8.2% for MST). BL ECT produces suprathreshold electric field in 100% of the brain; RUL and FEAST ECT produce suprathreshold electric field in over 90% of the brain.
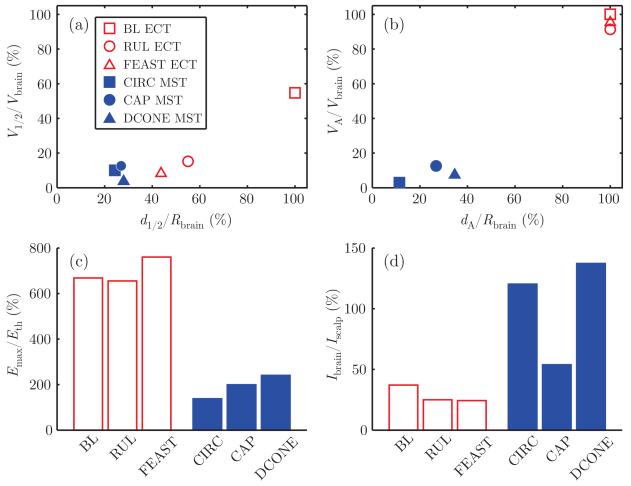
As illustrated in Fig. 2(c), all forms of ECT induced similar stimulation strength in the brain ranging from 620% to 720% of threshold, whereas the values for MST are much lower, ranging from 133% to 232% of threshold. Finally, the ratio of current induced in the brain to current induced in the scalp, Ibrain/Iscalp, is between 23% and 35% for ECT and between 55% and 134% for MST (Fig 2(d)), confirming that magnetic stimulation induces current in the brain with less scalp stimulation compared to electric stimulation. Finally, results for the nominal male head model are very similar to those for the female, and are shown in Fig. 5 in the Appendix.
Fig. 5.
Electric field characteristics of ECT and MST in the male nominal head model: (a) electric field half-strength volume, V1/2/Vbrain, as a function of half-strength depth, d1/2/Rbrain; (b) directly activated brain volume, VA/Vbrain, as a function of depth of activation, dA/Rbrain; (c) maximum electric field relative to neural activation threshold, Emax/Eth; (d) ratio of current induced in the brain to current induced in the scalp, Ibrain/Iscalp.
B. Effect of Anatomical Variation
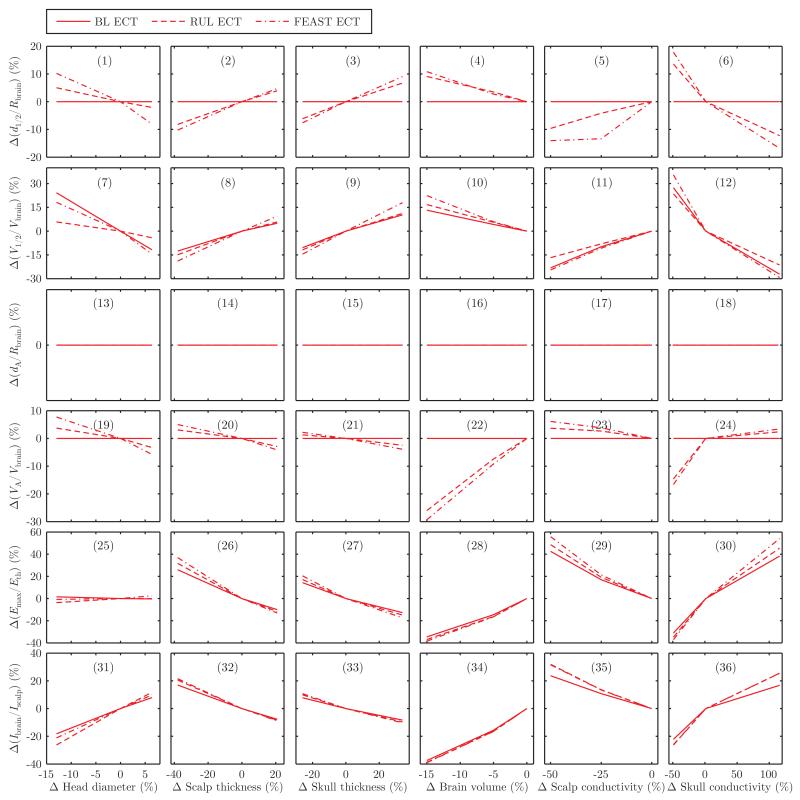
Fig. 3 and 4 show the sensitivity of the electric field characteristics to variations in head tissue layer thickness and conductivity in ECT and MST, respectively.
Fig. 3.
Sensitivity of the ECT electric field characteristics to head tissue thickness and conductivity variations: (1)–(6) change in electric field half-strength depth relative to brain radius, Δ(d1/2/Rbrain); (7)–(12) change in half-strength volume relative to total brain volume, Δ(V1/2/Vbrain); (13)–(18) change in direct activation depth relative to brain radius, Δ(dA/Rbrain); (19)–(24) change in directly activated brain volume relative to total brain volume, Δ(VA/Vbrain); (25)–(30) change in maximum electric field relative to neural activation threshold, Δ(Emax/Eth); (31)–(36) change in current induced in brain relative to current induced in scalp, Δ(Ibrain/Ibrain). The horizontal and vertical axes are both expressed as percentage change from the nominal model. In the nominal model, the ECT electrode current is fixed at 800 mA. For BL ECT, d1/2/Rbrain = 100% and VA/Vbrain = 100% for all anatomical parameters.
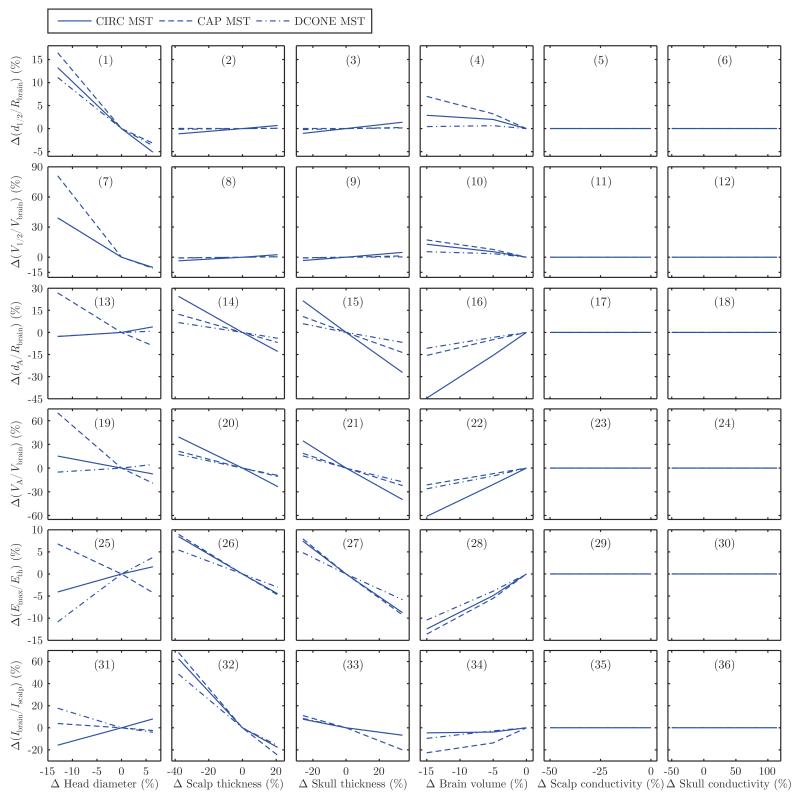
Fig. 4.
Sensitivity of the MST electric field characteristics to head tissue thickness and conductivity variations: (1)–(6) change in electric field half-strength depth relative to brain radius, Δ(d1/2/Rbrain); (7)–(12) change in half-strength volume relative to total brain volume, Δ(V1/2/Vbrain); (13)–(18) change in direct activation depth relative to brain radius, Δ(dA/Rbrain); (19)–(24) change in directly activated brain volume relative to total brain volume, Δ(VA/Vbrain); (25)–(30) change in maximum electric field relative to neural activation threshold, Δ(Emax/Eth); (31)–(36) change in current induced in brain relative to current induced in scalp, Δ(Ibrain/Ibrain). The horizontal and vertical axes are both expressed as percentage change from the nominal model. In the nominal model, the MST coil current is set to the maximum for a Magstim Theta MST device (1.65 kV initial coil voltage).
There are two ways an electric field metric can be insensitive to anatomical variability in our model. In the case of MST, the electric field is independent of the tissue conductivity values in the spherical head model since the electric field is directly induced by the changing magnetic flux and since the electric field is tangentially oriented everywhere and does not cross boundaries between regions with different conductivity. This explains the flat MST sensitivity curves in the last two columns in Fig. 4. In the case of ECT, an electric field metric can be saturated at its maximum value in the nominal model, and the metric remains saturated upon perturbation. For example, d1/2/Rbrain and VA/Vbrain are 100% for BL ECT in the nominal model. Similarly, dA/Rbrain = 100% for all ECT configurations. Therefore, these metrics are insensitive to anatomical variability by virtue of a ceiling effect. However, the Emax/Eth metric has no saturation and reflects the variation of the peak electric field strength with changing anatomy even when the focality and depth metrics saturate. Thus, collectively, the metrics we present capture various aspects of the effect of anatomical differences.
We compare the sensitivity to anatomical variability of ECT and MST configurations by counting the number of panels in Fig. 3 and 4 where the configuration shows the greatest change. Discounting the insensitive cases, FEAST is the ECT configuration most sensitive to variability in all anatomical parameters except head diameter. BL ECT is generally less sensitive to anatomical variability than RUL ECT, except for the V1/2/Vbrain metric in response to changes in head diameter as well as scalp and skull conductivity. The anatomical parameter that contributes the most variability to ECT is skull conductivity, although the percentage directly activated brain volume and the brain to scalp current ratio are most influenced by variation in brain volume. The anatomical parameter that contributes the most variability to MST is head diameter. Finally, the electric field induced in the brain by CAP and CIRC MST is more sensitive to anatomical variability than that of DCONE MST, with the exception of the effect of head diameter on the maximum electric field.
IV. Discussion
A. Comparison of Nominal Field Characteristics
The results for the nominal model are consistent with our previous study [8]; here we highlight the most important findings and discuss new results. MST provides weaker (lower Emax/Eth), more superficial (lower dA/Rbrain), and more focal stimulation (lower VA/Vbrain) than all forms of ECT. DCONE MST has the best intrinsic focality (Fig. 2(a)) and CIRC MST has the smallest stimulated brain volume (Fig. 2(b)). These characteristics of MST may be important for limiting side effects, and are consistent with in vivo data [39]. For all forms of ECT at 800 mA (the clinically used dosage), large portions of the brain (VA/Vbrain = 90%–100%) are stimulated at suprathreshold levels, and the maximum stimulation intensity is exceedingly high relative to neuronal threshold (Emax/Eth up to 720% for FEAST ECT). Importantly, the nonfocality and high intensity of the electric field generated by ECT are largely determined by the high conventional current amplitude and longer effective pulse width (0.3 ms rectangular ECT pulse compared to the cosine MST pulse with briefer stimulating phases). For example, the intrinsic focality of FEAST ECT is comparable to that of MST (see Fig. 2(a)), suggesting that focal ECT electrode configurations at lower electrode currents could provide stimulation focality similar to that of MST, consistent with our previous findings [12]. These observations suggest that using lower current amplitudes and/or briefer pulses would provide stimulation closer to the neural activation threshold, which would improve the focality of ECT and may reduce its side effects [12], [35], [40], [41].
The ECT electrode size and inter-electrode spacing can be manipulated to produce more focal stimulation [12]. However, decreasing the inter-electrode spacing results in more current shunting in the scalp, which could result in scalp burns if the electrode current is not low enough. For instance, Fig. 2(d) shows that the fractional ECT current entering the brain, Ibrain/Iscalp, is smaller for RUL and FEAST compared to BL ECT. Compared to ECT, the use of magnetic induction in MST reduces the relative strength of scalp currents, and therefore could be an advantage for high intensity, focal stimulation.
Finally, ECT can potentially stimulate deeper brain structures than MST, while remaining relatively focal. For example, Fig. 2(a) shows that FEAST ECT has similar intrinsic focality compared with CIRC and CAP MST configurations, while having nearly double the half-strength depth d1/2/Rbrain.
B. Impact of Anatomical Variation
Skull conductivity most strongly affects the ECT electric field characteristics in the brain. Our results for the ECT electric field sensitivity to skull conductivity and thickness are consistent with the numerical simulation of transcranial electric stimulation by Grandori and Rossini who found that the current density changes by 0.3% at the center of the brain and by 1% under the electrode per unit change in skull conductivity [42]. Similarly, our results show that the electric field strength at the brain surface changes by 0.68% per unit change in skull conductivity.
The original motivation for MST development hypothesized that magnetic induction is less sensitive to head tissue inhomogeneities, particularly to skull impedance [43]. Our results confirm that MST is independent of the tissue conductivity values in the spherical head model (see two rightmost columns in Fig. 4). Head diameter is the parameter that contributes most electric field variability in MST. Tissue thickness variation in MST amounts to changing the coil-to-cortex distance, which, in turn, leads to variation in the induced electric field magnitude. Head size variation significantly affects the coupling between the head and the stimulation coil, subsequently affecting the electric field distribution [44]. The high focality (small directly activated brain volume) in the MST nominal model also contributes to high sensitivity, since a small change in the absolute VA/Vbrain or dA/Rbrain results in a large percentage relative change.
In ECT, there appears to be a relationship between the focality of the electrode configuration and the sensitivity to anatomical variation. The configurations that are more focal—RUL and FEAST—are also more sensitive to anatomical differences than the less focal BL electrode placement, with FEAST having both the highest intrinsic focality and the most electric field variation. On the other hand, for MST there may be a relationship between the depth of penetration of the electric field and the anatomical sensitivity. The DCONE coil induces the most penetrating electric field (largest d1/2/Rbrain and dA/Rbrain) and is least sensitive to anatomical variation. This is consistent with the understanding that anatomical variation in MST amounts chiefly to changing the effective coil-to-cortex distance. Hence, coils with deeper electric field penetration, like DCONE, would be less sensitive to coil-to cortex distance variations compared to more superficial coils like CAP and CIRC.
The sensitivities of the ECT and MST electric field characteristics were expressed as percent deviations from the nominal model. The clinical significance of these deviations would depend, in part, on the nominal field values. Since the ECT nominal field strength and stimulated brain volume and depth are drastically greater than those of MST, sensitivities of their electric field characteristics cannot be directly compared. However, we have previously shown that when the focality of ECT is matched to that of MST by altering the electrode size, spacing, and current amplitude, ECT becomes more sensitive to variations of most anatomical except for head size [12].
C. Sex-Related Effects
Sex is a predictor of seizure threshold in ECT [45]. Sackeim et al. attributed the lower seizure threshold in women to their thinner skull thickness resulting in less current shunting [2]. This argument was based on skull thickness measurements in children (age 6 to 16) [46]. One study in adults has found the skull to be thicker in women than in men [18], resulting in lower electric field strength in the female brain.
It has been asserted that the sex difference in scalp composition could also contribute to the seizure threshold variation, though no supporting evidence was cited [2]. Women have thicker hypodermis than men [16], resulting in potentially lower scalp conductivity in women. This would lead to less current shunting during ECT, stronger stimulation strength and larger stimulated brain volume, and, consequently, lower seizure threshold. Our simulation data support the significance of the contribution of scalp conductivity: a 25% and 50% decrease in the scalp conductivity results respectively in 20% and 50% increase in maximum electric field strength in RUL ECT. The effect of scalp conductivity on the relative stimulated brain volume is curbed since this metric is at or close to 100%.
D. Age-Related Effects
After the age of 18, the cranium does not show significant increase in thickness with age [18], [47]. Therefore, we do not expect a large age-dependent effect on the induced electric field from the growth in skull thickness in adults. The hypodermis thickness does not undergo significant change with aging in men, whereas hypodermis thickness in women correlates positively with estrogen level during the lifetime [16], [48]. In addition to structural changes that accompany hormonal fluctuations, estrogen is also known to increase the number of hippocampal excitatory neuron synapses and increase seizure susceptibility in female epileptic patients—factors that could also affect seizure threshold [49].
Brain atrophy is a large source of variability in ECT focality. A 5% and 15% decrease in brain volume led to a 7% and 26% decrease in the stimulated brain volume and a 16% and 37% decrease in the maximum electric field in RUL ECT, respectively. This effect is consistent with the repeated observation that seizure threshold increases with age [3], [4], [50]. Atrophy affects MST as well. A 5% and 15% decrease in brain volume led to a 20% and 60% decrease in the stimulated brain volume and a 5% and 12% decrease in the maximum electric field, respectively. This is consistent with the observed increase in TMS motor threshold with age [51], although neural excitability could also be a contributing factor.
E. Implications for ECT and MST Technique
The sensitivity of the electric field characteristics to anatomical differences could result in undesirable interindividual variability in clinical outcomes. Van Waarde et al. demonstrated that CSF volume strongly predicted initial seizure threshold in both RUL and BL ECT [11]. However, since in conventional ECT and MST seizure threshold titration the current amplitude and pulse width are fixed for all patients, individuals with thicker CSF layer would have weaker and more focal electric field in the brain. That will remain the case even as the other pulse train parameters are titrated up. While titration in the train frequency and duration domains addresses the dynamic response of the brain, it fails to account for the anatomydependent spatial extent of stimulation which is driven by pulse amplitude and width. Thus, with the conventional dose titration, the variability of the electric field distribution due to anatomical variations is not compensated directly, but rather indirectly by adjusting the number of times the same spatial electric field is pulsed. This indirect compensation relies on the existence of a “strength-duration” relationship between current amplitude and train duration for seizure induction [52]. Therefore, in patients with less current reaching the brain, the number of pulses (and hence the seizure threshold in units of charge) is increased, because a weaker and more focal electric field requires more repetition to trigger a seizure.
To compensate directly for the variability in the electric field, pulse amplitude could be individualized [12], [53], for example by current amplitude titration of the seizure threshold [35], [40], [52], by patient-specific electric field simulation [54], or by setting the amplitude relative to the patient’s motor threshold [40] as is routinely done in clinical rTMS. The stimulus pulse parameters affect dA/Rbrain, VA/Vbrain, and Emax/Eth which could be normalized across patients by stimulus current adjustment [12]. On the other hand, d1/2/Rbrain, V1/2/Vbrain, and Ibrain/Iscalp depend only on the head anatomy and the ECT electrode or MST coil configuration, and are independent of the pulse waveform parameters. These metrics cannot be compensated by pulse amplitude titration.
While the exact relationship between the induced electric field and the pattern of seizure initiation and propagation has not been determined, there are some evidence that focal stimulation can trigger focal onsets of seizure activity. A study using single photon emission computed tomography (SPECT) in a depressed patient undergoing FEAST showed early ictal increases in regional cerebral blood flow confined to the right prefrontal cortex [55]. Since the site and focality of stimulation are related to those of seizure initiation, we conjecture that the variation in the induced electric field characteristics will likely contribute to variability in imaging results among individuals.
F. Limitations
Future work on the effect of anatomical variability could use more anatomically accurate head models and could analyze the electric field in specific brain regions, as we have demonstrated in an ECT modeling study [54]. However, this approach will require a large number of individual models to capture the anatomical variability of the ECT and MST clinical populations. Potential limitations of both spherical and anatomically realistic models include uncertainty about the various tissue conductivity values as well as errors in the tissue segmentation in realistic models or uncertainty in the assumptions of the shells’ thickness in the spherical model.
The skull has a multi-layer structure; however, boundaries between the spongiosa and the compacta layers of the skull are often difficult to determine on MRI scans [23], [56]. Further, in a spherical head model, an isotropic single-layer skull adequately approximates the multi-layer skull [57]. Therefore, we did not model the skull layers. The skull thickness variation in this study is based on the CT scan data by Li et al. [18] collected is a single city in China that may not be representative of skull geometric variation for other populations. Further, when varying the skull thickness in our models, we kept the gray matter-to-skull conductivity ratio constant at 40, an assumption that may not be accurate. Tang et al. found a negative correlation between skull resistivity and thickness [58]. This correlation may be included in future simulation studies. Finally, the head diameter estimates were also obtained from non-caucasian populations, although average interracial differences in head geometry should be covered by the range of perturbation in our model.
Finally, this model focuses exclusively on biophysical properties of the head and how their variation affects the induced electric field. Not modeled here, but important for physiological response, is neuronal excitability which is also influenced by drivers of anatomical variation such as age and sex. Nevertheless, we showed in this study that the sensitivity of the electric field characteristics to head anatomical differences is already relatively large.
V. Conclusions
Consistent with our previous findings, at conventional stimulus current strengths, ECT produces peak electric field far in excess of neural activation threshold, and exposes nearly 100% of the brain volume to suprathreshold field strengths. In comparison, MST induces much weaker, more superficial, and more focal electric field. For ECT, the degree to which neural activation threshold is exceeded is most sensitive to variation in skull conductivity. MST is unaffected by tissue conductivity; rather, the head diameter is the greatest contributor of variability. Focal ECT configurations such as FEAST are more sensitive to anatomical variability compared to unfocal configurations such as BL ECT. In MST, the CAP and CIRC coil configurations are more sensitive to anatomical variability than the DCONE configuration, possibly due to the more superficial electric field of the former. Higher sensitivity of the electric field characteristics to anatomical differences could potentially effect wider variation in clinical outcomes. The sensitivity to anatomical variability is significant not only for interindividual differences, but also for within-subject differences in the tissue layer thickness and conductivity in various parts of the head, as well as differences due to time-variant state of the tissues, such as the presence of perspiration in the scalp. These results are useful in interpreting differences in seizure threshold across patients and stimulation modalities. The sensitivity of the electric field characteristics to anatomical variation in both ECT and MST motivate the development of improved methods for dose individualization, such as current amplitude individualization, with the ultimate goal of better and consistent clinical outcomes. Finally, the analysis frame-work and the anatomical parameter ranges provided in this paper could be useful for anatomical variability analysis of other transcranial electric and magnetic stimulation modalities as well as of electroencephalography.
Acknowledgments
This work was supported in part by NIH grants 5TL1RR024158-03, R01MH60884, and R01MH091083. Dr. Deng is inventor on patent applications on TMS/MST technology assigned to Columbia and Duke. Dr. Lisanby has served as Principal Investigator on industry-sponsored research grants to Columbia/RFMH or Duke (Neuronetics (past), Brainsway, ANS/St. Jude Medical, Cyberonics (past), NeoSync); equipment loans to Columbia or Duke (Magstim, MagVenture); is co-inventor on a patent application on TMS/MST technology; is supported by grants from NIH (R01MH091083-01, 5U01MH084241-02, 5R01MH060884-09), Stanley Medical Research Institute, Brain and Behavior Research Foundation, and the Wallace H. Coulter Foundation; and has no consultancies, speakers bureau memberships, board affiliations, or equity holdings in related device industries. Dr. Peterchev is inventor on patents and patent applications on TMS technology assigned to Columbia and Duke, including technology licensed to Rogue Research; was Principal Investigator on a research grant to Duke from Rogue Research and equipment donations to Columbia and Duke by Magstim and MagVenture; and has received patent royalties and travel support from Rogue Research through Columbia and Duke for TMS technology.
Appendix
Electric field characteristics of ECT and MST in the male nominal head model are plotted in Fig. 5.
Contributor Information
Zhi-De Deng, Department of Psychiatry and Behavioral Sciences, Duke University, Durham, NC 27710, USA.
Sarah H. Lisanby, Departments of Psychiatry and Behavioral Sciences, and Neuroscience, Duke University, Durham, NC, 27710 USA (sarah.lisanby@duke.edu)
Angel V. Peterchev, Departments of Psychiatry and Behavioral Sciences, Biomedical Engineering, and Electrical and Computer Engineering, Duke University, Durham, NC 27710, USA (angel.peterchev@duke.edu).
References
- [1].Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357(19):1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- [2].Sackeim HA, Long J, Luber B, Moeller JR, Prohovnik I, Devanand DP, Nobler MS. Physical properties and quantification of the ECT stimulus: I. Basic principles. Convuls Ther. 1994;10(2):93–123. [PubMed] [Google Scholar]
- [3].Boylan LS, Haskett RF, Mulsant BH, Greenberg RM, Prudic J, Spicknall KE, Lisanby SH, Sackeim HA. Determinants of seizure threshold in ECT: benzodiazepine use, anesthetic dosage, and other factors. J ECT. 2000;16(1):3–18. doi: 10.1097/00124509-200003000-00002. [DOI] [PubMed] [Google Scholar]
- [4].van Waarde JA, van Oudheusden LJ, Verwey B, Giltay EJ, van der Mast RC. Clinical predictors of seizure threshold in electroconvulsive therapy: a prospective study. Eur Arch Psychiatry Clin Neurosci. 2013;263(2):167–175. doi: 10.1007/s00406-012-0342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28(10):1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- [6].Kayser S, Bewernick BH, Grubert C, Hadrysiewicz BL, Ax-macher N, Schlaepfer TE. Antidepressant effects, of magnetic seizure therapy and electroconvulsive therapy, in treatment-resistant depression. J Psychiatr Res. 2011;45(5):569–576. doi: 10.1016/j.jpsychires.2010.09.008. [DOI] [PubMed] [Google Scholar]
- [7].Fitzgerald PB, Hoy KE, Herring SE, Clinton AM, Downey G, Daskalakis ZJ. Pilot study of the clinical and cognitive effects of high-frequency magnetic seizure therapy in major depressive disorder. Depress Anxiety. 2013;30:129–136. doi: 10.1002/da.22005. [DOI] [PubMed] [Google Scholar]
- [8].Deng Z-D, Lisanby SH, Peterchev AV. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J Neural Eng. 2011;8(1):016007. doi: 10.1088/1741-2560/8/1/016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stecker MM. Transcranial electric stimulation of motor pathways: a theoretical analysis. Comput Biol Med. 2005;35(2):133–155. doi: 10.1016/j.compbiomed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [10].Rath WT. Master’s thesis. University of Pittsburgh, Department of Bioengineering; 2006. Modeling of transcranial electrical stimulation by finite element analysis. [Google Scholar]
- [11].van Waarde JA, van Oudheusden LJB, Tonino BAR, van der Wee NJA, Verwey B, van der Mast RC, Giltay EJ. MRI characteristics predicting seizure threshold in patients undergoing electroconvulsive therapy: a prospective study. Brain Stimul. 2013;6(4):607–614. doi: 10.1016/j.brs.2012.12.003. [DOI] [PubMed] [Google Scholar]
- [12].Deng Z-D, Lisanby SH, Peterchev AV. Controlling stimulation strength and focality in electroconvulsive therapy via current amplitude and electrode size and spacing: comparison with magnetic seizure therapy. J ECT. 2013;29(4):325–335. doi: 10.1097/YCT.10.1097/YCT.0b013e3182a4b4a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rudorfer MV, Henry ME, Sackeim HA. Psychiatry, chapter Electroconvulsive therapy. John Wiley & Sons Ltd; Chichester: 2003. pp. 1865–1901. [Google Scholar]
- [14].Örmeei AR, Gürbüz H, Ayata A, Çetin H. Adult head circumferences and centiles. J Turgut Özal Med Cent. 1997;4(3):261–264. [Google Scholar]
- [15].Manjunath KY. Estimation of cranial volume in dissecting room cadavers. J Anat Soc India. 2002;51(2):168–172. [Google Scholar]
- [16].Hori H, Moretti G, Rebora A, Crovato F. The thickness of human scalp: normal and bald. J Invest Dermatol. 1972;58(6):396–399. doi: 10.1111/1523-1747.ep12540633. [DOI] [PubMed] [Google Scholar]
- [17].Lupin AJ, Gardiner RJ. Scalp thickness in the temporal region: its relevance to the development of cochlear implants. Cochlear Implants Int. 2001;2(1):30–38. doi: 10.1179/cim.2001.2.1.30. [DOI] [PubMed] [Google Scholar]
- [18].Li H, Ruan J, Xie Z, Wang H, Liu W. Investigation of the critical geometric characteristics of living human skulls utilising medical image analysis techniques. Int J Vehicle Safety. 2007;2(4):345–367. [Google Scholar]
- [19].Rush S, Driscoll DA. Current distribution in the brain from surface electrodes. Anesth Analg. 1968;47(6):717–723. [PubMed] [Google Scholar]
- [20].Baumann SB, Wozny DR, Kelly SK, Meno FM. The electrical conductivity of human cerebrospinal fluid at body temperature. IEEE Trans Biomed Eng. 1997;44(3):220–223. doi: 10.1109/10.554770. [DOI] [PubMed] [Google Scholar]
- [21].Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41(11):2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- [22].Geddes LA, Baker LE. The specific resistance of biological material—a compendium of data for the biomedical engineer and physiologist. Med Biol Eng Comput. 1967;5(3):271–293. doi: 10.1007/BF02474537. [DOI] [PubMed] [Google Scholar]
- [23].Wolters CH, Anwander A, Tricoche X, Weinstein D, Koch MA, MacLeod RS. Influence of tissue conductivity anisotropy on EEG/MEG field and return current computation in a realistic head model: a simulation and visualization study using high-resolution finite element modeling. Neuroimage. 2006;30(3):813–826. doi: 10.1016/j.neuroimage.2005.10.014. [DOI] [PubMed] [Google Scholar]
- [24].Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron. 2007;55(5):809–823. doi: 10.1016/j.neuron.2007.07.027. [DOI] [PubMed] [Google Scholar]
- [25].Ross AH, Jantz RL, McCormick WF. Cranial thickness in American females and males. J Forensic Sci. 1998;43(2):267–272. [PubMed] [Google Scholar]
- [26].Smith P, Wax Y, Becker A, Einy S. Diachronic variation in cranial thickness of Near Eastern populations. Am J Phys Anthropol. 1985;67(2):127–133. doi: 10.1002/ajpa.1330670208. [DOI] [PubMed] [Google Scholar]
- [27].Buchsbaum MS, Henkin RI, Christiansen RL. Age and sex differences in averaged evoked responses in a normal population, with observations on patients with gonadal dysgenesis. Electroencephalogr Clin Neurophysiol. 1974;37(2):137–144. doi: 10.1016/0013-4694(74)90004-2. [DOI] [PubMed] [Google Scholar]
- [28].Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55(2):169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- [29].Oostendorp TF, Delbeke J, Stegeman DF. The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans Biomed Eng. 2000;47(10):1487–1491. doi: 10.1109/TBME.2000.880100. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Y, van Drongelen W, He B. Estimation of in vivo brain-to-skull conductivity ratio in human. Appl Phys Lett. 2006;89(22):223903–2239033. doi: 10.1063/1.2398883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lai Y, van Drongelen W, Ding L, Hecox HE, Towle VL, Frim DM, He B. Estimation of in vivo human brain-to-skull conductivity ratio by means of cortical potential imaging. Clin Neurophysiol. 2005;116(2):456–465. doi: 10.1016/j.clinph.2004.08.017. [DOI] [PubMed] [Google Scholar]
- [32].Baysal U, Hauseisen J. Use of a priori information in estimating tissue resistivities—application to human data in vivo. Physiol Meas. 2004;25(3):737–748. doi: 10.1088/0967-3334/25/3/013. [DOI] [PubMed] [Google Scholar]
- [33].Gonçalve SI, de Munck JC, Verbunt JPA, Bijma F, Heethaar RM, da Silva FHL. In vivo measurement of the brain and skull resistivities using an EIT-based method and realistic models for the head. IEEE Trans Biomed Eng. 2003;50(6):754–767. doi: 10.1109/tbme.2003.812164. [DOI] [PubMed] [Google Scholar]
- [34].Zhao X, Kinouchi Y, Yasuno E, Gao D, Iritani T, Morimoto T, Takeuchi M. A new method for noninvasive measurement of multilayer tissue conductivity and structure using divided electrodes. IEEE Trans Biomed Eng. 2004;51(2):362–370. doi: 10.1109/TBME.2003.820403. [DOI] [PubMed] [Google Scholar]
- [35].Nahas Z, Short B, Burns C, Archer M, Schmidt M, Prudic J, Nobler MS, Devanand DP, Fitzsimons L, Lisanby SH, Payne N, Perera T, George MS, Sackeim HA. A feasibility study of a new method for electrically producing seizures in man: focal electrically administered seizure therapy [FEAST] Brain Stimul. 2013;6(3):403–408. doi: 10.1016/j.brs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- [36].White PF, Amos Q, Zhang Y, Stool L, Husain MM, Thornton L, Downing M, McClintock S, Lisanby SH. Anesthetic considerations for magnetic seizure therapy: a novel therapy for severe depression. Anesth Analg. 2006;103(1):76–80. doi: 10.1213/01.ane.0000221182.71648.a3. [DOI] [PubMed] [Google Scholar]
- [37].Deng Z-D, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peterchev AV, Wagner TA, Miranda PC, Nitsche MA, Paulus W, Lisanby SH, Pascual-Leone A, Bikson M. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5(4):435–453. doi: 10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lisanby SH, Moscrip TD, Morales O, Luber B, Schroeder C, Sackeim HA. Neurophysiological characterization of magnetic seizure therapy (MST) in non-human primates. Clin Neurophysiol Suppl. 2003;56:81–99. doi: 10.1016/s1567-424x(09)70212-0. [DOI] [PubMed] [Google Scholar]
- [40].Peterchev AV, Chan B, Lisanby SH. Pulse amplitude adjustment: a novel means of individualizing and predicting dosage requirements for electroconvulsive therapy and magnetic seizure therapy. J ECT. 2010;26:154–155. [Google Scholar]
- [41].Rosa MA, Abdo GL, Lisanby SH, Peterchev AV. Seizure induction with low-amplitude-current (0.5 A) electroconvulsive therapy. J ECT. 2011;27:341–342. doi: 10.1097/YCT.0b013e31822149db. [DOI] [PubMed] [Google Scholar]
- [42].Grandori F, Rossini P. Electrical stimulation of the motor cortex: theoretical considerations. Ann Biomed Eng. 1988;16(6):639–652. doi: 10.1007/BF02368019. [DOI] [PubMed] [Google Scholar]
- [43].Sackeim HA. Magnetic stimulation therapy and ECT. Convuls Ther. 1994;10(4):255–258. [Google Scholar]
- [44].Weissman JD, Epstein CM, Davey KR. Magnetic brain stimulation and brain size: relevance to animal studies. Electroenceph Clin Neurophysiol. 1992;85(3):215–219. doi: 10.1016/0168-5597(92)90135-x. [DOI] [PubMed] [Google Scholar]
- [45].Sackeim HA, Decina P, Prohvnik I, Malitz S. Seizure threshold in electroconvulsive therapy: effects of sex, age, electrode placement, and number of treatments. Arch Gen Psychiatry. 1987;44(4):355–360. doi: 10.1001/archpsyc.1987.01800160067009. [DOI] [PubMed] [Google Scholar]
- [46].Riolo ML, Moyers RE, McNamara JAJ, Hunter WS. An Atlas of Craniofacial Growth. University of Michigan, Ann Arbor; 1974. [Google Scholar]
- [47].Todd TW. Thickness of the male white cranium. Anat Rec. 1924;27(5):245–256. [Google Scholar]
- [48].Pedersen-Bjergaard K, Tonnesen M. Oestrogenic, androgenic and gonadotrophic substances in the urine of normal women; sex hormone analyses. Acta Endocrinol. 1948;1(1):38–60. doi: 10.1530/acta.0.0010038. [DOI] [PubMed] [Google Scholar]
- [49].Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmocol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- [50].Coffey CE, Lucke J, Weiner RD, Krystal AD, Aque M. Seizure threshold in electroconvulsive therapy: I. Initial seizure thresh-old. Biol Psychiatry. 1995;37(10):713–720. doi: 10.1016/0006-3223(95)00262-F. [DOI] [PubMed] [Google Scholar]
- [51].Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, Risch SC, George MS. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12(3):376–384. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- [52].Liberson WT. Brief stimulus therapy—physiological and clinical observations. Am J Psychiatry. 1948;105(1):28–39. doi: 10.1176/ajp.105.1.28. [DOI] [PubMed] [Google Scholar]
- [53].Peterchev AV, Rosa MA, Deng Z-D, Prudic J, Lisanby SH. Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT. 2010;26(3):159–174. doi: 10.1097/YCT.0b013e3181e48165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lee WH, Deng Z-D, Kim T-S, Laine AF, Lisanby SH, Peterchev AV. Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: influence of white matter anisotropic conductivity. Neuroimage. 2012;59(3):2045–2048. doi: 10.1016/j.neuroimage.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chahine G, Short B, Spicer K, Schmidt M, Burns C, Atoui M, George MS, Sackeim HA, Nahas Z. Regional cerebral blood flow changes associated with focal electrically administered seizure therapy (FEAST) Brain Stimul. 2014;7(3):483–485. doi: 10.1016/j.brs.2014.02.011. [DOI] [PubMed] [Google Scholar]
- [56].Sadleir RJ, Argibay A. Modeling skull electrical properties. Ann Biomed Eng. 2007;35(10):1699–1712. doi: 10.1007/s10439-007-9343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rampersad S, Stegeman D, Oostendorp T. Single-layer skull approximations perform well in transcranial direct current stimulation modeling. IEEE Trans Neural Syst Rehabil Eng. 2013;21(3):346–53. doi: 10.1109/TNSRE.2012.2206829. [DOI] [PubMed] [Google Scholar]
- [58].Tang C, You F, Cheng G, Gao D, Fu F, Yang G, Dong X. Correlation between structure and resistivity variation of the live human skull. IEEE Trans Biomed Eng. 2008;55(9):2286–2292. doi: 10.1109/TBME.2008.923919. [DOI] [PubMed] [Google Scholar]