Abstract
Atopic dermatitis and psoriasis are the two most common immune-mediated inflammatory disorders affecting the skin. Genome-wide studies demonstrate a high degree of genetic overlap, but these diseases have mutually exclusive clinical phenotypes and opposing immune mechanisms. Despite their prevalence, atopic dermatitis and psoriasis very rarely co-occur within one individual. By utilizing genome-wide association study and ImmunoChip data from >19,000 individuals and methodologies developed from meta-analysis, we have identified opposing risk alleles at shared loci as well as independent disease-specific loci within the epidermal differentiation complex (chromosome 1q21.3), the Th2 locus control region (chromosome 5q31.1), and the major histocompatibility complex (chromosome 6p21–22). We further identified previously unreported pleiotropic alleles with opposing effects on atopic dermatitis and psoriasis risk in PRKRA and ANXA6/TNIP1. In contrast, there was no evidence for shared loci with effects operating in the same direction on both diseases. Our results show that atopic dermatitis and psoriasis have distinct genetic mechanisms with opposing effects in shared pathways influencing epidermal differentiation and immune response. The statistical analysis methods developed in the conduct of this study have produced additional insight from previously published data sets. The approach is likely to be applicable to the investigation of the genetic basis of other complex traits with overlapping and distinct clinical features.
Introduction
Atopic dermatitis (AD, synonymous with eczema [MIM 603165]) and psoriasis (psoriasis vulgaris [MIM 177900]) are the two most common chronic inflammatory skin conditions. They are associated with a significantly reduced quality of life and multiple comorbidities.1,2 Both diseases result from the interaction of genetic and environmental factors and are characterized by epidermal defects as well as local and systemic immunological abnormalities. Despite a lifetime prevalence of ∼2% for psoriasis and 10%–20% for AD,3,4 these diseases rarely co-occur within an individual5—an observation attributed to opposing immune response patterns.6 However, it has been reported that both Th1-cell-dominated autoimmune and Th2-cell-dominated allergic diseases aggregate within families7 and that parental psoriasis might increase the risk of AD in offspring.8 Furthermore, genome-wide linkage and association studies have shown genetic risk loci in each disease that map to similar regions of the genome. The epidermal differentiation complex (EDC) on chromosome 1q21.3 includes AD and psoriasis risk loci in close proximity.9–12 Null mutations in the gene encoding filaggrin (FLG [MIM 135940]) represent the strongest known risk factor for AD13,14 and account for at least a proportion of AD risk within the EDC, but FLG-null mutations are not associated with psoriasis.15,16 A deletion of the late cornified envelope genes LCE3B-LCE3C (MIM 612614, 612615) represents a genetic substrate for psoriasis within the EDC,17,18 but this deletion is not associated with AD.19 The cytokine cluster encoded at 5q23.1–5q31.1 includes variants showing association with both diseases,10,20,21 and an intergenic region of chromosome 20q13.2 has also shown association with both AD and psoriasis.22,23 Finally, a recent genome-wide association study (GWAS) on AD identified a strong association within the margins of the major histocompatibility complex (MHC)20 on chromosome 6p21.3, less than 2.4 kb from a variant associated with HLA-Cw6 (MIM 142840),24 the strongest known psoriasis-risk locus.
In order to gain insight into overlapping and specific genetic mechanisms, we systematically compared and contrasted AD and psoriasis via analytical techniques developed from meta-analysis.
Subjects and Methods
Study Subjects
Genome-wide genotype data were obtained on samples from six case-control cohorts (three each of AD and psoriasis), totaling 2,262 AD and 4,489 psoriasis case subjects and 12,333 control subjects (Table S1 available online).
The German AD case subjects were recruited from tertiary dermatology clinics at Munich, as part of the GENEVA study, University of Kiel, University of Bonn, and the University Children’s Hospital of Charité Universitätsmedizin Berlin. AD was diagnosed by experienced dermatologists and/or pediatricians according to the UK Diagnostic Criteria.25 German control subjects were obtained from the PopGen biorepository,26 the population-based KORA study in southern Germany,27 and the German part of ISAAC II to assess the prevalence of asthma and allergies in schoolchildren.28 The Irish AD case collection was recruited from the secondary and tertiary pediatric dermatology clinic at Our Lady’s Children’s Hospital, Crumlin, Dublin. Irish control individuals were obtained from healthy adult blood donors as part of the Trinity Biobank, Dublin.29
The German psoriasis case subjects were recruited from the tertiary dermatology clinic at the University of Kiel and German controls were again obtained from the PopGen biorepository and the KORA study (independent from those used as controls for AD). The British psoriasis case-control study is part of the Welcome Trust Case Control Consortium 224 and the US psoriasis study has been described elsewhere.21
ImmunoChip data on 2,425 AD case subjects, 3,580 psoriasis case subjects, and 9,061 control subjects were obtained from previous studies,11,12 including data on a subset of case and control individuals also analyzed by GWAS. Results of analysis of the four most prevalent FLG (RefSeq accession number NM_002016.1) loss-of-function mutations were obtained for a total of 2,865 case subjects and 5,540 control subjects as data generated for previous studies;11,20 the FLG mutations in these analyses are as follows: p.Arg501∗ (c.1501C>T), p.Ser761Cysfs∗36 (c.2282_2285del), p.Arg2447∗ (c.7339C>T), and p.Ser3247∗ (c.9740C>A) (R501X, 2282del4, R2447X, and S3247X, respectively).
The institutional review board in each contributing center approved these studies. All participants (or their parents or guardians) gave written informed consent.
Study Design
The study design is summarized in Figure 1.
Figure 1.
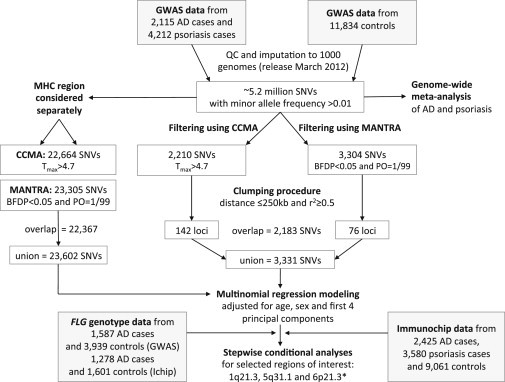
Study Design
Abbreviations are as follows: CCMA, case control meta-analysis; MANTRA, meta-analysis of trans-ethnic association studies; BFD, Bayesian false discovery; PO, prior odds; ∗conditional analysis for the MHC was also carried out with imputed classical HLA-allele (detailed in the Subjects and Methods).
Quality Control
Quality control and standard GWAS analysis of genotyped single-nucleotide variants (SNVs) was carried out with PLINK30 and R. Samples with extensive missing data (rate >5%), excess of heterozygosity or homozygosity, and discrepant gender determined on the basis of average X-chromosomal heterozygosity compared to the gender recorded in the database were excluded. We then examined identity-by-state (IBS) sharing and estimated identity-by-decent (IBD) on a pruned SNV set between all pairs of individuals and deleted resulting duplicates or closely related samples with PI_HAT > 0.1875 (halfway between expected IBD for third- and second-degree relatives). Multidimensional scaling (MDS) of the pairwise IBS matrix was carried out to identify and delete outliers of unusual ancestry and to calculate genome-wide principal-component scores for each individual. We excluded 894 samples because of SNVs showing a missing rate of >5%, deviation of Hardy-Weinberg equilibrium pHWE < 10−8, or minor allele frequency (MAF) <5% (summarized in Table S2). After quality control, the resulting SNVs and samples were analyzed for association via logistic regression with age, sex, and principal-component scores as covariates. Results from each panel were investigated to determine whether established GWAS loci were identified for the respective trait of interest, and genomic control inflation factors were calculated.
Imputation of SNVs and Classical HLA Alleles
Any SNVs showing significant association were checked (e.g., by visual inspection of the intensity cluster plots and investigation of consistency of LD with surrounding markers) and those SNVs deemed unreliable were removed. The final data sets of high-quality SNVs were prephased with SHAPEIT31 and subsequently used to perform imputation with IMPUTE2,32 the 1000 Genomes reference panel (integrated variant set, release March 2012).33 In the Irish AD collection (Table S1), case and control subjects were genotyped on different platforms, and therefore only the 131,692 SNVs in common between the platforms were used to inform imputation.
Postimputation SNVs with low imputation quality (info score < 0.4), call rate <95%, deviation from pHWE < 10−8, or MAF < 5% were excluded. A final data set of approximately 5.2 million SNVs in 2,079 AD case subjects, 3,867 control subjects, 4,212 psoriasis case subjects, and 8,032 control subjects were eligible for subsequent analysis (Table S3).
Classical alleles for HLA-A, HLA-B, and HLA-C were imputed for each case-control cohort separately by HLA∗IMP34,35 and best guess genotypes with probability >0.9. Additional classical HLA-DQA1, HLA-DQB1, and HLA-DRB1 alleles were imputed in each case-control cohort with the exception of the Irish samples, in which there were insufficient informative SNPs. Alleles with a frequency >1% were put forward for analysis. For each individual, alleles were coded as having no, one, or two copies of the respective allele via allele probability >0.9. We obtained high-quality data at the four-digit level with call rates of 92%–100% and accuracy of 92%–98%.
Statistical Analysis
Meta-GWAS was performed on each disease, via standard methodologies. To analyze these findings further, we developed two different meta-analysis-based approaches to filter SNVs and model the contrasting effects in each disease. The first was a compare and contrast meta-analysis (CCMA) approach inspired by a subset-based method.36 The second used transethnic meta-analysis implemented in the MANTRA software,37 combining all six studies by using prior clustering to reflect the ethnic difference and the disease type. The MHC region was reserved for separate analysis because of its unique and complex variability and patterns of strong linkage disequilibrium (LD).
The CCMA approach is based on an adaptation of an idea of Bhattacharjee et al.,36 who modeled association with heterogeneous traits. With METAL,38 we calculated z-scores signed positive or negative with respect to the same reference allele for two meta-analyses, T1, combining AD studies only, and T2, combining psoriasis studies only. We then calculated the overall test statistic Tmax with the formula , where and . We categorized the effect of each SNV as corresponding to an effect on AD only, to an effect on psoriasis only, to a shared effect (in the same direction on AD and psoriasis), or to opposing effects, according to which of the four test statistics was the largest. In order to derive a p value for Tmax, we worked out an empirical null distribution by simulating 10,000,000 realizations of two normally distributed random variables, Z1 and Z2. Then we calculated , , and . The emprical p values can be derived as .
In a separate simulation of 1,000,000,000 replicates, we derived a calibration curve for the p values and found it suitable up to a p value of 10−9. Hence with the calibration curve we can derive Zmax thresholds corresponding to standard genome-wide “suggestive” (10−5) and genome-wide “significant” (10−8) thresholds, corresponding to Tmax values of approximately 4.7 and 6.0, respectively (Figure S1).
In the second approach we used the MANTRA software37 developed for transethnic meta-analysis. MANTRA uses a Bayesian partition model for grouping studies according to their ethnicity. We adopted this idea and worked out a prior distribution to cluster studies according to both our phenotypes of interest and the genetic distance between the studies derived from our MDS analysis based on the pairwise IBS matrix: DTotal = DDisease + DEthnicity, where DEthnicity is a diagonal matrix of Euclidean distances between study centers. To distinguish the two diseases (psoriasis and AD), we set the corresponding cells of the DDisease matrix to Dij = 2 × max(DEthnicity) and to account for the different subphenotype in AD (AD in general versus childhood AD), we set the corresponding cells of the DDisease matrix to Dij = max(DEthnicity), resulting in the prior components shown in Table S4.
We calibrated the resulting log10BFMANTRA = log10(Bayes Factors) from the MANTRA software in order to find a threshold for filtering SNVs, which were compared with the CCMA top SNVs and subsequently carried forward to multinomial regression modeling. To perform this calibration, we calculated the Bayesian False Discovery Probability proposed by Wakefield39 with diverse prior odds (PO) in favor of H0:
Sensitivity analysis was performed with only the DEthnicity as prior matrix and we observe high correlation (r2 > 0.99) of the top-ranked SNVs (BFDP < 0.05; PO = 99) with our analysis (data not shown).
Finally, we carried forward a filtered set of SNVs from CCMA and MANTRA for modeling via a multinomial regression model, adjusted for sex and the first four genome-wide principal-component scores. The multinomial model involves three outcome categories: the “baseline” category into which all controls are categorized, a “psoriasis” case category, and an “AD” case category (modeled through regression coefficients βPSO and βAD, respectively). This analysis makes use of individual-level genotypes and is thus more computationally intensive (although arguably more powerful and more statistically satisfactory) than CCMA and MANTRA. We calculated p values for tests that were designed to be sensitive to the following situations: an overall SNV effect (on either or both diseases, in either direction), an individual SNV effect on one disease (but not on the other), a shared SNV effect (operating in the same direction for both diseases), and a contrasting SNV effect (operating in opposing directions between both diseases), by performing Wald tests of the following linear hypotheses:
The overall significance of the SNV was assessed through the 2 degree of freedom (df) test of overall effect, which compares the null hypothesis that the SNV has no effect on either psoriasis or AD with the alternative hypothesis that it has an effect on one or both diseases. The other four 1 df tests were used to categorize the effect (in analogy to CCMA) in four categories—AD only, psoriasis only, shared effect, and opposing effects—by categorizing according to the minimum of the p values: pMNM = min(pAD, pPSO, pSHARED, pOPPOSING). The rationale for the use of the minimum of these 1 df tests for categorization is as follows: if a SNV is associated with one disease but not the other, the test of a nonzero regression coefficient for that disease (even while unnecessarily also allowing for a nonzero coefficient for the other disease, as is done in the psoriasis effect and AD effect tests), should be more powerful than a test that erroneously groups together the coefficients of the associated and the nonassociated disease (as is done in the shared and opposing effect tests). This is on account of the fact that grouping together these coefficients will incur a penalty in terms of increasing the variance, while not incurring any greater expected magnitude of effect since the expected value of the regression coefficient for the nonassociated disease is zero. If, on the other hand, the SNV has effects that operate in the same direction on both diseases, then a test based on adding together these effects (as is done in the shared effect test) should be more powerful than considering each effect on its own, or subtracting one effect from the other (as is done in the opposing effect test), because adding together the coefficients induces the greatest magnitude of effect. Finally, if the SNV has effects that operate in opposite directions in the two diseases, then a test based on subtracting one effect from the other (as is done in the opposing effect test) should be most powerful because it induces the greatest magnitude of effect.
All analyses if not explicitly stated were carried out with R. For the purposes of this analysis, we distinguished between a shared genetic “region” and a shared genetic “locus.” We arbitrarily designated a shared region as a block of genomic DNA spanning 2 Mb with association signals for both traits. We defined a genetic locus as the lead SNV and all SNVs with r2 > 0.5.
Predicted Protein Network Analysis and Gene Ontology Analysis
Functional protein association networks were investigated in silico and gene ontology analyses were performed with STRING9.1.
Results
Filtering Variants to Define Risk Effects
Quality control and imputation generated 5.2 million SNVs with a minor allele frequency >0.01 for further analysis (Figure 1). GWASs within each cohort resulted in genomic inflation factors λ between 1.03 and 1.08. Meta-GWAS performed on each disease confirmed previously reported risk loci in AD and psoriasis and illustrated areas of colocalization on chromosomes 1, 5, and 6 (Figure 2A).
Figure 2.
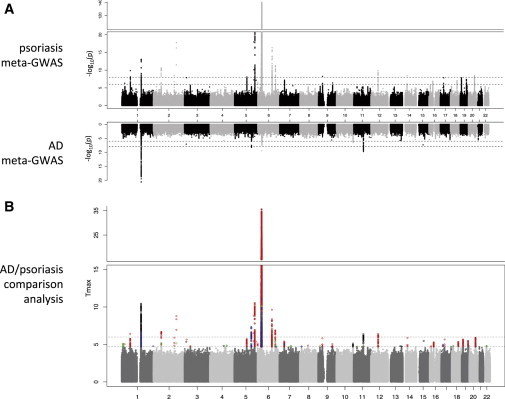
Genome-wide Comparison of AD and Psoriasis
(A) Mirrored Manhattan plots showing results of AD meta-GWAS (top) and psoriasis meta-GWAS (bottom).
(B) Comparative analysis of AD and psoriasis in which SNVs are color coded to show AD-specific effect (black), psoriasis-specific effect (red), shared effects defined as alleles operating in the same direction (green), and opposing effects (blue). The genome-wide significance level is marked at p = 0.5 × 10−8 (Tmax = 6.0).
Excluding the MHC, 2,210 SNVs were identified with shared (by which we mean alleles having effects operating in the same direction in both diseases), opposing, and disease-specific SNVs with CCMA test statistic Tmax > 4.7. This threshold was defined to correspond to a suggestive significance of p < 10−5 in order to reduce the probability of false negatives. The 2,210 SNVs were condensed to 142 distinct loci after an LD-based clumping procedure30 with the following parameters: distance ≤ 250 kb and r2 ≥ 0.5.
Analysis with MANTRA revealed 3,304 SNVs with Bayesian false discovery probability (BFDP) < 0.05 with prior odds (PO) 1/99 resulting in 76 distinct loci after clumping. The overlap of CCMA and MANTRA gave 2,183 SNVs and the union of both methods resulted in 3,331 SNVs that were carried forward for multinomial regression modeling (MNM), which was adjusted for sex and the first four genome-wide principal-component scores. The results are displayed in Figure 2B, in which disease-specific, shared, and opposing loci are coded by color. SNVs showing genome-wide significance in at least one of the three methods of analysis (Tmax > 6, BFDP < 0.05 with PO = 1/999, or pMNM < 10−8) map to 144 distinct loci (Table S5). Comparison of effect classification (AD, psoriasis, shared, opposing) in CCMA and MNM (Figure S2) showed an agreement of 94.8% when excluding the MHC region (Figure S3). For further investigation, we considered only loci containing more than one SNV and an effect classified in the same direction by CCMA and MNM.
Validation of Previously Reported AD- and Psoriasis-Risk Loci
15 European and 9 Asian loci have previously been reported in GWASs on AD, and 44 European and 9 Asian loci have been reported in association with psoriasis (Table S6). In our disease-specific meta-analysis individuals of white European descent, 14 of the European AD loci as well as 43 of the psoriasis loci are replicated. Furthermore, 4 AD and 4 psoriasis loci so far reported only in Asians showed evidence for association in European populations (p < 10−3): CCDC80 (MIM 608298)/CD200R1L at 3q13.2, CARD11 (MIM 607210) at 7p22.2, ZNF365 (MIM 607818) at 10q21.2, and BCAS1 (MIM 602968) at 20q13.2 in AD; CSMD1 (MIM 608397) at 8p23.2, SERPINB8 (MIM 601697) at 18q22.1, MAMSTR (MIM 610349)/RASIP1 (MIM 609623) at 19q13.33, and ZNF816A at 19q13.41 in psoriasis (Table S6).
New Opposing-Effect Loci Identified by Genome-wide Comparative Analysis
Excluding the MHC, 25 loci showed a genome-wide significant association with either skin disorder, defined by all three methods of analysis (CCMA Tmax > 6 and MANTRA BFDP < 0.05 with PO = 1/999, and pMNM < 10−8) including six loci that were coassociated with both AD and psoriasis. Each coassociated locus displayed opposing effects and two of these loci (2q31.2, 5q33.1) have not previously been reported as showing coassociation with AD and psoriasis (Table 1).
Table 1.
Loci Showing Genome-wide Significant Association with Either AD or Psoriasis Defined by All Three Methods of Comparative Analysis
| Chr Band | Reference SNV Number(s) | Position (hg19) | Nearest Gene(s) or Transcript(s) | Effect Observed in GW Analyses |
Estimated Odds Ratio (95% CI) |
Previous Report(s) of Association at This Locus | |
|---|---|---|---|---|---|---|---|
| AD | Psoriasis | ||||||
| 1p31.3 | rs77614545 (del) | 67749581 | retro-DNAJB6 and IL23R (MIM 607562) | psoriasis | 0.99 (0.92–1.07) | 1.21 (1.15–1.28) | psoriasis: 1p31.3 locus, IL28RA21,24,40 |
| 1q21.3a |
rs55879323 | 152168740 | within FLG-AS1 | opposing | 0.76 (0.70–0.82) | 1.05 (1.00–1.12) | AD and psoriasis: 1q21.3 locus, HRNR, FLG;41FLG;20,42LCE3B, LCE3C,18LCE gene cluster;17LCE3D24 |
| rs11205006, rs12144049 | 152440176, 152440910 | RP1-91G5.3, CRNN (MIM 611312), LCE5A (MIM 612619) | AD | 1.52 (1.41–1.64), 1.53 (1.42–1.64) | 0.97 (0.92–1.03), 0.98 (0.92–1.03) | AD: 1q21.3 locus, HRNR, FLG;41FLG20,42 | |
| rs471144 | 152454255 | LCE5A (MIM 612619) | AD | 1.54 (1.37–1.73) | 1.03 (0.94–1.14) | AD: 1q21.3 locus, HRNR, FLG;41FLG20,42 | |
| rs10888499 | 152532742 | LCE3E (MIM 612617) | AD | 1.49 (1.38–1.61) | 0.98 (0.93–1.04) | AD: 1q21.3 locus, HRNR, FLG;41FLG20,42 | |
| rs4112788 | 152551276 | LCE3D (MIM 612616) | psoriasis | 0.97 (0.90–1.05) | 1.22 (1.15–1.28) | psoriasis: LCE gene cluster;17LCE3D24 | |
| rs1581803 | 152592281 | LCE3A (MIM 612613) | psoriasis | 0.97 (0.90–1.04) | 1.22 (1.15–1.30) | psoriasis: LCE gene cluster17 | |
| rs77199844 (del) | 152757094 | LCE1E (MIM 612607) | [AD] | 2.01 (1.72–2.35) | 1.16 (1.01–1.33) | AD: 1q21.3 locus, HRNR, FLG;41FLG20,42 psoriasis: LCE gene cluster17 |
|
| rs4363385 | 152989321 | SNORA31, SPRR3 (MIM 182271), SPRR1B (MIM 182266) | [opposing] | 1.23 (1.15–1.32) | 0.89 (0.85–0.94) | AD: SPRR3 repeat number variant43 | |
| 2p16.1 | rs35741374 | 61072567 | within lincRNA AC010733.4 | psoriasis | 1.09 (1.01–1.63) | 1.20 (1.15–1.27) | psoriasis: REL;24NR23 |
| 2q31.2 | rs62176107 | 179300971 | exonic PRKRA and within miRNA548n and AC009948.5 | opposing | 0.55 (0.46–0.65) | 1.42 (1.32–1.53) | – |
| 5q31.1a |
rs1295686 | 131952222 | intronic IL13 (MIM 147683) and within AC004041.2 | [opposing] | 1.27 (1.17–1.38) | 0.88 (0.82–0.94) | AD and psoriasis: IL13;21KIF3A, IL13;22KIF3A, IL4, IL13-RAD50;10 multiple effect locus RAD50/IL13;20C5orf56 |
| rs6596086 | 131995843 | intronic RAD50 (MIM 604040) | opposing | 1.30 (1.20–1.41) | 0.85 (0.8–0.91) | AD and psoriasis: IL13-RAD50;10 multiple effect locus RAD50/IL1320 | |
| 5q33.1 | rs17728338 | 150478318 | ANXA6 (MIM 114070) | opposing | 0.70 (0.59–0.84) | 1.77 (1.61–1.95) | psoriasis: TNIP121,42 |
| 5q33.3 |
rs10515778, rs7715173, rs7719425b | 158658012, 158664631, 158670938 | within CTB-11I22.1 | psoriasis | 1.07 (0.98–1.17) | 1.29 (1.21–1.38) | psoriasis: 5q33.3 locus, IL12B17,21,24,44; AD: PTTG142 |
| rs11135056, rs4921442b | 158687281, 158694100 | intronic UBLCP1 (MIM 609867) | psoriasis | 0.97 (0.89–1.05) | 1.45 (1.35–1.56) | ||
| rs2546890 | 158759900 | within AC008697.1 | psoriasis | 1.01 (0.94–1.06) | 1.39 (1.32–1.47) | ||
| rs5872599 (indel) | 158859989 | lincRNA AC008703.1, IL12B (MIM 161561) | psoriasis | 0.82 (0.73–0.93) | 1.54 (1.45–1.64) | ||
| 6q21 |
rs9487605 | 111582885 | intronic KIAA1919 | psoriasis | 1.06 (0.98–1.14) | 1.27 (1.20–1.35) | – |
| rs240993 | 111673714 | intronic REV3L (MIM 602776) | psoriasis | 1.05 (0.97–1.13) | 1.29 (1.22–1.36) | – | |
| rs9481169 | 111929862 | TRAF3IP2 (MIM 607043) | psoriasis | 0.98 (0.86–1.11) | 1.58 (1.45–1.72) | psoriasis and psoriatic arthritis: TRAF3IP224,44,45 | |
| 6q23.2 | rs643177, rs582757b | 138195693, 138197824 | TNFAIP3 (MIM 610669) | psoriasis | 1.05 (0.97–1.14) | 1.27 (1.20–1.34) | psoriasis: TNFAIP321,24 |
| 11q13.5 | rs2212434, rs7126418b | 76281593, 76292573 | c11orf30 (MIM 608574) | AD | 1.29 (1.21–1.39) | 1.05 (1.00–1.11) | AD: c11orf30-LRRC32;10,41c11orf30;22 11q13 locus20 |
| 12q13.3 | rs36207871 (del) | 56684496 | intronic CS (MIM 118950) | psoriasis | 0.94 (0.83–1.06) | 1.47 (1.33–1.67) | psoriasis: 12q13.3 locus, IL23A, STAT2;21IL23A24 |
| rs11575234 | 56744276 | intronic STAT2 (MIM 600556) | psoriasis | 0.90 (0.79–1.02) | 1.47 (1.32–1.64) | psoriasis: STAT221 | |
Genome-wide significance is defined as CCMA Tmax > 6 and MANTRA BFDP < 0.05 with PO = 1/999 and multinomial model p < 10−8; genes and transcripts identified from UCSC Genome Browser Human Feb. 2009 (GRCh37/hg19) Assembly accessed 21 March 2014; this variant of FLG-AS1 extends across HRHR and FLG; RP1-91G5.3 extends across CRNN; AC004041.2 extends across RAD50 and IL13; CTB-11I22.1 overlaps RNF145.
1q21.3 and 5q31.1 were further investigated via stepwise conditional analysis (the results are shown in Table 2); square brackets indicate results from the univariate analysis that were subsequently accounted for by other nearby variants when examined by stepwise conditional analysis.
Multiple SNVs are assigned to the same LD block but odds ratios and 95% CI are presented only for the first SNV.
2q31.2 demonstrates an opposing effect at rs62176107 (MNM p = 1.08 × 10−34; Table S5); this variant is within exon 6 of PRKRA (MIM 603424) and also within microRNA 548n (MIR548N) and a noncoding transcript, AC009948.5. PRKRA encodes protein kinase interferon-inducible double-stranded RNA-dependent activator (PACT), a cellular dsRNA-binding protein originally identified as a binding partner and activator of PKR in response to extracellular stress.46 More recently, it has been shown to be an essential factor in the PKR-independent initiation of RIG-I-induced antiviral response.47 Of note, individuals with AD are known to be susceptible to viral skin infections, but cutaneous infections rarely occur in psoriasis.48 MicroRNAs play a role in posttranscriptional regulation of gene expression by affecting the stability and translation of mRNAs, but the specific role of miRNA548n has not been defined. The most significantly associated (“lead”) SNV at 2q31.2 (rs62176107, G>A, having the smallest p value from MNM) is a synonymous SNV with predicted effects on 12 transcripts, including PRKRA splice variants’ UTR and intronic regions and a variant predicted to undergo nonsense-mediated decay (Ensembl release 75). Gene expression profiling data show downregulation of both PRKRA mRNA and miRNA548n in psoriatic lesions compared to nonlesional skin, but no significant differences in AD (Table S7).
The most highly significant variant at 5q33.1 (rs17728338) shows opposing effects on AD and psoriasis (MNM p = 3.96 × 10−38; Table S5) and lies 2 kb upstream of ANXA6 (MIM 114070) and 8 kb downstream of TNIP1 (MIM 607714). LD analysis in 1000 Genomes (release August 2009) via LocusZoom49 showed that rs17728338 is located within a 25-kb block containing both TNIP1 and ANXA6. The locus has previously been associated with psoriasis in European and Chinese populations but has not been implicated in AD. TNIP1 is involved in TNF signaling and regulation of the transcription factor NF-κB;21,50 it shows increased expression both in AD and psoriatic lesions compared to control skin (Table S7). In contrast, ANXA6, which encodes a calcium-dependent membrane and phospholipid binding protein, shows significant upregulation of expression in atopic skin compared to control skin (fold change 1.3, FDR p = 0.016) and lesional to nonlesional AD skin (fold change 2.4, p = 0.027), whereas expression is decreased in psoriatic versus healthy skin (fold change 0.7, p = 6.38 × 10−13) (Table S7). Clearly, further fine mapping is necessary to identify the causal variant that exerts opposing effects on AD and psoriasis, but we speculate that ANXA6 might be a switch-point differentiating AD from psoriasis that reflects the importance of calcium-dependent effects in keratinocyte differentiation.
Opposing effect loci were also identified within regions characterized by complex patterns of LD within the EDC (Figure S4), the cytokine cluster on 5q31.1 (Figures S4 and S5), and the MHC. These regions were therefore investigated further via conditional analysis.
Stepwise Conditional Analysis within 1q21.3 and 5q31.1 Identifies Opposing and Disease-Specific Risk Variants
Coverage of the EDC was achieved via GWAS data (Figure 3), whereas ImmunoChip data provided better coverage for the cytokine cluster on 5q31.1 (Figures 4 and S5).
Figure 3.
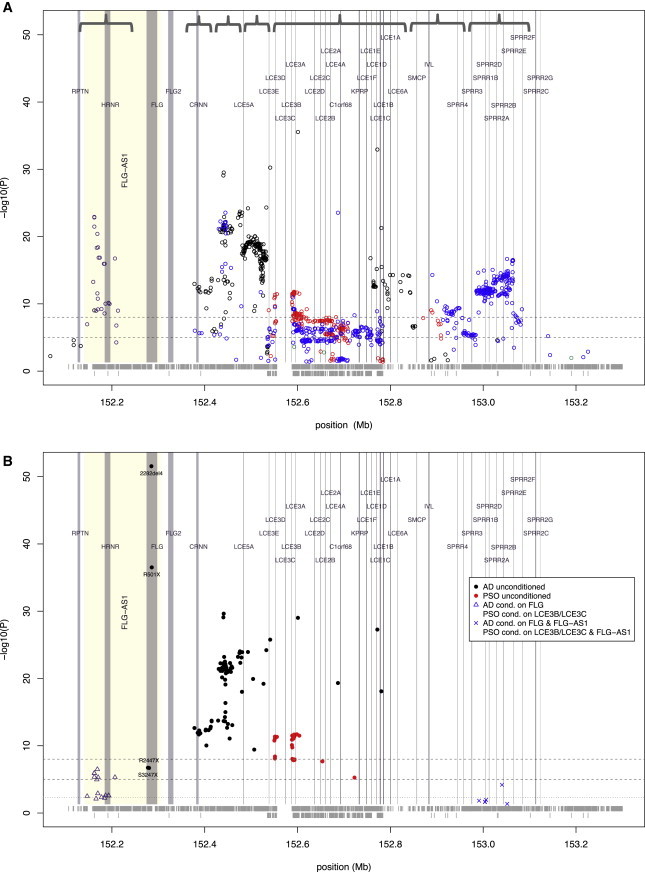
Regional Association within the Epidermal Differentiation Complex at 1q21.3
(A) Multinomial regression model with GWAS and ImmunoChip data. Seven blocks of linkage disequilibrium are indicated by curly brackets; black circles indicate AD-specific association, red circles indicate a psoriasis-specific association, blue circles represent opposing effects in AD and psoriasis, and green circles indicate shared effects. Vertical lines have been drawn to mark the positions of known genes and transcripts (identified from UCSC Genome Browser, GRCh37/hg19 accessed Feb. 2009) and the horizontal dotted lines indicate thresholds of suggestive and genome-wide significance (p = 10−5 and 10−8). The horizontal gray bands at the bottom indicate the coverage of the region by GWAS SNVs (upper row) and ImmunoChip SNVs (lower row).
(B) Conditional regional association plot of stepwise logistic regression using GWAS and ImmunoChip data. The different colored symbols indicate association results after each step of analysis, as follows. Unconditioned results are shown by black dots to indicate association with AD and red dots to indicate association with psoriasis; blue triangles and blue crosses represent results after conditioning on the known disease-associated variants, FLG in AD and LCE3B-LCE3C deletion in psoriasis; SNVs indicated by the same symbol are in LD with the lead SNV of each stepwise conditional analysis (defined as r2 ≥ 0.5). Vertical lines are drawn to mark the positions of known genes and transcripts (identified from the UCSC Genome Browser GRCh37/hg19 accessed Feb. 2009), and horizontal dotted lines indicate significance thresholds of p = 0.005, 10−5, and 10−8. The horizontal gray bands at the bottom indicate the coverage of the region by GWAS SNVs (upper row) and ImmunoChip SNVs (lower row).
Figure 4.
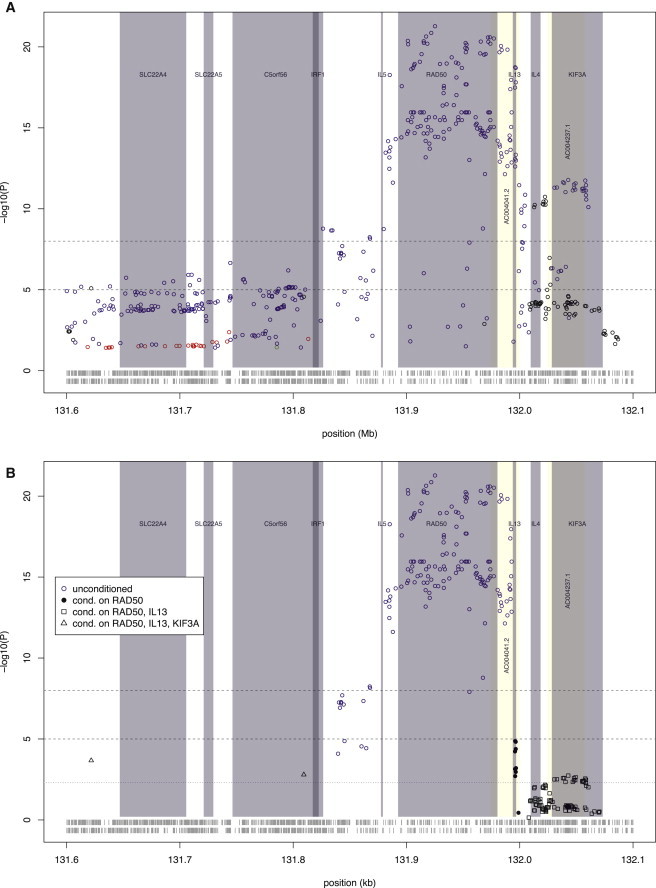
Regional Association within the Cytokine Cluster at 5q31.1
(A) Multinomial regression model with GWAS and ImmunoChip data. Black circles indicate AD-specific association, red circles indicate psoriasis-specific association, blue circles represent opposing effects in AD and psoriasis, and green circles indicate shared effects. Vertical gray shading marks the positions of known genes (identified from the UCSC Genome Browser GRCh37/hg19 accessed Feb. 2009), and horizontal dotted lines indicate suggestive and genome-wide significance thresholds (p = 10−5 and 10−8, respectively); results are shown for SNVs in LD with the lead SNV (defined as r2 ≥ 0.5). The horizontal bands at the bottom indicate the coverage of the region by GWAS SNVs (upper row) and ImmunoChip SNVs (lower row).
(B) Conditional regional association plot of the EDC by multinomial regression of GWAS and ImmunoChip data. Different symbols indicate association results after each step of analysis, as follows. Unconditioned results are shown by blue circles representing opposing effects in AD and psoriasis; black dots show AD-specific association results after conditioning on the lead SNV in RAD50 (a gene reported to be associated with AD and psoriasis); black squares indicate the residual AD-specific association after conditioning on the lead SNVs in RAD50 and IL13 (genes reported to be associated with AD); and black triangles indicate the residual AD-specific association after additionally conditioning on the lead SNV in KIF3A (a gene reported to be associated with AD). SNVs indicated by the same symbol are in LD with the lead SNV of each stepwise conditional analysis (defined as r2 ≥ 0.5). Vertical gray shading marks the positions of known genes (identified from the UCSC Genome Browser GRCh37/hg19 accessed Feb. 2009), and horizontal dotted lines indicate significance thresholds of p = 0.005, 10−5, and 10−8; results are shown for SNVs in LD with the lead SNV (defined as r2 ≥ 0.5). The horizontal bands at the bottom indicate the coverage of the region by GWAS SNVs (upper row) and ImmunoChip SNVs (lower row).
Within 1q21.3 we identified seven LD blocks with disease-specific or opposing signals (Figure 3A). Stepwise conditional analysis on the four most prevalent FLG-null mutations and variants tagging the LCE3B-LCE3C deletion identified one AD-specific locus mapping to FLG, a psoriasis-specific locus mapping to LCE3B-LCE3C, and a locus with opposing effects on both diseases mapping to RPTN (MIM 613259)/HRNR/FLG-AS1 (Figure 3B and Table 2). After conditioning on the four FLG-null mutations and the LCE3B-LCE3C deletion, the G allele of the lead MNM SNV rs12130219 decreases the risk for AD (ORADcond = 0.812, pADcond = 0.0018) and increases the risk for psoriasis (ORPSOcond = 1.119, pPSOcond = 3.68 × 10−4) (Table 2). Filaggrin, repetin, and hornerin are all members of the S100 fused-type protein family and each contribute to the cornified cell envelope, a functional component of the epidermal barrier. Both FLG and HRNR show reduced expression in AD51–53 whereas RPTN shows no significant difference (Table S7). In psoriasis HRNR expression can be downregulated53 or upregulated,54 RPTN expression might be upregulated, and FLG expression might be downregulated55 or dysregulated15 (Table S7). The function of FLG-AS1 (FLG antisense RNA1) is currently undefined, but its proximity to FLG and HRNR suggests a role in coordinating keratinocyte terminal differentiation. FLG-AS1 expression is increased in psoriasis lesional compared with nonlesional skin, whereas in AD lesional skin, expression is reduced (Table S7). Together, our results confirm the role of the LCE3B-LCE3C deletion in psoriasis and support the presence of genetic risk mechanisms for AD within the EDC in addition to the predominant effect of FLG-null mutations, with opposing effects on psoriasis.
Table 2.
Conditional Analysis of the 1q21.3 and 5q31.1 Regions Showing Disease-Specific and Opposing Risk Effects in AD and Psoriasis
| Data Source | Effect | SNV | Pos (hg19) | Allele | Candidate Genes |
AD |
Psoriasis |
poveralla |
AD |
Psoriasis |
Poveralla | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||||||||
| Chromosome 1q21.3 | |||||||||||||||
| Unconditioned Analysis | Conditional Analysisb | ||||||||||||||
| GWAS | Opposing | rs12130219 | 152162106 | G/A | FLG-AS1/RPTN/HRNR | 0.66 (0.60–0.73) | 1.1 × 10−16 | 1.15 (1.09–1.224) | 4.0 × 10−6 | 1.2 × 10−23 | 0.812 (0.71–0.93) | 0.0018 | 1.119 (1.05–1.19) | 3.68 × 10−4 | 2.4 × 10−6 |
| GWAS | AD | rs12144049 | 152440910 | C/T | FLG | 1.53 (1.42–1.64) | 2.7 × 10−30 | 0.98 (0.92–1.03) | 0.4140 | 3.0 × 10−30 | – | – | – | – | |
| GWAS | Psoriasis | rs1581803c | 152592281 | G/T | LCE3B/LCE3D | 0.97 (0.90–1.04) | 0.4396 | 1.22 (1.16–1.30) | 1.5 × 10−12 | 1.6 × 10−12 | – | – | – | – | |
| GWAS | Opposing | rs35722864 | 153040505 | G/GA | SPRR cluster | 0.81 (0.75–0.88) | 1.0 × 10−7 | 1.129 (1.07–1.20) | 2.1 × 10−5 | 4.8 × 10−13 | 0.851 (0.71–0.93) | 0.0019 | 1.074 (1.01–1.14) | 0.0211 | 1.3 × 10−4 |
| Chromosome 5q31.1 | |||||||||||||||
| Conditional Modelsd | Full Model | ||||||||||||||
| Ichip | Opposing | rs6596086 | 131952222 | C/T | RAD50 | 1.31 (1.22–1.41) | 1.7 × 10−13 | 0.86 (0.80–0.92) | 1.7 × 10−5 | 5.7 × 10−21 | 1.17 (1.07–1.28) | 4.04 × 10−4 | 0.88 (0.81–0.96) | 0.0023 | 6.3 × 10−7 |
| Ichip | AD | rs848 | 131996500 | A/C | IL13 | 1.20 (1.10–1.30) | 5.6 × 10−5 | 0.96 (0.89–1.04) | 0.3375 | 4.14 × 10−5 | 1.12 (1.02–1.23) | 0.0197 | 0.96 (0.88–1.05) | 0.3515 | 0.0204 |
| Ichip | AD | rs2299009 | 132042813 | G/T | IL4/KIF3A | 1.14 (1.06–1.23) | 7.9 × 10−4 | 0.99 (0.92–1.06) | 0.7392 | 0.0018 | 1.16 (1.07–1.25) | 2.03 × 10−4 | 0.99 (0.92–1.06) | 0.6657 | 4.1 × 10−4 |
| Ichip | AD | rs74458173 | 131621731 | A/C | SLC22A4 | 1.57 (1.26–1.96) | 6.1 × 10−5 | 1.02 (0.80–1.30) | 0.8590 | 2.14 × 10−4 | 1.57 (1.26–1.96) | 5.71 × 10−5 | 1.02 (0.80–1.30) | 0.8683 | 2.0 × 10−4 |
Full model incorporates the combined effects of independent SNVs identified by stepwise analyses.
poverall represents the overall opposing signal calculated using the T12opposing statistic and derive the p value from the normal distribution.
Conditional analysis of chr1q21.3 was conditioned on FLG for AD and LCE3B/LCE3D for psoriasis.
rs1581803 tags the previously reported psoriasis SNV rs4112788 (r2 = 0.995).
Stepwise conditional analysis at chr5q31.1 was carried out using multinomial regression models and resulted in three additional signals for AD; this table shows only independent loci (r2 < 0.5) and the SNV with the strongest association; the effect allele is underlined.
Conditional analysis at 5q31.1 revealed three independent loci specifically contributing to AD risk: IL13 (MIM 147683, rs848, ORADfull = 1.12, p = 0.0204), KIF3A (MIM 604683, rs 2299009, ORADfull = 1.16, p = 4.1 × 10−4), and SLC22A4 (MIM 604190)/C5orf56 (rs74458173, ORADfull = 1.57, p = 2.0 × 10−4) (Figure 4A, Table 2). None of these loci showed significant effects on psoriasis. However, a fourth independent locus has opposing effects on AD and psoriasis. The most highly significant variant maps to RAD50 (MIM 604040, rs6596086, ORADfull = 1.17, ORPSOfull = 0.88, p = 6.3 × 10−7); this variant is associated with increased risk of AD but is protective against psoriasis (Figure 4B, Table 2).
Analysis of the MHC Confirms Multiple Psoriasis-Risk Loci and Identifies Opposing Effects
In the extended HLA region, we took forward 23,479 SNVs with Tmax > 4.7 or BFDP < 0.05 (PO = 1/99) and 75 variables representing the classical HLA alleles obtained from HLA imputation for multinomial modeling, of which 18,515 SNVs were classified as specific to psoriasis by CCMA and MNM. To reduce the data set for post hoc analysis, we considered only SNVs with effect classification in the same direction by CCMA and MNM, meeting the p < 10−5 threshold in MNM. Within the psoriasis-specific markers, we excluded all tagging SNVs (r2 > 0.8 with the lead SNV), resulting in 1,503 SNVs, including those previously reported for AD.20
The strongest and most significant association was observed for psoriasis, a variant (rs111576655 ORPSOfull = 3.32, p = 3.2 × 10−65) tagging the well-known psoriasis-risk allele HLA-C∗06:02 (ORPSOfull = 3.59, p = 8.7 × 10−154). Conditional analysis revealed two additional independent loci contributing to psoriasis risk at MICA (MIM 600169, rs201374403, ORPSOfull = 1.65, p = 1.0 × 10−26) and HLA-A (MIM 142800, rs113573479, ORPSOfull = 1.41, p = 2.7 × 10−17), as well as two loci with opposing effects at HLA-C (MIM 142840, rs1793889, ORADfull = 0.6, ORPSOfull = 1.18, p = 1.1 × 10−9) and HLA-DRB1 (MIM 142857, rs28383201, ORADfull = 0.61, ORPSOfull = 1.18, p = 6.5 × 10−9) (Figure 5, Table 3). Conditional analysis with imputed classical alleles identified five independent HLA-class I alleles contributing to psoriasis risk in addition to HLA-C∗06:02 and two alleles with opposing effects: HLA-C∗03:03 (ORADfull = 0.71, ORPSOfull = 1.27, p = 2.3 × 10−5) and HLA-DQA1∗02:01 (ORADfull = 0.64, ORPSOfull = 1.09, p = 6.0 × 10−8; r2 = 0.405 with rs28383201) (Table 3).
Figure 5.
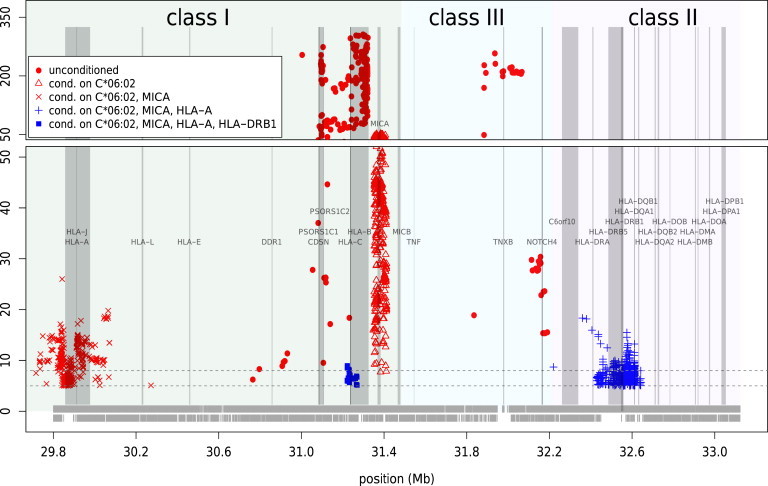
Conditional Regional Association within the Major Histocompatibility Complex at 6p21–22 via GWAS and ImmunoChip Data
Symbols indicate association results after each step of analysis, as follows. Unconditioned psoriasis-specific results are shown by red dots; red triangles show psoriasis-specific association results after conditioning on C∗06:02 (known to be strongly associated with psoriasis); red ×s indicate psoriasis-specific association after conditioning on C∗06:02 and MICA; blue +s indicate the association after conditioning on C∗06:02, MICA, and HLA-A with opposing effects on AD and psoriasis; and blue squares indicate the residual association after conditioning on C∗06:02, MICA, HLA-A, and HLA-DRB1 with opposing effects on AD and psoriasis. SNVs indicated by the same symbol are in LD with the lead SNV of each stepwise conditional analysis (defined as r2 ≥ 0.5). Vertical shading marks the positions of known genes (identified from the UCSC Genome Browser GRCh37/hg19 accessed Feb. 2009) and HLA classes; horizontal dotted lines indicate significance thresholds of p = 10−5 and p = 10−8; results are shown for SNVs in LD with the lead SNV (defined as r2 ≥ 0.5). The horizontal bands at the bottom indicate the coverage of the region by GWAS SNVs (upper row) and ImmunoChip SNVs (lower row).
Table 3.
Conditional Analysis of the MHC Region on 6p21–22 Showing Psoriasis-Specific and Opposing Risk Effects in AD and Psoriasis
| Data Source | Effect | SNV | Pos (hg19) | Allele | HLA Allele/Candidate Genes |
Conditional Modelsa |
Full Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AD |
Psoriasis |
poverall |
AD |
Psoriasis |
poverall | ||||||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||||||||
| GWAS | PSO | rs111576655 | 31242731 | A/T | C∗06:02 | 0.84 (0.74–0.95) | 0.0053 | 4.41 (4.10–4.74) | 3.1 × 10−376 | 9.8 × 10−380 | 1.12 (0.81–1.54) | 0.5071 | 3.32 (2.90–3.81) | 2.3 × 10−69 | 3.2 × 10−65 |
| GWAS | PSO | rs201374403 | 31383754 | T/TAG | MICA | 0.78 (0.7–0.88) | 6.4 × 10−5 | 1.68 (1.56–1.8) | 7.9 × 10−48 | 2.2 × 10−53 | 0.81 (0.67–0.96) | 0.0174 | 1.65 (1.50–1.81) | 1.8 × 10−25 | 1.0 × 10−26 |
| GWAS | PSO | rs113573479 | 29842444 | A/G | HLA-A | 0.89 (0.81–0.97) | 0.0109 | 1.39 (1.30–1.49) | 6.6 × 10−25 | 1.0 × 10−26 | 0.92 (0.81–1.04) | 0.1948 | 1.41 (1.30–1.52) | 2.8 × 10−17 | 2.7 × 10−17 |
| GWAS | opposing | rs28383201 | 32574869 | C/G | HLA-DRB1 | 0.59 (0.51–0.68) | 4.6 × 10−13 | 1.15 (1.06–1.24) | 4.5 × 10−4 | 3.3 × 10−16 | 0.61 (0.52–0.71) | 3.4 × 10−10 | 1.18 (1.08–1.28) | 1.0 × 10−4 | 6.5 × 10−14 |
| GWAS | opposing | rs1793889 | 31222181 | A/G | HLA-C | 0.60 (0.50–0.73) | 2.5 × 10−7 | 1.18 (1.07–1.31) | 0.0011 | 1.1 × 10−9 | 0.60 (0.50–0.73) | 2.5 × 10−7 | 1.18 (1.07–1.31) | 0.0011 | 1.1 × 10−9 |
| Data Source | Effect | HLA Allele | HLA Allele Frequency in Ps/AD/Controls |
Conditional Modelsa |
Full Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
AD |
Psoriasis |
poverall |
AD |
Psoriasis |
poverall | ||||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||||||
| GWAS | PSO | C∗06:02 | 0.271/0.075/0.089 | 0.81 (0.71–0.91) | 8.48 × 10−4 | 4.28 (3.98–4.61) | 2.9 × 10−362 | 1.30 × 10−368 | 0.97 (0.82–1.15) | 0.7475 | 3.59 (3.26–3.95) | 2.1 × 10−159 | 8.7 × 10−154 |
| GWAS | PSO | A∗02:01 | 0.28/0.227/0.239 | 0.95 (0.88–1.03) | 0.1793 | 1.32 (1.24–1.40) | 4.1 × 10−20 | 1.1 × 10−20 | 0.99 (0.90–1.08) | 0.7739 | 1.32 (1.24–1.41) | 1.8 × 10−17 | 1.1 × 10−16 |
| GWAS | PSO | B∗57:01 | 0.13/0.017/0.032 | 0.61 (0.46–0.80) | 3.83 × 10−4 | 1.63 (1.44–1.84) | 8.0 × 10−15 | 5.8 × 10−20 | 0.60 (0.42–0.85) | 0.0039 | 1.58 (1.38–1.81) | 3.8 × 10−11 | 1.1 × 10−13 |
| GWAS | PSO | C∗12:03 | 0.048/0.044/0.038 | 0.87 (0.74–1.03) | 0.1169 | 1.74 (1.54–2.00) | 5.2 × 10−18 | 3.5 × 10−19 | 0.86 (0.72–1.04) | 0.1295 | 1.85 (1.62–2.11) | 7.5 × 10−20 | 1.8 × 10−20 |
| GWAS | PSO | B∗27:05 | 0.032/0.02/0.025 | 0.80 (0.63–1.01) | 0.0619 | 1.59 (1.37–1.86) | 2.9 × 10−9 | 3.2 × 10−10 | 0.74 (0.55–1.00) | 0.0499 | 1.50 (1.28–1.76) | 7.3 × 10−7 | 1.8 × 10−7 |
| GWAS | PSO | A∗01:01 | 0.221/0.154/0.166 | 1.03 (0.94–1.13) | 0.5475 | 1.25 (1.17–1.35) | 2.3 × 10−10 | 1.7 × 10−9 | 1.08 (0.96–1.21) | 0.2013 | 1.23 (1.14–1.33) | 6.8 × 10−8 | 4.0 × 10−7 |
| GWAS | opposing | C∗03:03 | 0.037/0.025/0.038 | 0.66 (0.54–0.81) | 9.71 × 10−5 | 1.30 (1.13–1.49) | 2.5 × 10−4 | 5.9 × 10−8 | 0.71 (0.56–0.90) | 0.0040 | 1.27 (1.10–1.47) | 0.0011 | 2.3 × 10−5 |
| GWAS | opposing | DQA1∗02:01 | 0.186/0.055/0.098 | 0.64 (0.54–0.76) | 3.44 × 10−7 | 1.09 (1.01–1.19) | 0.0385 | 6.0 × 10−8 | 0.64 (0.54–0.76) | 3.4 × 10−7 | 1.09 (1.01–1.19) | 0.0385 | 6.0 × 10−8 |
Effect allele is underlined. Abbreviations are as follows: PSO, psoriasis; AD, atopic dermatitis. Table shows only independent loci (r2 < 0.5) and the SNV with the strongest association.
Stepwise conditional analysis was carried out with multinomial regression models and resulted in three psoriasis-specific and two opposing signals.
Ontology and Network Analysis of Genes Indicate Effects in the Skin Barrier and Immune Response
Genes implicated from genome-wide and conditional analyses (identified from Tables 1, 2, and 3) were investigated via predicted protein network and gene ontology (GO) analysis. The results are summarized in Figure S6. The GO term “keratinocyte differentiation” (GO:0030216) is enriched in genes implicated in AD and psoriasis risk (FDR p = 4.3 × 10−4 in AD; p = 6.9 × 10−4 in psoriasis; and p = 2.7 × 10−3 in opposing effects). The GO term “response to interferon-gamma” (GO:0034341) is also significantly enriched in psoriasis (FDR p = 1.9 × 10−3).
Discussion
This genome-wide comparative analysis confirms a high degree of genomic coincidence between AD and psoriasis, suggesting that common molecular mechanisms are involved. This agrees with the central role of epidermal barrier defects and T-cell-dominated inflammation in both diseases.48 Within the six regions of colocalization, we demonstrate coassociated and independent disease-specific loci. Of note, all coassociated loci display opposing (antagonistic) effects on AD and psoriasis, in agreement with the epidemiological observations of lower-than-expected coincidence between these diseases in the population.5 Within these loci, specific variants including chromosome 2q31.2 (rs62176107), chromosome 5q33.1 (rs17728338), and within RAD50 on chromosome 5q33.3 (rs6596086) demonstrate opposing effects on risk of AD and psoriasis. This raises the intriguing possibility that the same biological mechanisms might act differentially on AD versus psoriasis. However, our current data cannot distinguish this specific opposing mechanism from the possibility that each lead variant is in LD with other variants having opposing effects in each disease.
The majority of the opposing effect loci are implicated in pathways related to adaptive immunological functions, which potentially mirrors the polarized immune mechanisms.6 It might further be speculated whether the presence of multiple opposing alleles reflects balancing selection as a response to heterogeneity in environmental pressures. Balancing selection is particularly common within the extended MHC region and has been proposed as a potential explanation for antagonistic effects at multiple loci in different autoimmune diseases.56
Two of the loci displaying opposing effects (ANXA6/TNIP1 and PRKRA) have not previously been reported in association with psoriasis and/or AD. Formal external validation is limited by the requirement for additional independent, population-matched GWAS data for AD and psoriasis, but data available from RNA sequencing and microarray analyses provide some support for the differential expression of ANXA6/TNIP1 and PRKRA in AD and psoriasis, relative to normal or uninvolved skin. The lead variant within PRKRA might mediate opposing effects in AD and psoriasis via miRNA processing and/or cellular response to environmental stress, and we hypothesize that this reflects the striking differential susceptibility to viral and bacterial skin infections observed in AD and psoriasis. The opposing effect of variation in ANXA6 suggests a role for calcium-dependent effects in defining patterns of skin inflammation.
On chromosome 1q21.3, apart from well-established AD-associated FLG mutations and psoriasis-associated deletion of LCE3B-LCE3C, FLG-AS1 is a plausible candidate to mediate differential AD/psoriasis risk via the network of regulatory elements coordinating gene expression.57 Natural antisense transcripts contribute to gene regulation via a variety of transcriptional and posttranscriptional mechanisms58 and include effects on human epidermal differentiation.59 The proximity of FLG-AS1 to FLG and HRNR, combined with data showing coordinated differential expression of these genes, supports a role in control of keratinocyte terminal differentiation.
On chromosome 5q31.1, antagonistic signals for AD and psoriasis have previously been attributed to IL13.10,11,21 We here show that IL13 polymorphisms specifically influence AD risk, whereas opposing signals map to RAD50. The Rad50 protein, a component of the MRN complex (Mre11, Rad50, and Nbs1), is involved in DNA double-strand break repair but has no known function directly related to AD or psoriasis. However, RAD50 mRNA shows significantly increased expression in psoriasis lesional skin and a trend to reduced expression in AD lesional skin (Table S7). Of note, RAD50 is located in the center of the Th2-cytokine cluster and its 3′ end is part of a locus control region regulating expression of these cytokine genes.60 AD and psoriasis represent opposing extremes of Th2 cell dysregulation, and therefore we hypothesize that RAD50 polymorphisms might exert opposing effects on AD and psoriasis through variation in DNA repair resulting in a differential skew in Th2 cell response.
Our dissection of the MHC locus confirms the presence of multiple independent psoriasis-risk loci. Markers tagging HLA-Cw∗0602 generate the strongest effects, which is in line with previous reports.17,21,24,44,61 CD8+ T cells are increased in the epidermis of lesional psoriatic skin, and the association of psoriasis susceptibility primarily with class I HLA alleles might reflect the critical role of psoriasis-associated (auto-)antigen presentation to pathogenic CD8+ T cells.62 CD8+ T cells are also increased in the epidermis of AD skin, but with strikingly different cytokine profiles compared to psoriasis.63 The opposing effects of class II HLA alleles in AD and psoriasis might represent the differential responses to pathogenic and allergenic peptides presented to CD4+ T cells.64 GWASs in AD by univariate and multivariate models have reported association signals in the MHC class I and II regions22,65 and two specific HLA class II haplotypes, HLA-DRB1∗0701 (a protective effect) and HLA-B∗4402 (a risk effect).20 Our analysis confirms the association of classical HLA class II alleles with AD, but in the conditional analysis, only HLA-DQA1∗02:01 remained, showing a significant protective effect on AD and a significant opposing effect on psoriasis. A further opposing locus mapped to HLA-C∗03:03 (Table 3).
The reported observation of AD occurring within the offspring of parents with psoriasis8 is not supported by our findings, and the observation that both Th1-cell-dominated autoimmune and Th2-cell-dominated allergic diseases can show aggregation within families7 also presents a discrepancy with our analyses. It is possible that there are shared risk loci for AD and psoriasis that were not detected in our current study because of lack of power, if the shared effect is not strong; alternatively, there might be hereditary risk factors associated with predisposition to any chronic inflammatory (auto-)immune disease. It is also possible that diagnostic misclassification occurs, particularly in pediatric cases, where the clinical signs of psoriasis are more difficult to distinguish from AD than is the case in adult disease,66 or by recall bias for disease in parents.
It is interesting to estimate the extent to which our findings can explain the mutual exclusivity of AD and psoriasis, but an accurate assessment is hindered by the lack of published data on the proportion of AD and psoriasis cases where the diseases do and do not co-occur. Henseler et al. report a 25-fold lower prevalence of AD occurring in psoriasis cases5 and assuming a prevalence of 10% and 2% for AD and psoriasis, respectively,3,4 we estimate that the effects at the six opposing loci listed in Tables 1, 2, and 3 would result in a reduction in prevalence of AD from 10% to 8% within the group of individuals with psoriasis. This 2% reduction contrasts with the 25-fold reduction reported by Henseler et al.,5 which is equivalent to a reduction of 9.6%, from 10% in the population to 0.4%. Our results have therefore explained approximately 21% (2/9.6 × 100) of the mutual exclusivity of AD and psoriasis.
Taken together, our comparative analyses of AD and psoriasis support a paradigm in which genetic factors determining keratinocyte differentiation and cutaneous barrier function have particularly strong effects on AD risk, whereas in psoriasis genetic factors influencing (auto-)antigen recognition are of paramount importance. Furthermore, multiple pleiotropic loci with antagonistic effects contribute to opposing mechanisms of adaptive immunity in both AD and psoriasis.
The meta-analysis-inspired methodology developed in the course of this study has demonstrated the power to leverage additional information from GWAS and high-density SNV data and to dissect cross-phenotype associations. AD and psoriasis are particularly well suited to the compare/contrast approach, but this methodology will be applicable to many other complex traits with overlapping and disease-specific phenotypic features. Characterizing shared and opposing molecular mechanisms across complex phenotypes will expand our understanding of biology and disease and will have implications for treatment and drug discovery.
Consortia
Membership of the PAGE (Population Architecture using Genomics and Epidemiology) consortium is as follows: Trilokraj Tejasvi, Johann E. Gudjonsson, John J. Voorhees, Jun Ding, Yanming Li, Hyun M. Kang, Goncalo R. Abecasis, Dafna D. Gladman, Fawnda J. Pellett, Vinod Chandran, Cheryl F. Rosen, Proton Rahman, Sulev Koks, Külli Kingo, Tonu Esko, Andres Metspalu, Peter Gregersen, Andrew Henschel, Marin Aurand, Bruce Bebo, and Henry W. Lim.
Acknowledgments
We are grateful to the individuals who provided clinical data and DNA for research. This study makes use of genome-wide analysis data generated by the Wellcome Trust Case-Control Consortium (WTCCC); a full list of investigators is available at http://www.wtccc.org.uk. Funding for the WTCCC was provided by Wellcome Trust awards 076113 and 085475. This work was additionally supported by the Wellcome Trust through an Intermediate Clinical Fellowship (WT086398MA) to S.J.B., Senior Research Fellowship in Basic Biomedical Science (087436/Z/08/Z and 102858/Z/13/Z) to H.J.C., Programme Grant (092530/Z/10/Z) to W.H.I.M. and A.D.I., and Strategic Award (098439/Z/12/Z) to W.H.I.M. The KORA research platform was initiated and financed by the Helmholtz Zentrum München - German Research Center for Environmental Health, funded by the German Federal Ministry of Education and Research and by the State of Bavaria. KORA research was supported within the Munich Center of Health Sciences (MC Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ. The project received infrastructure support through DFG Clusters of Excellence “Inflammation at Interfaces” (grants EXC306 and EXC306/2) and the German Federal Ministry of Education and Research within the framework of e:Med research and funding concept (sysINFLAME, 01ZX1306A). M.M.N. is a member of the Cluster of Excellence “ImmunoSensation.” The NCRC-ADC is supported by the National Children’s Research Centre, Dublin. We also acknowledge use of Trinity Biobank samples from the Irish Blood Transfusion Service. This work was supported by the NIH (R01AR042742, R01AR050511, R01AR054966, R01AR062886-01, R01AR062382) and by the Babcock Memorial Trust. J.T.E. is supported by the Ann Arbor Veterans Affairs Hospital.
Published: January 8, 2015
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Contributor Information
Stephan Weidinger, Email: sweidinger@dermatology.uni-kiel.de.
Sara J. Brown, Email: s.j.brown@dundee.ac.uk.
Supplemental Data
Lead SNP defined by clumping procedure (Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81, 559-75 2007); genomewide significant results defined as Tmax>6, BFDP<0.05 with PO=1/999 or PMNM<10-8 are marked in bold; genes and transcripts identified from UCSC Genome Browser Human Feb. 2009 (GRCh37/hg19) Assembly accessed 21 March 2014.
Gene expression data available from published and publically available data sets are summarized for candidate genes.
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes, http://browser.1000genomes.org
Ensembl Genome Browser, http://www.ensembl.org/index.html
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org/
R statistical software, http://www.r-project.org/
STRING 9.1, http://www.string-db.org/
References
- 1.Deckert S., Kopkow C., Schmitt J. Nonallergic comorbidities of atopic eczema: an overview of systematic reviews. Allergy. 2014;69:37–45. doi: 10.1111/all.12246. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths C.E., Barker J.N. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 3.Parisi R., Symmons D.P., Griffiths C.E., Ashcroft D.M., Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J. Invest. Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 4.Deckers I.A., McLean S., Linssen S., Mommers M., van Schayck C.P., Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS ONE. 2012;7:e39803. doi: 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henseler T., Christophers E. Disease concomitance in psoriasis. J. Am. Acad. Dermatol. 1995;32:982–986. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 6.Eyerich S., Onken A.T., Weidinger S., Franke A., Nasorri F., Pennino D., Grosber M., Pfab F., Schmidt-Weber C.B., Mempel M. Mutual antagonism of T cells causing psoriasis and atopic eczema. N. Engl. J. Med. 2011;365:231–238. doi: 10.1056/NEJMoa1104200. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki K., Li X., Sundquist K., Sundquist J. Shared familial aggregation of susceptibility to autoimmune diseases. Arthritis Rheum. 2009;60:2845–2847. doi: 10.1002/art.24749. [DOI] [PubMed] [Google Scholar]
- 8.Maas T., Nieuwhof C., Passos V.L., Robertson C., Boonen A., Landewé R.B., Voncken J.W., Knottnerus J.A., Damoiseaux J.G. Transgenerational occurrence of allergic disease and autoimmunity: general practice-based epidemiological research. Prim. Care Respir. J. 2014;23:14–21. doi: 10.4104/pcrj.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cookson W.O., Ubhi B., Lawrence R., Abecasis G.R., Walley A.J., Cox H.E., Coleman R., Leaves N.I., Trembath R.C., Moffatt M.F., Harper J.I. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat. Genet. 2001;27:372–373. doi: 10.1038/86867. [DOI] [PubMed] [Google Scholar]
- 10.Paternoster L., Standl M., Chen C.M., Ramasamy A., Bønnelykke K., Duijts L., Ferreira M.A., Alves A.C., Thyssen J.P., Albrecht E., Australian Asthma Genetics Consortium (AAGC) Genetics of Overweight Young Adults (GOYA) Consortium. EArly Genetics & Lifecourse Epidemiology (EAGLE) Consortium Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat. Genet. 2012;44:187–192. doi: 10.1038/ng.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellinghaus D., Baurecht H., Esparza-Gordillo J., Rodríguez E., Matanovic A., Marenholz I., Hübner N., Schaarschmidt H., Novak N., Michel S. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat. Genet. 2013;45:808–812. doi: 10.1038/ng.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsoi L.C., Spain S.L., Knight J., Ellinghaus E., Stuart P.E., Capon F., Ding J., Li Y., Tejasvi T., Gudjonsson J.E., Collaborative Association Study of Psoriasis (CASP) Genetic Analysis of Psoriasis Consortium. Psoriasis Association Genetics Extension. Wellcome Trust Case Control Consortium 2 Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P., Goudie D.R., Sandilands A., Campbell L.E., Smith F.J. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez E., Baurecht H., Herberich E., Wagenpfeil S., Brown S.J., Cordell H.J., Irvine A.D., Weidinger S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J. Allergy Clin. Immunol. 2009;123:1361–1370.e7. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Hüffmeier U., Traupe H., Oji V., Lascorz J., Ständer M., Lohmann J., Wendler J., Burkhardt H., Reis A. Loss-of-function variants of the filaggrin gene are not major susceptibility factors for psoriasis vulgaris or psoriatic arthritis in German patients. J. Invest. Dermatol. 2007;127:1367–1370. doi: 10.1038/sj.jid.5700720. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y., Terron-Kwiatkowski A., Liao H., Lee S.P., Allen M.H., Hull P.R., Campbell L.E., Trembath R.C., Capon F., Griffiths C.E. Filaggrin null alleles are not associated with psoriasis. J. Invest. Dermatol. 2007;127:1878–1882. doi: 10.1038/sj.jid.5700817. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X.J., Huang W., Yang S., Sun L.D., Zhang F.Y., Zhu Q.X., Zhang F.R., Zhang C., Du W.H., Pu X.M. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat. Genet. 2009;41:205–210. doi: 10.1038/ng.310. [DOI] [PubMed] [Google Scholar]
- 18.de Cid R., Riveira-Munoz E., Zeeuwen P.L., Robarge J., Liao W., Dannhauser E.N., Giardina E., Stuart P.E., Nair R., Helms C. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat. Genet. 2009;41:211–215. doi: 10.1038/ng.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergboer J.G., Zeeuwen P.L., Irvine A.D., Weidinger S., Giardina E., Novelli G., Den Heijer M., Rodriguez E., Illig T., Riveira-Munoz E. Deletion of Late Cornified Envelope 3B and 3C genes is not associated with atopic dermatitis. J. Invest. Dermatol. 2010;130:2057–2061. doi: 10.1038/jid.2010.88. [DOI] [PubMed] [Google Scholar]
- 20.Weidinger S., Willis-Owen S.A., Kamatani Y., Baurecht H., Morar N., Liang L., Edser P., Street T., Rodriguez E., O’Regan G.M. A genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum. Mol. Genet. 2013;22:4841–4856. doi: 10.1093/hmg/ddt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair R.P., Duffin K.C., Helms C., Ding J., Stuart P.E., Goldgar D., Gudjonsson J.E., Li Y., Tejasvi T., Feng B.J., Collaborative Association Study of Psoriasis Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota T., Takahashi A., Kubo M., Tsunoda T., Tomita K., Sakashita M., Yamada T., Fujieda S., Tanaka S., Doi S. Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nat. Genet. 2012;44:1222–1226. doi: 10.1038/ng.2438. [DOI] [PubMed] [Google Scholar]
- 23.Stuart P.E., Nair R.P., Ellinghaus E., Ding J., Tejasvi T., Gudjonsson J.E., Li Y., Weidinger S., Eberlein B., Gieger C. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat. Genet. 2010;42:1000–1004. doi: 10.1038/ng.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strange A., Capon F., Spencer C.C., Knight J., Weale M.E., Allen M.H., Barton A., Band G., Bellenguez C., Bergboer J.G., Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2 A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams H.C., Burney P.G., Pembroke A.C., Hay R.J. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br. J. Dermatol. 1994;131:406–416. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 26.Krawczak M., Nikolaus S., von Eberstein H., Croucher P.J., El Mokhtari N.E., Schreiber S. PopGen: population-based recruitment of patients and controls for the analysis of complex genotype-phenotype relationships. Community Genet. 2006;9:55–61. doi: 10.1159/000090694. [DOI] [PubMed] [Google Scholar]
- 27.Wichmann H.E., Gieger C., Illig T., MONICA/KORA Study Group KORA-gen—resource for population genetics, controls and a broad spectrum of disease phenotypes. Gesundheitswesen. 2005;67(1):S26–S30. doi: 10.1055/s-2005-858226. [DOI] [PubMed] [Google Scholar]
- 28.Weiland S.K., von Mutius E., Hirsch T., Duhme H., Fritzsch C., Werner B., Hüsing A., Stender M., Renz H., Leupold W., Keil U. Prevalence of respiratory and atopic disorders among children in the East and West of Germany five years after unification. Eur. Respir. J. 1999;14:862–870. doi: 10.1034/j.1399-3003.1999.14d23.x. [DOI] [PubMed] [Google Scholar]
- 29.O’Donovan M.C., Craddock N., Norton N., Williams H., Peirce T., Moskvina V., Nikolov I., Hamshere M., Carroll L., Georgieva L., Molecular Genetics of Schizophrenia Collaboration Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat. Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 30.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delaneau O., Marchini J., Zagury J.F. A linear complexity phasing method for thousands of genomes. Nat. Methods. 2012;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 32.Marchini J., Howie B., Myers S., McVean G., Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 33.Abecasis G.R., Auton A., Brooks L.D., DePristo M.A., Durbin R.M., Handsaker R.E., Kang H.M., Marth G.T., McVean G.A., 1000 Genomes Project Consortium An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dilthey A.T., Moutsianas L., Leslie S., McVean G. HLA∗IMP—an integrated framework for imputing classical HLA alleles from SNP genotypes. Bioinformatics. 2011;27:968–972. doi: 10.1093/bioinformatics/btr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leslie S., Donnelly P., McVean G. A statistical method for predicting classical HLA alleles from SNP data. Am. J. Hum. Genet. 2008;82:48–56. doi: 10.1016/j.ajhg.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharjee S., Rajaraman P., Jacobs K.B., Wheeler W.A., Melin B.S., Hartge P., Yeager M., Chung C.C., Chanock S.J., Chatterjee N., GliomaScan Consortium A subset-based approach improves power and interpretation for the combined analysis of genetic association studies of heterogeneous traits. Am. J. Hum. Genet. 2012;90:821–835. doi: 10.1016/j.ajhg.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris A.P. Transethnic meta-analysis of genomewide association studies. Genet. Epidemiol. 2011;35:809–822. doi: 10.1002/gepi.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willer C.J., Li Y., Abecasis G.R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakefield J. Bayes factors for genome-wide association studies: comparison with P-values. Genet. Epidemiol. 2009;33:79–86. doi: 10.1002/gepi.20359. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y., Helms C., Liao W., Zaba L.C., Duan S., Gardner J., Wise C., Miner A., Malloy M.J., Pullinger C.R. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4:e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esparza-Gordillo J., Weidinger S., Fölster-Holst R., Bauerfeind A., Ruschendorf F., Patone G., Rohde K., Marenholz I., Schulz F., Kerscher T. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat. Genet. 2009;41:596–601. doi: 10.1038/ng.347. [DOI] [PubMed] [Google Scholar]
- 42.Sun L.D., Xiao F.L., Li Y., Zhou W.M., Tang H.Y., Tang X.F., Zhang H., Schaarschmidt H., Zuo X.B., Foelster-Holst R. Genome-wide association study identifies two new susceptibility loci for atopic dermatitis in the Chinese Han population. Nat. Genet. 2011;43:690–694. doi: 10.1038/ng.851. [DOI] [PubMed] [Google Scholar]
- 43.Marenholz I., Rivera V.A., Esparza-Gordillo J., Bauerfeind A., Lee-Kirsch M.A., Ciechanowicz A., Kurek M., Piskackova T., Macek M., Lee Y.A. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J. Invest. Dermatol. 2011;131:1644–1649. doi: 10.1038/jid.2011.90. [DOI] [PubMed] [Google Scholar]
- 44.Ellinghaus E., Ellinghaus D., Stuart P.E., Nair R.P., Debrus S., Raelson J.V., Belouchi M., Fournier H., Reinhard C., Ding J. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat. Genet. 2010;42:991–995. doi: 10.1038/ng.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hüffmeier U., Uebe S., Ekici A.B., Bowes J., Giardina E., Korendowych E., Juneblad K., Apel M., McManus R., Ho P. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat. Genet. 2010;42:996–999. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel C.V., Handy I., Goldsmith T., Patel R.C. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J. Biol. Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- 47.Kok K.H., Lui P.Y., Ng M.H., Siu K.L., Au S.W., Jin D.Y. The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe. 2011;9:299–309. doi: 10.1016/j.chom.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Guttman-Yassky E., Nograles K.E., Krueger J.G. Contrasting pathogenesis of atopic dermatitis and psoriasis—part I: clinical and pathologic concepts. J. Allergy Clin. Immunol. 2011;127:1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 49.Pruim R.J., Welch R.P., Sanna S., Teslovich T.M., Chines P.S., Gliedt T.P., Boehnke M., Abecasis G.R., Willer C.J. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elder J.T., Bruce A.T., Gudjonsson J.E., Johnston A., Stuart P.E., Tejasvi T., Voorhees J.J., Abecasis G.R., Nair R.P. Molecular dissection of psoriasis: integrating genetics and biology. J. Invest. Dermatol. 2010;130:1213–1226. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- 51.Henry J., Hsu C.Y., Haftek M., Nachat R., de Koning H.D., Gardinal-Galera I., Hitomi K., Balica S., Jean-Decoster C., Schmitt A.M. Hornerin is a component of the epidermal cornified cell envelopes. FASEB J. 2011;25:1567–1576. doi: 10.1096/fj.10-168658. [DOI] [PubMed] [Google Scholar]
- 52.Guttman-Yassky E., Suárez-Fariñas M., Chiricozzi A., Nograles K.E., Shemer A., Fuentes-Duculan J., Cardinale I., Lin P., Bergman R., Bowcock A.M., Krueger J.G. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J. Allergy Clin. Immunol. 2009;124:1235–1244.e58. doi: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 53.Wu Z., Hansmann B., Meyer-Hoffert U., Gläser R., Schröder J.M. Molecular identification and expression analysis of filaggrin-2, a member of the S100 fused-type protein family. PLoS ONE. 2009;4:e5227. doi: 10.1371/journal.pone.0005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takaishi M., Makino T., Morohashi M., Huh N.H. Identification of human hornerin and its expression in regenerating and psoriatic skin. J. Biol. Chem. 2005;280:4696–4703. doi: 10.1074/jbc.M409026200. [DOI] [PubMed] [Google Scholar]
- 55.Kim B.E., Howell M.D., Guttman-Yassky E., Gilleaudeau P.M., Cardinale I.R., Boguniewicz M., Krueger J.G., Leung D.Y. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier. J. Invest. Dermatol. 2011;131:1272–1279. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang K., Baldassano R., Zhang H., Qu H.Q., Imielinski M., Kugathasan S., Annese V., Dubinsky M., Rotter J.I., Russell R.K. Comparative genetic analysis of inflammatory bowel disease and type 1 diabetes implicates multiple loci with opposite effects. Hum. Mol. Genet. 2010;19:2059–2067. doi: 10.1093/hmg/ddq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Guzman Strong C., Conlan S., Deming C.B., Cheng J., Sears K.E., Segre J.A. A milieu of regulatory elements in the epidermal differentiation complex syntenic block: implications for atopic dermatitis and psoriasis. Hum. Mol. Genet. 2010;19:1453–1460. doi: 10.1093/hmg/ddq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faghihi M.A., Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat. Rev. Mol. Cell Biol. 2009;10:637–643. doi: 10.1038/nrm2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kretz M., Siprashvili Z., Chu C., Webster D.E., Zehnder A., Qu K., Lee C.S., Flockhart R.J., Groff A.F., Chow J. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493:231–235. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee G.R., Spilianakis C.G., Flavell R.A. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nat. Immunol. 2005;6:42–48. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- 61.Knight J., Spain S.L., Capon F., Hayday A., Nestle F.O., Clop A., Barker J.N., Weale M.E., Trembath R.C., Wellcome Trust Case Control Consortium. Genetic Analysis of Psoriasis Consortium. I-chip for Psoriasis Consortium Conditional analysis identifies three novel major histocompatibility complex loci associated with psoriasis. Hum. Mol. Genet. 2012;21:5185–5192. doi: 10.1093/hmg/dds344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gunderson A.J., Mohammed J., Horvath F.J., Podolsky M.A., Anderson C.R., Glick A.B. CD8(+) T cells mediate RAS-induced psoriasis-like skin inflammation through IFN-γ. J. Invest. Dermatol. 2013;133:955–963. doi: 10.1038/jid.2012.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hijnen D., Knol E.F., Gent Y.Y., Giovannone B., Beijn S.J., Kupper T.S., Bruijnzeel-Koomen C.A., Clark R.A. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J. Invest. Dermatol. 2013;133:973–979. doi: 10.1038/jid.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mangalam A.K., Taneja V., David C.S. HLA class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile. J. Immunol. 2013;190:513–518. doi: 10.4049/jimmunol.1201891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okada Y., Han B., Tsoi L.C., Stuart P.E., Ellinghaus E., Tejasvi T., Chandran V., Pellett F., Pollock R., Bowcock A.M. Fine mapping major histocompatibility complex associations in psoriasis and its clinical subtypes. Am. J. Hum. Genet. 2014;95:162–172. doi: 10.1016/j.ajhg.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kapila S., Hong E., Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas. J. Dermatol. 2012;53:98–105. doi: 10.1111/j.1440-0960.2012.00878.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lead SNP defined by clumping procedure (Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81, 559-75 2007); genomewide significant results defined as Tmax>6, BFDP<0.05 with PO=1/999 or PMNM<10-8 are marked in bold; genes and transcripts identified from UCSC Genome Browser Human Feb. 2009 (GRCh37/hg19) Assembly accessed 21 March 2014.
Gene expression data available from published and publically available data sets are summarized for candidate genes.


