Abstract
Plants emit a plethora of volatile organic compounds, which provide detailed information on the physiological condition of emitters. Volatiles induced by herbivore-feeding are among the best studied plant responses to stress and may constitute an informative message to the surrounding community and function in the process of plant defence. However, under natural conditions, plants are potentially exposed to multiple concurrent stresses, which can have complex effects on the volatile emissions. Atmospheric pollutants are an important facet of the abiotic environment and can impinge on a plant’s volatile-mediated defences in multiple ways at multiple temporal scales. They can exert changes in volatile emissions through oxidative stress, as is the case with ozone pollution. They may also react with volatiles in the atmosphere; such is the case for ozone, nitrogen oxides, hydroxyl radicals and other oxidizing atmospheric species. These reactions result in breakdown products, which may themselves be perceived by community members as informative signals. In this review we demonstrate the complex interplay between stress, emitted signals and modification in signal strength and composition by the atmosphere, collectively determining the responses of the biotic community to elicited signals.
Keywords: Abiotic stress, biotic stress, cross-stress tolerance, induced volatiles, interactive stresses, multiple stresses, volatile-mediate interactions
INTRODUCTION
Plants communicate with other community members by emitting a blend of volatile organic compounds (VOCs). The chemical composition and ratios of compounds in the blend constitute the plant scent. The constitutively emitted VOC blend and ratios of compounds in that blend are often taxonomically or even species-specific (Bruce et al. 2005). However, there is sometimes a large degree of within-species variation in volatile emissions, which can include general heterogeneity, geographical heterogeneity or quite well defined chemotypes (Bäck et al. 2012; Loreto et al. 2009). Variation in the scent of plants can be induced by different stresses, both biotic and abiotic, and can provide detailed information on the plants physiological condition and phenology (Takabayashi et al. 1994; Kuhn et al. 2004, Loreto & Schnitzler 2010). Furthermore, the severity of both biotic and abiotic stresses can modulate the intensity of VOC emissions (Toome et al. 2010; Brilli et al. 2011; Copolovici et al. 2011; Niinemets et al. 2013; Opriş et al. 2013), implying that stress alters both the plant scent bouquets and intensity.
Numerous ecological processes are to varying extents facilitated by volatiles, which are transported from the emitting to receiving organisms in air currents. The dilution of volatiles in air clearly affects the distance over which they can act as effective signals. However, the composition of that air and how it both impacts directly on the emitter (in this review plants) and on their volatile emissions has long been overlooked. In more recent times a growing body of work has focussed on how atmospheric pollutants may exert oxidative stress and induce plants to emit volatiles (Vuorinen et al. 2004), while additional work has described the degradation of volatiles by oxidising pollutants and how that process may reduce signalling efficiency (Himanen et al. 2009; Pinto et al. 2010; McFrederick et al. 2009).
In this review, we take an approach based around the idea that emission of VOCs constitutes a form of ‘plant language’. We outline some of the volatiles induced by oxidising pollutants, the phenomenon of degradation of volatiles by atmospheric pollutants and the known impact of the process on volatile-mediated interactions. We go on to examine how differences in the reactivity of volatiles within a blend may result in evolution of a volatile signal. We also examine how the responses of plants to oxidizing pollutants overlap with those that are regulated by volatiles in the process of inter- and intra-plant signalling. Additional insight as to the implications of atmospheric pollutants on chemical ecology will be provided, with particular focus on Brassicaceae and their associated community. We argue that for gaining full mechanistic insight into plant-plant and plant-insect interactions understanding volatile signal reactions is of paramount significance.
PLANT LANGUAGES
When thinking of VOCs emitted by plants and their ecological functions, we can liken the emission of volatiles to a plant “language”. Similar analogies have been coined previously, such as the ‘cry for help’ synonym for indirect defence, which is the attraction of herbivore natural enemies to herbivore-damaged plants (Dicke et al. 1990; Dicke 2009). The phenomenon of “talking trees” also draws upon a linguistic analogy to describe plants altering their defences upon exposure to volatiles from biotically-stressed neighbours (Baldwin & Schultz 1983; Rhoades 1983; Fowler & Lawton 1985). The intricacies of such interactions have been subject to immense scrutiny from ecologists and evolutionary scientists. The degree of ‘speaking’ and ‘listening’ and the capabilities of plants to do both has been a focal issue of chemical ecology and chemical biology (Dicke et al. 2003).
The intended recipient of volatile signals is also a critical issue from an evolutionary point of view. This is particularly true for plant-plant interactions, for which it has been posited that between-plant interactions – the original ‘talking trees’ hypothesis – is actually an example of eavesdropping, whereby the neighbouring plants are responding to a message meant for a different recipient (Karban & Baxter 2001; Karban & Maron 2002). Even when merging these few simple analogies it starts to become clear that plant languages are complex. To this end, plant defence and the interactions between plants, neighbouring plants and the multitude of cohabiting arthropods, have been the focus of numerous studies and reviews (Dicke 2009; Kant et al. 2009; Dicke & Baldwin 2010; de Rijk et al. 2013). VOCs have been shown to be either attractive or repellent to arthropods foraging for food or hosts. These may be herbivorous, predatory, parasitic or pollinating arthropods, which may all utilise VOCs as cues for locating their required feeding resource, prey or oviposition target, but can also serve for avoidance of inappropriate or hazardous situations (Turlings et al. 1990; Vet & Dicke 1992; Oluwafemi et al. 2011; Kessler et al. 2013). Plant-emitted VOCs may also signal to vascularly distant parts of the same plant, resulting in systemic responses (Heil & Silva Bueno 2007; Frost et al. 2007; Niinemets et al. 2013) and in doing so warn of impending attacks to the neighbouring plants, which may eavesdrop on the signal (Baldwin & Schultz 1983; Rhoades 1983; Kost & Heil 2006; Karban & Maron 2002, Karban et al. 2006). Yet, an important aspect often overlooked is that plants in a community form their specific microclimate characterized by greater humidity and less severe temperature gradients (Niinemets & Anten 2009; Kegge & Pierik 2010; Gommers et al. 2013). Induced defences in eavesdropping receiver plants make these more resistant to ongoing and future attacks. Thus, signalling to neighbours can play an important role in preserving the integrity of the vegetation and reducing the risk of more severe abiotic stresses in the community, implying that “eavesdropping” can benefit the signal sending plants as well.
Complexity of “language” vs. “loudness” of talk
To expand upon the language analogy we may think of the different compounds emitted by plants as words and the blend of compounds emitted constituting sentences. In general, plants emit compounds belonging to a few ubiquitous compound classes such as terpenoids, benzenoids, aliphatic alcohols and aldehydes (Niinemets et al. 2013; Pichersky & Gershenzon 2002). On the other hand, individual plant species frequently emit volatile blends that are specific to the given species, and often specific to the stress acting upon it (Bruce et al. 2005; Niinemets et al. 2013). The ratios of compounds in the blend provide sufficient information for herbivorous insects to locate their host plants within complex environments (Bruce et al. 2005), while individual components of a blend may constitute non-host cues if other blend components are not present (Webster et al. 2010a). Furthermore, the “loudness” of the talk, the magnitude of the emissions induced in response to stress is often quantitatively linked to the severity of the stress, e.g. to the amount of leaf area consumed by herbivores (Copolovici et al. 2011; Niinemets et al. 2013) or damaged by pathogens (Steindel et al. 2005; Toome et al. 2010; Niinemets et al. 2013). As well as the ratios of compounds, the qualitative blend of volatiles can be rather specific with some compounds common to almost all plants, while others may be specific to one or few related taxa (Pichersky & Gershenzon 2002; Karban 2011; McCormick et al. 2012). However, species-specific constitutive blends of volatiles may have substantial variation in the ratio of dominating compounds when populations of a larger geographical scale are compared (Semiz et al. 2007), and the responses of the blends induced by elicitors can also widely vary (Semiz et al. 2012), collectively constituting a large evolutionary pool for stress response and adaptation. In the case of the Brassicaceae, a range of volatile glucosinolate breakdown products are emitted when plants are damaged (e.g., Gols et al. 2009). Isothiocyanates are one such breakdown product and represent a major component of the odour characteristic of damaged brassicaceous plants. Individual isothiocyanates, and particularly 3-butenyl isothiocyanate, have been shown to attract aphid parasitoids without the presence of other compounds (Blande et al. 2007a).
Signal fidelity and longevity
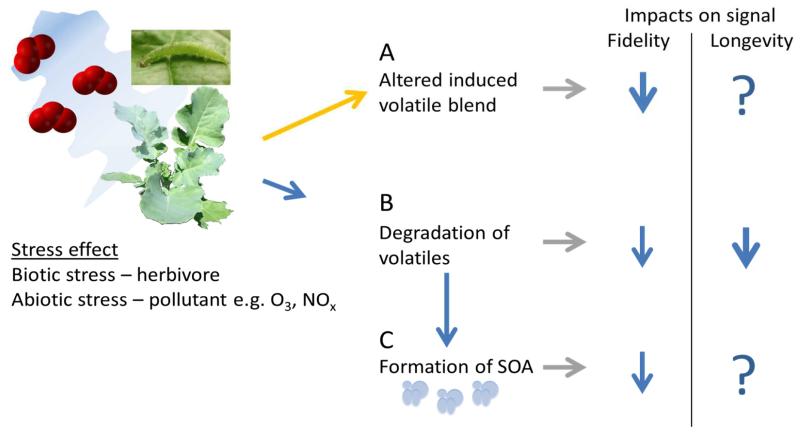
Two critical issues relating to the efficiency of communication between one organism and another are the fidelity and longevity of the signal (fig. 1). The majority of previous studies reporting the responses of arthropods and plants to VOCs have focused on emitter plants subjected to either no controlled stress or else to single stresses (Glinwood et al. 2009; Holopainen & Gershenzon 2010). However, natural environments are exceedingly more complicated, incorporating multiple biotic and abiotic factors that act simultaneously on plants and have the potential to induce blends of VOCs that are different in composition to those induced by the individual stressors (de Rijk et al. 2013). The effect of multiple stresses may be to induce different volatile blends, with the consequence being that the ‘listening’ arthropod or plant community will respond differently to volatile blends from plants stressed by multiple factors compared with the response to the volatiles induced by each stress occurring individually (Ponzio et al. 2013). There may also be an overlap in the volatiles induced by different stresses. It has recently been shown that signalling in Arabidopsis in response to egg deposition by Pieris brassicae is similar to that triggered by recognition of pathogen associated molecular patterns (Gouhier-Darimont et al. 2013), which highlights a common defence response to multiple biotic factors. Modification of induced volatile blends constitutes one way that abiotic factors may critically disturb volatile mediated interactions. Induction of volatile emissions by oxidising pollutants, modification of not-yet-airborne volatiles by oxidants within tissues and degradation of airborne volatiles by oxidants in the atmosphere are potentially co-occurring effects of the abiotic environment. In the following each of these areas will be considered in terms of how they affect a volatile blend and the related interactions between organisms.
Fig. 1.
Effects of pollutants on the fidelity and longevity of herbivore-induced volatile signals. A) Indicates the effects of pollutants on the blend of volatiles emitted in response to herbivore-feeding. B) Indicates the degradation of a volatile blend. C) Indicates the formation of secondary organic aerosols (SOA). The predicted effects of these processes on the blend composition (fidelity) and overall blend degradation (longevity) are represented by arrows. Arrow thickness indicates the predicted strength of effect with thicker arrows corresponding with a stronger effect.
ABIOTIC STRESS – VOLATILE INDUCTION
Plant responses to environmental factors
Typically, abiotic stresses, especially long-term sustained stress events, weaken plants and can make them more vulnerable to any subsequent or simultaneous stress such as pathogen or herbivore attack (e.g., Niinemets 2010). A range of abiotic factors are known to have significant effects on the volatile emissions of plants. Among the key environmental and stress factors, drought, humidity, light intensity and quality, ozone, CO2, temperature and nutrient availability all have some impact on the volatile emission dynamics, or on the ratios of compounds in a volatile blend (Gouinguené & Turlings 2002; Staudt & Lhoutellier 2011; Pinto et al. 2010). These factors may also impact on plant quality, which can affect herbivore performance and also the performance of predators and parasitoids. Consequently, the abiotic environment has enormous potential to interfere with multitrophic interactions, including those mediated by volatiles, blends of which may be altered in plants subjected to oxidative stress (Blande et al. 2007b). Some abiotic factors may be classified as stresses, such as enhanced levels of ozone, drought or flooding, whilst others are more difficult to classify based on their effects. Humidity and increased CO2 are environmental factors, which may alter the metabolism of a plant, but do not necessarily constitute a stress. Even enhanced UV-B, long thought to be a plant stress, may not be as unequivocally stressful as previously postulated, a hypothesis that is presented in recent reviews (Hideg et al. 2013; Wargent & Jordan 2013). Therefore, both stressful and other abiotic factors affecting plant metabolism, especially volatile metabolism, should be considered as obstacles to the fidelity of volatile-mediated interactions. In the following, we consider the effects of some prevalent air pollutants on plant volatile emissions, but recognise the significance of other facets of the abiotic environment as drivers of volatile emissions. Previous reviews have provided detailed accounts of the roles of abiotic factors as inducers and regulators of volatile emissions and should be referred to for additional information (e.g., Niinemets et al. 2010; Grote et al. 2013).
Impact of key air pollutants on volatile emissions
Air pollutants are mostly chemical compounds of anthropogenic origin that affect biotic systems due to their enrichment in areas of human activity. Many of the air pollutants also have natural origins (e.g. sulphurous compounds released during volcanic activity, CO2, and oxides of nitrogen and methane released as a result of microbial activity) and participate in normal atmospheric processes. Higher concentrations of these compounds are often a result of human activity, but they can be dispersed to remote areas or undergo atmospheric reactions to form breakdown products or secondary pollutants such as in the photochemical formation of ozone from oxides of nitrogen (Sitch et al 2007).
Ozone has been shown to both induce and reduce volatile emissions from different plant species depending on the severity and duration of exposure (Calfapietra et al. 2013). Exposing Brassica napus plants to 100 nmol mol−1 ozone reduced the emission of two monoterpenes, sabinene and δ-3-carene (Himanen et al. 2009), but this had virtually no effect on the tritrophic interaction incorporating Plutella xylostella as a herbivore and the parasitoid Cotesia vestalis. Hybrid poplar (Populus 10eltoids x maximowiczii) clones have been shown to have increased emissions of hydrocarbons and oxygenated sesquiterpenes upon exposure to 80 nmol mol−1 ozone for five hours per day for 10 days (Pellegrini et al. 2012). In a hybrid aspen clone, Populus tremula L. x P. tremuloides Michx., moderate increases in ozone of 1.3-1.4 times the ambient level induced an increase in total monoterpene emissions (Blande et al., 2007b). On the other hand, strong elicitation of emissions of stress volatiles, including green leaf volatiles, methyl salicylate and sesquiterpenes have been observed in tobacco (Nicotiana tabacum) (Beauchamp et al. 2004; Beauchamp et al. 2005). These emissions were quantitatively associated with ozone dose according to a threshold-type response, characterized by moderately elevated emissions at lower ozone doses and massively enhanced emissions when a certain stress threshold was exceeded (Beauchamp et al. 2005). Such quantitative responses can serve as a valuable resource for constructing induced emission models quantitatively linking stress severity and induced emission responses (Niinemets 2010a; Grote et al. 2013; Niinemets et al. 2013).
Nitrogen oxides – or NOx – are chiefly comprised of nitric oxide, NO, and nitrogen dioxide, NO2. NOx are major components of vehicle exhaust fumes and are involved in the reactions leading to the formation of tropospheric ozone. However, vegetation, especially under stress conditions, can also release NO (Wildt et al. 1997; Copolovici & Niinemets 2010). Exposure to NO2 and NO in fumigation studies showed that there can be variable effects on plant growth depending upon species (Bell et al. 2011). However, there is very limited information on the effects of Nox on volatile emissions of plants. There is indication that NO might induce emissions of some terpenoids from lima bean in exposure studies, but the degree of induction was quite low (Souza et al. 2013). Fumigation of plants with NO prior to oxidative stress induced by either ozone or singlet oxygen generated by Rose Bengal resulted in reduced emission of LOX compounds produced by the octadecanoid pathway and reduced levels of hydrogen peroxide (H2O2) and malondialdehyde (MDA), which are indicative of oxidative stress (Souza et al. 2013; Velikova et al. 2008).
The above examples are non-exhaustive, but highlight that atmospheric pollutants can induce plants to emit volatile compounds whereas the intensity of the emissions and emission composition importantly depend on past stress history and stress severity. Although the complexity of abiotic influences makes evaluation of abiotic stress effects difficult, we argue that the quality and quantity of these induced volatiles do have an effect on the volatile-mediated interactions between different biotic components of a plant-based community.
Overlap of abiotically-induced volatiles and biotically-induced volatiles
The volatiles emitted by plants in response to abiotic factors may constitute either informative cues to other community members, or alternatively, may act as a false signal to receivers searching for a particular resource. Under certain abiotic stress conditions, plants may become a better quality host for herbivores than under regular conditions. An example is leaves that have accelerated senescence caused by excess water stress, which in silver birch (Betula pendula) were observed to be colonised more by aphids than the non-senescing green leaves (Holopainen et al. 2009). The reallocation of nutrients during the senescence process could be the reason for the preferences displayed by the aphids (White 1993; Holopainen et al. 2009), but it could also be that reduced defence in the yellowing leaves underlies the observation. It is likely that in this case visual cues are utilised by the aphids in the foraging process as yellow colouration is known to be much more attractive than green colouration to numerous aphid species (Döring et al. 2009; Debarro 1991). However, if volatiles induced by the abiotic stress are perceived by aphids, they may also constitute a useful cue to the surrounding community. Alternatively, volatile profiles that closely resemble those induced by herbivores – but with no herbivores present – could be misleading to predators and parasitoids searching for prey or hosts. Certain abiotic and biotic stresses induce plants via the same phytohormonal pathways. In fact, there is a convergence of various stress pathways at the level of oxidative signalling (Fujita et al. 2006; Mittler 2006; Koornneef & Pieterse 2008), resulting in development of induced resistance that is effective against a broad variety of attackers (Koornneef & Pieterse 2008). For example, ozone and aphid feeding both induce the salicylic acid pathway, which may lead to overlap in stress-induced volatile blends. Furthermore, both ozone (Beauchamp et al. 2004; Beauchamp et al. 2005) and aphid feeding (Blande et al. 2010b) also induce emissions of sesquiterpenes, further underscoring the overlap of stress pathways. In addition, exposure to ozone at concentrations sufficient to cause visible symptoms on leaves and exposure to spider mite feeding both induce the emission of green leaf volatiles (GLVs) and homoterpenes in lima bean, but emission of the monoterpene β-ocimene was only induced by spider mite feeding (Vuorinen et al. 2004). When exposed to both stresses simultaneously, there was a direct additive effect in stress-induced volatile blends (Vuorinen et al. 2004).
In addition to the overlap between volatiles induced by abiotic factors and those induced by herbivores and other biotic agents, a cross-talk between phytohormone regulated pathways induced by these different agents could result in novel blends of compounds that are different from those induced when either of the factors acted independently. Interestingly, the changes in volatile emissions may not always be a cumulative response to an increasing stress load, but may be indicative of a reduction in stress. This process is known as a cross-stress tolerance, in which prior exposure to one stress provides a degree of resistance to another. As mentioned above, exposure to nitric oxide and ozone in sequence results in a lower volatile emission and less oxidative stress than exposure to the individual pollutants in isolation (Souza et al. 2013). However, it may be important which stress comes first (Zhang et al. 2009; Niinemets 2010) and the effect can also be significantly altered by the timing and severity of the subsequent stress (Niinemets 2010). Any degree of cross stress tolerance involving abiotic factors and herbivore-feeding could affect the volatile emissions of the plant and again impact on the fidelity of volatile-mediated interactions.
However, sometimes, sequential stresses can act almost independently. For instance, ozone exposure of bean (Phaseolus vulgaris) did not alter the vulnerability to Botrytis cinerea infections (Tonneijck 1994). Furthermore, effects of simultaneous exposure to multiple stresses is generally additive or even interactive, but again the effects may be importantly determined by past stress history resulting in either priming or exhaustion of plant defences due to reduction of soluble and storage carbohydrate pools (Richardson et al. 2004; Myers 2005; Niinemets 2010). Especially, sustained exposure to some abiotic stresses such as drought may predispose plant stands to insect damage or fungal attacks (Schoeneweiss 1983; Appel 1984; Mattson & Haack 1987; Wargo 1996; Bigler et al. 2007).
THE ECOLOGICAL EFFECTS OF VOLATILE UPTAKE AND DEGRADATION
Once volatiles have been released from plants, the plant’s control over those volatiles is effectively over. The volatiles have numerous potential fates based on their reactivity with atmospheric pollutants and their degree of volatility. In the following we focus on the uptake of volatiles by vegetation, degradation of volatile compounds by pollutants, the effects of volatile degradation on volatile mediated interactions and some of the biological and ecological effects of degradation products.
Uptake, modification and release of volatiles by vegetation
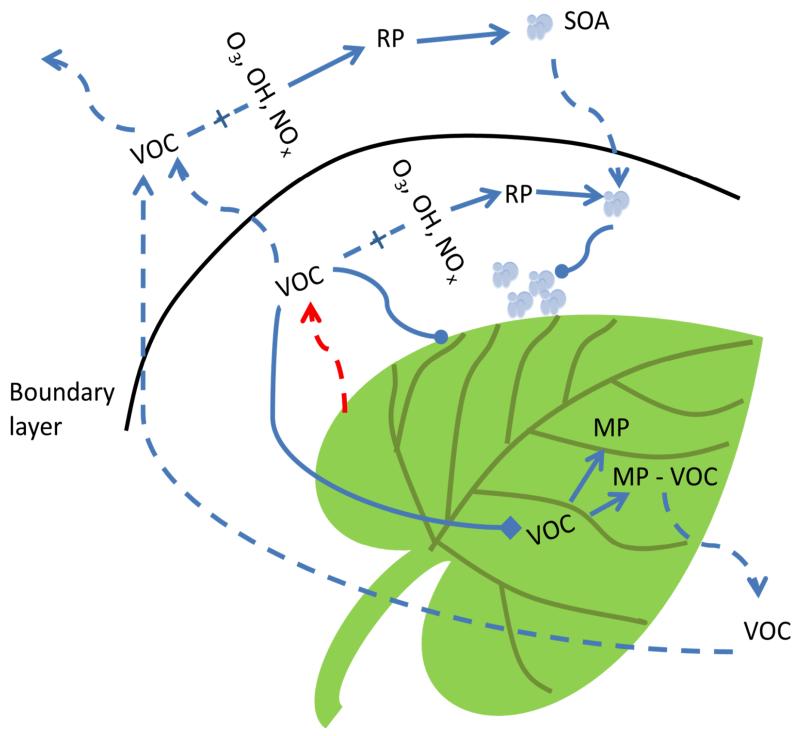
The passage that volatiles take after emission from the plant takes them first to the boundary layer, or the leaf-atmosphere interface (fig 2). The physical and chemical properties of this zone, which envelopes the aerial parts of plants, have been assigned particular importance to exchange of chemical signals between organisms (Riederer et al. 2002). The vapour pressure of a compound largely governs the passage of compounds from the plant into the boundary layer while volatility, lipophilicity and stomatal opening affect whether volatiles will remain in the boundary layer, be taken up by the plant either through stomata or diffusion, or enter the turbulent atmosphere (Riederer et al. 2002).
Fig. 2.
Transport, reaction, deposition and metabolism of volatiles after emission from plants. Volatile emission begins with the red arrow and movement of volatiles is represented by the dashed blue arrows. Reaction of VOCs with oxidants is represented by the line and plus sign. Solid lined arrows represent resulting products, RP denotes reaction products, MP denotes products of metabolism, MP-VOC denotes volatile products of metabolism and the SOA drawing represents secondary organic aerosol. The lines with round ends represent adsorption to surfaces. The line with a diamond at the end represents VOC uptake.
Semi-volatile compounds, such as sesquiterpenes, are more likely to remain in the boundary layer or to be readsorbed to the surfaces of vegetation according to equilibrium partition coefficients (Noe et al. 2008; Fowler et al. 2009; Niinemets et al. 2011), where they may have further active roles in between-species interactions (Himanen et al. 2010).
Abiotic factors also have a decisive role in the route that volatile emissions will take. Humidity affects the deposition of atmospheric constituents such as ozone to surfaces, which may react with volatiles within the boundary layer (Altimir et al. 2006), while temperature is a critical factor in the volatility of chemical compounds (Copolovici & Niinemets 2005). Sesquiterpenes are known to be ‘sticky’ compounds that adsorb to surfaces at low temperatures and are re-released as temperatures increase (Schaub et al. 2010). This adsorption phenomenon has been directly linked to the observations of associational resistance in birch (Betula spp.) neighbouring Rhododendron tomentosum (Himanen et al. 2010) and may also have a role in recent observations of associational resistance to oviposition by Spodoptera littoralis in cotton (Gossypium hirsutum) and alfalfa (Medicago sativa) plants adjacent to S. littoralis-damaged cotton plants (Zakir et al. 2013). It is clear that there is great potential for different abiotic factors to alter the atmospheric behaviour of volatiles and in doing so, to contribute to a change in volatile-mediated processes. Indeed, the adsorption of sesquiterpenes could change the mode of action of the molecule from being a component of a volatile blend to be perceived by foraging predators and parasitoids into a plant surface constituent that may have an impact on host selection by herbivores and their subsequent feeding.
Uptake of volatile compounds into plants could have a role to play in the process of between-plant signalling. One of the most intriguing molecules with respect to inter-plant signalling is methyl jasmonate (MeJA), which has a key role in regulation of plant defence responses. MeJA has been shown to be taken up by plants and metabolised into jasmonic acid and jasmonoyl isoleucine and to a lesser extent jasmonoyl leucine (Tamogami et al. 2008). These metabolic conversions lead to the activation of VOC emissions, which are part of a plants indirect defence response. In addition to uptake of signalling molecules, such as MeJA, plants may take up other volatile compounds, which may be re-emitted or transformed into other by-products. This process has been explored with respect to phytoremediation of indoor air by common houseplants, with aldehydes and ketones shown to be taken up (Tani et al. 2007; Tani & Hewitt 2009). The catabolism of volatiles in plant and emission of reaction products can have a significant impact on the composition of volatiles emitted. VOC catabolism and degradation can affect a range of processes including plant C balance, tolerance to environmental stress, plant signaling, and plant–atmosphere interactions (Oikawa & Lerdau 2013). However, the effects on foraging arthropods have not been studied extensively. Clearly, this is an important gap that needs filling in the near future.
Degradation of herbivore-induced plant volatiles and effects on volatile-mediated interactions
Oxidizing pollutants and atmospheric constituents, including ozone, OH radicals and NO3 radicals can react with VOCs in the atmosphere and impact the dynamics and fidelity of interactions between volatile emitting plants and the volatile-receiving community (Pinto et al. 2007a; Pinto et al. 2010) (fig. 1). Nitrate radicals (NO3) formed by oxidation of NO2 by O3 is considered to be the most important night-time oxidant of VOCs (Monks 2005). NO3 radicals can initiate, but not catalyse removal of VOCs from the atmosphere, which leads to removal of NO3 radicals in the presence of highly reactive VOCs. In day-time reactions, the abundant OH radicals catalyse several atmospheric reactions leading to the rapid removal of numerous volatile compounds (Atkinson & Arey 2003; Monks 2005). Changes to the volatile blend can potentially render the ‘sentence’ conveyed by VOCs distorted or indecipherable to some or all recipients in the community. In this situation the altered blend may just be inactivated, or else it may provide different information to that of the volatile blend actually emitted by the plant. The reaction products formed in atmospheric reactions can be extraordinarily diverse, with ozonolysis of limonene alone shown to result in nearly 1200 different organic compounds, although with some having very short lifetimes (Kundu et al. 2012). In a recent review (Holopainen & Blande 2013), the various fates of volatile organic compounds after release to the atmosphere and the potential for atmospheric oxidants to degrade a volatile signal and thus confer temporal and spatial dimensions upon a volatile blend was outlined, underscoring the importance of the aerial environment in signal propagation and composition.
The majority of studies addressing the effects of oxidizing air pollutants on volatile-mediated interactions have focussed on the role of tropospheric ozone, with a number of the most widely studied volatile-mediated interactions shown to be reduced in efficiency by elevated ozone levels. The process of indirect defence, whereby herbivore-damaged plants emit volatiles that attract natural enemies of the herbivores, has been shown to be reduced in efficiency in a system comprising cabbage (Brassica oleracea), Plutella xylostella and the foraging parasitoid Cotesia vestalis (Pinto et al. 2007b), while a further study from the same group (Pinto et al. 2007c) showed no disruption of foraging in the same system, nor in a system comprising lima bean (Phaseolus lunatus), two-spotted spider mites (Tetranychus urticae) and the foraging predatory mite (Phytoseiulus persimilis), possibly due to the degree of volatile degradation not being sufficient to completely eliminate foraging behaviours in laboratory-based tests. Gate et al. (1995) conducted one of the earliest studies on the effects of air pollutants on the searching behaviour of parasitoids using a system comprising Drosophila subobscura larvae and the braconid parasitoid Asobara tabida. They examined the efficiency of host searching by A. tabida for differing densities of hosts feeding on an artificial diet and the effects of three different pollutants, ozone, nitrogen dioxide (NO2) and sulphur dioxide (SO2) on parasitism rates and searching efficiency. The key observation was that ozone significantly reduced searching efficiency, while there also appeared to be a negative effect of NO2 on the abilities of parasitoids to distinguish between patch sizes of their hosts. Interestingly, Himanen et al. (2009) observed that ozone affected the orientation of C. vestalis searching for P. xylostella feeding on transgenic BT plants, but had no effect on non-transgenic lines.
The host-finding behaviour of the striped cucumber beetle (Acalymma vittatum) has also been shown to be reduced in efficiency by ozone (Fuentes et al. 2013). This herbivore feeds on the foliage, flowers and pollen of cucurbit crops and is attracted by the floral volatiles (Andrews et al. 2007). However, mixing those volatiles with 80ppb ozone appeared sufficient to disrupt orientation towards the floral volatiles in Y-tube bioassays. Beyond the effects of ozone on plant-arthropod interactions, ozone has also been shown to reduce the distance of communication between plants (Blande et al. 2010a), with a concentration of 80ppb having a significant effect in reducing volatile-mediated signalling between lima bean plants in chamber experiments. The value of 80ppb has been identified as important in two laboratory studies of different systems and using different methods, which is suggestive of it being a potential threshold value that results in rapid degradation of biologically important compounds in the plant volatile blends. It is feasible that under field conditions with greater volatile dilution in air currents that lower ozone concentrations have a similar impact.
In addition to degradation of plant volatiles, ozone also degrades the aggregation pheromone of Drosophila melanogaster with negative effects on the attractiveness of the pheromone (Arndt 1995), which emphasises the broader biological consequences of increasing concentrations of atmospheric pollutants.
While the majority of past studies on the effects of pollutants on volatile-mediated interactions have focussed on the effects of ozone, a recent study by Girling et al (2013) concentrated on the effects of diesel engine exhaust fumes on the degradation of volatiles in the blend emitted by oilseed rape (Brassica napus) flowers and the responses of the honeybee Apis mellifera to intact and modified blends. They found that exhaust fumes rapidly and extensively degraded the volatile blend, with two terpenes, α-terpinene and α-farnesene subject to particularly rapid degradation. The fractions of the exhaust fumes responsible for the degradation were primarily NO2 and NO, which at concentration ratios of 1ppm:10ppm and 10ppm:10ppm resulted in substantial degradation of both terpenes. By conditioning the proboscis extension reflex of honeybees to the intact volatile blend, the authors were able to determine that removal of the two reactive terpenes from the volatile blend significantly reduced the ability of honeybees to recognise the odour. This could have profound effects on the abilities of honeybees to utilise volatile cues in urban areas, while the subsequent reactions between NOx and VOCs will in the presence of sunlight lead to the formation of ground-level ozone which could further degrade volatiles with further ecological implications. It should be noted that the products of reactions between the terpenes and NOx were not included in the assessment of odour recognition by Girling et al. (2013), which should be explored further in future studies. The need to consider the ecological effects of both primary and secondary pollutants is emphasised by this work and should be a key future area of research.
Biological effects of plant VOC degradation products
As already mentioned we are unaware of studies that have directly elucidated the effects of VOC degradation products on insects and other herbivorous arthropods. However, some first phase reaction products of VOCs are known to be synthesized directly by various organisms and their biological functions could hint at the potential biological effects of the same compounds forming after atmospheric transformation of plant emissions. The isoprene oxidation product methacrolein is emitted by mechanically damaged sagebrush (Artemisia tridentata tridentata) plants and this compound has the capacity to prime trypsin proteinase inhibitor (TPI) activity and reduce herbivory in nearby Nicotiana attenuata plants (Kessler et al. 2006). Another isoprene degradation product, formaldehyde, when encapsulated in fertilizer pellets and released from soil has been shown to reduce activity of herbivorous slugs (Schuder et al. 2004). In contrast, addition of formaldehyde to artificial diet of moth larvae has been shown to stimulate their growth (Assemi et al. 2012).
Some monoterpene oxidation products are known to elicit biological responses in herbivores. A common monoterpene of conifers and many other plants, α-pinene, is oxidised in atmospheric reactions to form several major first-generation gas or particle phase products such as verbenene, pinic acid, inonic acid and pinonaldehyde (Lee et al. 2006). Verbenone and pinonaldehyde were detected in the secondary aerosol particle mass of conifer forests (Rissanen et al. 2006), but pinonaldehyde formation is related to higher humidity and it is detectable mostly during nights (Zhao et al. 2013). Verbenone is also internally synthesised by aggregating bark beetles (Gitau et al. 2013) and is formed through microbial processes of soil comprising a fraction of decomposing α-pinene–rich pine needle litter (Kainulainen and Holopainen 2002). Verbenone can act as a repellent anti-aggregation signal chemical for some bark beetles species (Gitau et al. 2013), when populations become too crowded. So far, it has not been shown that natural atmospheric oxidation of α-pinene emission from damaged trees could lead to reduced bark beetle attack in nearby trees, but synthetically produced and released verbenone has been an efficient repellent for certain bark beetle species (Gitau et al. 2013). Interestingly, a common bark beetle predating beetle, Temnochila chlorodia, is attracted to high release rates of verbenone (Fettig et al. 2007) suggesting that some of the atmospheric oxidation products of herbivore-induced monoterpenes could act as multitrophic signalling compounds and be efficient attractants of natural enemies of herbivores.
In early oxidation of the monoterpene limonene, formaldehyde and limonoaldehyde have been detected with minor amounts of formic acid, acetone and acetic acid (Lee et al. 2006). Formaldehyde, formic acid, acetone and acetic acid are also common degradation products of other monoterpenes such as β-pinene, myrcene and 3-carene (Lee et al. 2006). Formic acid is an ant venom and it has acaricidal properties (Boncristiani et al. 2012). Acetic acid is a strong attractant of fruit flies (Drosophila sp.) (Landolt et al. 2012).
Sesquiterpenes are rapidly degraded in the presence of ozone (Pinto et al. 2007c). Key reaction products of ozone-initiated oxidation of β-caryophyllene, a common sesquiterpene, are β -caryophyllonic acid, β-caryophyllene aldehyde and formaldehyde (Zhao et al. 2010). β-caryophyllene aldehyde and β-nocaryophyllone aldehyde are degradation products that were identified in ambient SOA samples from a forest site in Southern Finland (Parshintsev et al. 2008). Parshintsev et al. (2008) were able to produce these compounds in experimental ozonolysis reactions of β-caryophyllene in laboratory conditions, but the biological effects of these compounds are unknown. Jaoui et al. (2003) found small amounts of β-caryophyllene oxide, which is a common biogenic VOC emission (e.g. in Betula) as an ozonolysis product of β-caryophyllene. The formation of β-caryophyllon aldehyde and β-caryophyllonic acid has also been reported in ozonolysis studies (Jenkin et al. 2012). Photo-oxidation and β-caryophyllene degradation in the presence of OH radicals can result in formation of a wide range of tertiary and secondary peroxy radicals (Jenkin et al. 2012), which could cause oxidative stress in various biological systems. First phase photo-oxidation products of other sesquiterpenes include formaldehyde from aromadendrene and formic acid from longifolene (Lee et al. 2006). Photooxidation of humulene did not produce formaldehyde, but a C15 keto-aldehyde was detected (Lee et al. 2006).
Green leaf volatiles are an important group of C6 compounds that are active in plant-plant and plant-carnivore interactions. Their atmospheric behaviour is not very well known, but GLVs such as cis-3-hexenol and cis-3-hexenyl acetate are among the first compounds to disappear when a herbivore-induced VOC plume is exposed to ozone (Pinto et al. 2007c).
Induction of sesquiterpene and monoterpene emissions by herbivore-feeding is generally thought to increase the formation of SOA, especially if the result of feeding is an overall increase in emission rather than a qualitative alteration of the volatile blend (Mentel et al. 2013; Amin et al. 2012). However, strong emissions of GLVs rather complicate matters by suppressing the formation of SOAs, which has been presumed to be through the process of scavenging OH radicals (Mentel et al. 2013). This is at least partly supported by the demonstration that in reaction chamber experiments cis-3-hexenol and cis-3-hexenyl acetate produce a significantly higher SOA yield when exposed to ozone than to OH radicals (Hamilton et al.2009). The atmospheric chemistry of VOC degradation is extremely complicated, with many gaps in our knowledge regarding the end products (Kroll & Seinfeld 2008) of oxidation reactions and their ecological effects. However, it is clear that attention should be given to the roles that those reaction products play and the potential effects of adsorbed SOA on interactions between plants and their community.
Modelling the effects of atmospheric reactions on infochemical signals
McFrederick et al. (2008) modelled the oxidation of a few common plant-emitted terpenes using Lagrangian diffusion models and indicated that atmospheric oxidants including ozone, hydroxyl and nitrate radicals would significantly reduce the distance over which volatiles would signal to pollinating insects. The empirical studies mentioned earlier tend to lack information about how combinations of different oxidants act, which is the situation encountered in nature (McFrederick et al. 2009). The modelling route is able to incorporate multiple oxidising species in making predictions, but generally lacks empirical information of how pollinating insects actually respond to the changes in volatile diffusion patterns.
The potential responses of pollinators to reaction products or more stable volatile compounds are not considered in such a model. Therefore, measuring the responses of insects and plants to volatile signals under conditions incorporating multiple oxidants is a key step for future studies. Experiments involving field-scale enrichment of more than one oxidant have not been conducted to date. Therefore, those experiments conducted with enrichment of individual oxidants, such as by Pinto et al. (2008) in the ozone Free Air Concentration Enrichment (O3-FACE) facility based in Kuopio, Finland, offer the best reference points available to date as to how foraging insects are affected by the presence of ozone in their short-range foraging for hosts. In the case of the parasitoid Cotesia vestalis foraging for Plutella xylostella feeding on cabbage (Brassica oleracea), it seems that moderate enrichment of ozone to 1.5 times the ambient level has little effect on foraging success (Pinto et al. 2008). The effects on longer distance foraging are not known to date. However, such effects might be important under low host and parasitoid population densities, which regularly occur for insects with stochastic population dynamics.
In their review, McFrederick et al. (2009) used the available evidence to estimate the ecological interactions most at threat from different oxidising pollutants. Many of the interactions listed are still untested, but the recent observations made since their review include the use of volatiles by foraging herbivores (Fuentes et al. 2013) and plant-plant interactions (Blande et al. 2010a). The short distance foraging by herbivores, which was best represented in the Y-tube study conducted by Fuentes et al. (2013) and the short distance between-plant signalling investigated by Blande et al. (2010a) were both thought to be at low risk by McFrederick et al. (2009) and were both significantly affected by ozone concentrations of around the 80 nmol mol−1 level. This suggests that the effects of oxidising pollutants on volatile-mediated interactions could be more dramatic than earlier thought. It also highlights the need for a holistic view of the effects of ozone on ecological systems, whereby the direct effects of ozone and other abiotic stresses on plants are looked at in tandem with the oxidising effects of the same agents on the volatiles in the air.
Recent advances in the ecology of plant-plant signalling have indicated a degree of complexity that was not previously expected. In both sagebrush (Artemisia tridentata) (Karban & Shiojiri, 2009; Karban et al. 2013) and willow (Salix spp.) (Pearse et al. 2013), receiver plants have been shown to respond more vigorously to volatiles from related plants than to unrelated conspecific plants, which demonstrates a degree of self or kin recognition. The deep mechanics underlying such recognition patterns have yet to be elucidated, but the essential information seems to be somehow encoded in the composition or ratios of compounds in a volatile blend. Therefore, small changes to that blend over short distances may result in considerable alteration to the signal quality and may play a large role in determining the distances over which volatile-mediated plant-plant interactions occur in nature.
OVERLAPS BETWEEN PLANT RESPONSES TO OXIDATIVE STRESS AND PLANT RESPONSES TO VOLATILE SIGNALS
Plant-plant interactions mediated by volatiles are among the most sensitive interactions observed in nature (Blande et al. 2010a). Consequently, they are particularly vulnerable to environmental perturbation. While foraging insects have great potential to learn different odours and associate them with a positive experience (Hoedjes et al. 2011; Allison & Hare 2009; Vet & Dicke 1992; Trowbridge & Stoy 2013), plants do not process information in the same way, and consequently, they could benefit from an alternative strategy for coping with the breakdown of important signalling chemicals by ozone. It seems that one strategy may be to have a large degree of commonality in response to herbivore-induced volatiles and ozone itself. Numerous studies have been conducted on responses of undamaged plants to volatiles from a damaged neighbour, with several defensive traits shown to be modified in response to such an exposure. Some of the defensive attributes include an increase in lipoxygenase gene transcripts, which has been observed in Brassica oleracea plants exposed to herbivore-induced plant volatiles (HIPVs) from conspecifics (Peng et al. 2011) and in tomato (Solanum lycopersicum) and potato (Solanum tuberosum) plants exposed to volatiles from damaged emitter plants (Peng et al. 2005). Genes for the pathogenesis-related proteins PR-1 (Peng et al. 2005), PR-2 (Yi et al. 2009; Arimura et al. 2000), PR-3 and PR-4 (Arimura et al. 2000) have all been shown to be upregulated in response to exposure to HIPVs. Phenylalanine-ammonia lyase (PAL) is also upregulated in response to HIPV exposure (Peng et al. 2005; Arimura et al. 2000). Exposure to herbivore-induced green leaf volatiles has further been shown to induce emissions of other volatiles including the homoterpene (E)-DMNT and acetylated green-leaf volatile derivatives (Yan & Wang 2006; Ruther & Furstenau 2005). In addition, lima bean (Phaseolus lunatus) plants exposed to herbivore-induced plant volatiles have a greater quantity of soluble sugars in extrafloral nectar secretions, which is an indirect defence trait that attracts and arrests natural enemies of herbivores (Kost & Heil 2006). However, the concentration threshold for elicitation of such systemic responses and for how long the systemic elicitation of emissions is preserved is still unclear (Niinemets et al. 2013 for a discussion). Although the systemic elicitation is silenced after the eliciting signal is extinguished, plants may still maintain a certain memory effect, priming, implying a faster and often also a stronger response upon a subsequent volatile signal or biotic attack (Karban & Niiho 1995; Conrath et al. 2006; Heil & Silva Bueno 2007; Ton et al. 2007; Choudhary et al. 2008; Frost et al. 2008).
There is also evidence of many of these HIPV-induced defensive traits being directly induced by exposure to ozone. Such an induction could to some extent negate the need to receive a signal from a damaged neighbour in order to fine-tune defensive responses, although the blend of ozone-induced emissions may be different and accordingly, the response may not necessarily be identical to the response elicited upon exposure to plant stress volatiles. In soybean (Glycine max), lipoxygenase (LOX) activity was doubled by three hours of ozone treatment, with ozone found to modulate LOX expression and to act on the transcription of the LOX genes (Maccarrone et al. 1992). In tomato (Solanum lycopersicum), exposure to ozone reduced soluble sugars and free amino acids, and increased expression of PAL and PR-1 (Cui et al. 2012), and such an activation of the shikimic acid pathway is also compatible with the evidence of enhanced emissions of methyl salicylate (Beauchamp et al. 2005). Ozone exposure also triggers increased expression of PR-1 in tobacco (Nicotiana tabacum) (Pasqualini et al. 2012). Additionally, exposing rice (Oryza sativa) seedlings to enhanced ozone for two days significantly induced PR-5 and two PR-10 proteins (Feng et al. 2008). In a study of the effects of ozone on plant-plant signalling, it was found that a level of 80 nmol mol−1 was sufficient to significantly reduce the effective distance of plant to plant signalling via HIPVs, but that at 120 nmol mol−1 and higher, extrafloral nectar was secreted in response to ozone exposure alone (Blande et al. 2010a). Thus, although the plants were likely to show symptoms of physical damage in response to ozone exposure, the ozone had effectively switched on one of the main induced defences associated with plant-plant signalling, albeit possibly not as a targeted response to the stress but as a metabolic overflow after stoppage of growth. There is a large within-species variability in ozone tolerance associated with the sensitivity of plants to upregulation of the salicylic acid pathway (Rao & Davis 1999; Koch et al. 2000; Vahala et al. 2003), and it remains to be tested whether this variation in abiotic stress tolerance is also associated with differences in engagement of defence pathways upon biotic stress. In particular, whether more abiotic-stress resistant genotypes are more resistant to biotic attacks or whether reduced level of induced emissions reduces plant fitness due to reduced capacity to attract enemies of herbivores.
SUMMARY AND FUTURE DIRECTIONS
There are numerous routes via which abiotic factors and particularly oxidising atmospheric pollutants may impact on volatile-mediated interactions. Factors that combine to influence the blend of volatiles emitted by plants and make them deviate from those induced by herbivore-feeding could have a direct effect on indirect defence and on plant-plant interactions. Atmospheric oxidants that react with a volatile blend will rapidly alter the strength of the signal to other community members. These factors may operate in tandem, with potentially large consequences for the fidelity and longevity of volatile-mediated signalling. However, there is still a lot to be investigated before we really know the effects of such disturbances on interactions between organisms and on broader ecosystem function. One particularly important, but technically challenging focus is the impact of atmospheric pollutants on foraging by pollinators. Long distance foraging via volatile cues is particularly vulnerable to the effects of oxidising pollutants. Therefore, it is important to carefully select systems whereby pollinators utilise volatiles during foraging, and also come into contact with varying levels of pollutants. Although a previous study by McFrederick et al. (2008) offers an excellent indication of how volatiles may be degraded and consequently be reduced in concentration at distances from a point source, there is no consideration of the potential for pollinators to utilise the degradation products, which may be more stable and travel further than their precursor molecules. Future research should focus on understanding the effects of multiple stresses on the information wrapped up in the volatile blends emitted by plants and in how foraging arthropods utilise the emissions directly from plants and their degradation products as well as in understanding how systemic elicitation by volatiles from stressed neighbours is propagated and how these secondary inductions increase the fitness of receiver and transmitter plants.
ACKNOWLEDGMENTS
This work, as part of the European Science Foundation EUROCORES Programme EuroVOL was supported from funds by the Academy of Finland and the Estonian Ministry of Science and Education. Additional support from the Estonian Ministry of Science and Education (institutional grant IUT-8-3), the European Commission through European Regional Fund (the Centre of Excellence in Environmental Adaptation), the European Research Council (advanced grant 322603, SIP-VOL+), the Academy of Finland, project nos. 251898, 141053 and 133322 and the University of Eastern Finland (spearhead project CABI) are acknowledged.
REFERENCES
- Allison JD, Hare JD. Learned and naive natural enemy responses and the interpretation of volatile organic compounds as cues or signals. New Phytologist. 2009;184:768–782. doi: 10.1111/j.1469-8137.2009.03046.x. [DOI] [PubMed] [Google Scholar]
- Altimir N, Kolari P, Tuovinen J-, Vesala T, Back J, Suni T, Kulmala M, Hari P. Foliage surface ozone deposition: A role for surface moisture? Biogeosciences. 2006;3:209–228. [Google Scholar]
- Amin H, Atkins PT, Russo RS, Brown AW, Sive B, Hallar AG, Huff Hartz KE. Effect of bark beetle infestation on secondary organic aerosol precursor emissions. Environmental Science and Technology. 2012;46:5696–5703. doi: 10.1021/es204205m. [DOI] [PubMed] [Google Scholar]
- Andrews ES, Theis N, Adler LS. Pollinator and herbivore attraction to Cucurbita floral volatiles. Journal of Chemical Ecology. 2007;33:1682–1691. doi: 10.1007/s10886-007-9337-7. [DOI] [PubMed] [Google Scholar]
- Appel DN. Canker expansion on water stressed pin oaks colonized by Endothia gyrosa. Plant Disease. 1984;68:851–853. [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- Arndt U. Air-pollutants and pheromones - a problem. Chemosphere. 1995;30:1023–1031. [Google Scholar]
- Assemi H, Rezapanah M, Vafaei-Shoushtari R, Mehrvar A. Modified artificial diet for rearing of tobacco budworm, Helicoverpa armigera, using the Taguchi method and Derringer’s desirability function. Journal of Insect Science. 2012;12:100. doi: 10.1673/031.012.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson R, Arey J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmospheric Environment. 2003;37:S197–S219. [Google Scholar]
- Baldwin I, Schultz J. Rapid changes in tree leaf chemistry induced by damage - evidence for communication between plants. Science. 1983;221:277–279. doi: 10.1126/science.221.4607.277. [DOI] [PubMed] [Google Scholar]
- Beauchamp J, Hansel A, Kleist E, Miebach M, Niinemets Ü, Wisthaler A, Wildt J. Stress induced emission of biogenic volatile organic compounds (VOC) - temporal behaviour and relations between VOC emissions from different biosynthetic pathways. 2004 [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relations between ozone uptake and emission of LOX products. Plant, Cell and Environment. 2005;28:1334–1343. [Google Scholar]
- Bell JNB, Honour SL, Power SA. Effects of vehicle exhaust emissions on urban wild plant species. Environmental Pollution. 2011;159:1984–1990. doi: 10.1016/j.envpol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Bigler C, Gavin DG, Gunning C, Veblen TT. Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos. 2007;116:1983–1994. [Google Scholar]
- Blande JD, Holopainen JK, Li T. Air pollution impedes plant-to-plant communication by volatiles. Ecology Letters. 2010a;13:1172–1181. doi: 10.1111/j.1461-0248.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- Blande JD, Korjus M, Holopainen JK. Foliar methyl salicylate emissions indicate prolonged aphid infestation on silver birch and black alder. Tree Physiology. 2010b;30:404–416. doi: 10.1093/treephys/tpp124. [DOI] [PubMed] [Google Scholar]
- Blande JD, Pickett JA, Poppy GM. A comparison of semiochemically mediated interactions involving specialist and generalist Brassica-feeding aphids and the braconid parasitoid Diaeretiella rapae. Journal of Chemical Ecology. 2007a;33:767–779. doi: 10.1007/s10886-007-9264-7. [DOI] [PubMed] [Google Scholar]
- Blande JD, Tiiva P, Oksanen E, Holopainen JK. Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula × tremuloides) clones under ambient and elevated ozone concentrations in the field. Global Change Biology. 2007b;13:2538–2550. [Google Scholar]
- Boncristiani H, Underwood R, Schwarz R, Evans JD, Pettis J, van Engelsdorp D. Direct effects of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. Journal of Insect Physiology. 2012;58:613–620. doi: 10.1016/j.jinsphys.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “time-of-flight” mass spectrometry (PTR-TOF) PloS ONE. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce T, Wadhams L, Woodcock C. Insect host location: A volatile situation. Trends in Plant Science. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Bäck J, Aalto J, Henriksson M, Hakola H, He Q, Boy M. Chemodiversity of a Scots pine stand and implications for terpene air concentrations. Biogeosciences. 2012;9:689–702. [Google Scholar]
- Calfapietra C, Pallozzi E, Lusini I, Velikova V. Modification of BVOC emissions by changes in atmospheric [CO2] and air pollution. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 253–284. [Google Scholar]
- Choudhary DK, Johri BN, Prakash A. Volatiles as priming agents that initiate plant growth and defence responses. Current Science. 2008;94:595–604. [Google Scholar]
- Conrath U, Beckers GJM, Flors V, García-Agustín P, Jakab G, Mauch F, Mauch-Mani B. Priming: getting ready for battle. Molecular Plant-Microbe Interactions. 2006;19:1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant, Cell and Environment. 2010;33:1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici LO, Niinemets Ü. Temperature dependencies of Henry’s law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere. 2005;61:1390–1400. doi: 10.1016/j.chemosphere.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Cui H, Sun Y, Su J, Ren Q, Li C, Ge F. Elevated O3 reduces the fitness of Bemisia tabaci via enhancement of the SA-dependent defense of the tomato plant. Arthropod-Plant Interactions. 2012;6:425–437. [Google Scholar]
- Debarro P. Attractiveness of 4 colors of traps to cereal aphids (hemiptera, aphididae) in south-australia. Journal of the Australian Entomological Society. 1991;30:263–264. [Google Scholar]
- de Rijk M, Dicke M, Poelman EH. Foraging behaviour by parasitoids in multiherbivore communities. Animal Behaviour. 2013;85:1517–1528. [Google Scholar]
- Dicke M, Sabelis MW, Takabayashi J. Do plants cry for help? evidence related to a tritrophic system of predatory mites, spider mites and their host plants. Symposia Biologica Hungarica. 1990;39:127–134. [Google Scholar]
- Dicke M, Agrawal AA, Bruin J. Plants talk, but are they deaf? Trends in Plant Science. 2003;8:403–405. doi: 10.1016/S1360-1385(03)00183-3. [DOI] [PubMed] [Google Scholar]
- Dicke M. Behavioural and community ecology of plants that cry for help. Plant Cell and Environment. 2009;32:654–665. doi: 10.1111/j.1365-3040.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends in Plant Science. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Döring TF, Archetti M, Hardie J. Autumn leaves seen through herbivore eyes. Proceedings of the Royal Society B-Biological Sciences. 2009;276:121–127. doi: 10.1098/rspb.2008.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Komatsu S, Furukawa T, Koshiba T, Kohno Y. Proteome analysis of proteins responsive to ambient and elevated ozone in rice seedlings. Agriculture Ecosystems & Environment. 2008;125:255–265. [Google Scholar]
- Fettig CJ, McKelvey SR, Dabney CP, Borys RR. The response of Dendroctonus valens and Temnochila chlorodia to Ips paraconfusus pheromone components and verbenone. Canadian Entomologist. 2007;139:141–145. [Google Scholar]
- Fowler D, Pilegaard K, Sutton MA, Ambus P, Raivonen M, Duyzer J, Erisman JW. Atmospheric composition change: ecosystems - atmosphere interactions. Atmospheric Environment. 2009;43:5193–5267. [Google Scholar]
- Fowler S, Lawton J. Rapidly induced defenses and talking trees - the devils advocate position. American Naturalist. 1985;126:181–195. [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiology. 2008;146:818–824. doi: 10.1104/pp.107.113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost CJ, Appel M, Carlson JE, De Moraes CM, Mescher MC, Schultz JC. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecology Letters. 2007;10:490–498. doi: 10.1111/j.1461-0248.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- Fuentes JD, Roulston TH, Zenker J. Ozone impedes the ability of a herbivore to find its host. Environmental Research Letters. 2013;8:014048. [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Current Opinion in Plant Biology. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gate I, McNeill S, Ashmore M. Effects of air pollution on the searching behaviour of an insect parasitoid. Water Air and Soil Pollution. 1995;85:1425–1430. [Google Scholar]
- Girling RD, Lusebrink I, Farthing E, Newman TA, Poppy GM. Diesel exhaust rapidly degrades floral odours used by honeybees. Scientific Reports. 2013;3:2779. doi: 10.1038/srep02779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau CW, Bashford R, Carnegie AJ, Gurr GM. A review of semiochemicals associated with bark beetle (coleoptera: Curculionidae: Scolytinae) pests of coniferous trees: A focus on beetle interactions with other pests and their associates. Forest Ecology and Management. 2013;297:1–14. [Google Scholar]
- Glinwood R, Ahmed E, Qvarfordt E, Ninkovic V, Pettersson J. Airborne interactions between undamaged plants of different cultivars affect insect herbivores and natural enemies. Arthropod-Plant Interactions. 2009;3:215–224. [Google Scholar]
- Gols R, van Dam NM, Raaijmakers CE, Dicke M, Harvey JA. Are population differences in plant quality reflected in the preference and performance of two endoparasitoid wasps? Oikos. 2009;118:733–743. [Google Scholar]
- Gommers CMM, Visser EJW, St Onge KR, Voesenek LACJ, Pierik R. Shade tolerance: when growing tall is not an option. Trends in Plant Science. 2013;18:65–71. doi: 10.1016/j.tplants.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Gouhier-Darimont C, Schmiesing A, Bonnet C, Lassueur S, Reymond P. Signalling of arabidopsis thaliana response to pieris brassicae eggs shares similarities with PAMP-triggered immunity. Journal of Experimental Botany. 2013;64:665–674. doi: 10.1093/jxb/ers362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouinguené S, Turlings T. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiology. 2002;129:1296–1307. doi: 10.1104/pp.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Hamilton JF, Lewis AC, Carey TJ, Wenger JC, Garcia EBI, Munoz A. Reactive oxidation products promote secondary organic aerosol formation from green leaf volatiles. Atmos Chem. Phys. 2009;9:3815–3823. [Google Scholar]
- Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Jansen MAK, Strid A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends in Plant Science. 2013;18:107–115. doi: 10.1016/j.tplants.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Himanen SJ, Blande JD, Klemola T, Pulkkinen J, Heijari J, Holopainen JK. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants--a mechanism for associational herbivore resistance? The New Phytologist. 2010;186:722–732. doi: 10.1111/j.1469-8137.2010.03220.x. [DOI] [PubMed] [Google Scholar]
- Himanen SJ, Nerg A, Nissinen A, Pinto DM, Stewart CN, Jr., Poppy GM, Holopainen JK. Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus) New Phytologist. 2009;181:174–186. doi: 10.1111/j.1469-8137.2008.02646.x. [DOI] [PubMed] [Google Scholar]
- Hoedjes KM, Kruidhof HM, Huigens ME, Dicke M, Vet LEM, Smid HM. Natural variation in learning rate and memory dynamics in parasitoid wasps: Opportunities for converging ecology and neuroscience. Proceedings of the Royal Society B-Biological Sciences. 2011;278:889–897. doi: 10.1098/rspb.2010.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JK, Blande JD. Where do herbivore-induced plant volatiles go? Frontiers in Plant Science. 2013;4(185) doi: 10.3389/fpls.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends in Plant Science. 2010;15:176–184. doi: 10.1016/j.tplants.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Holopainen JK, Semiz G, Blande JD. Life-history strategies affect aphid preference for yellowing leaves. Biology Letters. 2009;5:603–605. doi: 10.1098/rsbl.2009.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaoui M, Leungsakul S, Kamens R. Gas and particle products distribution from the reaction of beta-caryophyllene with ozone. Journal of Atmospheric Chemistry. 2003;45:261–287. [Google Scholar]
- Jenkin ME, Wyche KP, Evans CJ, Carr T, Monks PS, Alfarra MR, Rickard AR. Development and chamber evaluation of the MCM v3.2 degradation scheme for beta-caryophyllene. Atmospheric Chemistry and Physics. 2012;12:5275–5308. [Google Scholar]
- Kainulainen P, Holopainen J. Concentrations of secondary compounds in Scots pine needles at different stages of decomposition. Soil Biology & Biochemistry. 2002;34:37–42. [Google Scholar]
- Kant MR, Bleeker PM, Van Wijk M, Schuurink RC, Haring MA. Plant volatiles in defence. Plant Innate Immunity. 2009;51:613–666. [Google Scholar]
- Karban R. The ecology and evolution of induced resistance against herbivores. Functional Ecology. 2011;25:339–347. [Google Scholar]
- Karban R, Baxter KJ. Induced resistance in wild tobacco with clipped sagebrush neighbors: the role of herbivore behavior. Journal of Insect Behavior. 2001;14:147–156. [Google Scholar]
- Karban R, Maron J. The fitness consequences of interspecific eavesdropping between plants. Ecology. 2002;83:1209–1213. [Google Scholar]
- Karban R, Shiojiri K. Self-recognition affects plant communication and defense. Ecology Letters. 2009;12:502–506. doi: 10.1111/j.1461-0248.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- Karban R, Shiojiri K, Huntzinger M, McCall AC. Damage-induced resistance in sagebrush: Volatiles are key to intra- and interplant communication. Ecology. 2006;87:922–930. doi: 10.1890/0012-9658(2006)87[922:drisva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Karban R, Shiojiri K, Ishizaki S, Wetzel WC, Evans RY. Kin recognition affects plant communication and defence. Proceedings of the Royal Society B-Biological Sciences. 2013;280:20123062. doi: 10.1098/rspb.2012.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R, Niiho C. Induced resistance and susceptibility to herbivory: plant memory and altered plant development. Ecology. 1995;76:1220–1225. [Google Scholar]
- Kegge W, Pierik R. Biogenic volatile organic compounds and plant competition. Trends in Plant Science. 2010;14:126–132. doi: 10.1016/j.tplants.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Kessler A, Halitschke R, Diezel C, Baldwin I. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia. 2006;148:280–292. doi: 10.1007/s00442-006-0365-8. [DOI] [PubMed] [Google Scholar]
- Kessler D, Diezel C, Clark DG, Colquhoun TA, Baldwin IT. Petunia flowers solve the defence/apparency dilemma of pollinator attraction by deploying complex floral blends. Ecology Letters. 2013;16:299–306. doi: 10.1111/ele.12038. [DOI] [PubMed] [Google Scholar]
- Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR. Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiology. 2000;123:487–496. doi: 10.1104/pp.123.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef A, Pieterse CMJ. Cross talk in defense signaling. Plant Physiology. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost C, Heil M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. Journal of Ecology. 2006;94:619–628. [Google Scholar]
- Kroll JH, Seinfeld JH. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmospheric Environment. 2008;42:3593–3624. [Google Scholar]
- Kuhn U, Rottenberger S, Biesenthal T, Wolf A, Schebeske G, Ciccioli P, Kesselmeier J. Strong correlation between isoprene emission and gross photosynthetic capacity during leaf phenology of the tropical tree species Hymenaea courbaril with fundamental changes in volatile organic compounds emission composition during early leaf development. Plant Cell and Environment. 2004;27:1469–1485. [Google Scholar]
- Kundu S, Fisseha R, Putman AL, Rahn TA, Mazzoleni LR. High molecular weight SOA formation during limonene ozonolysis: insights from ultrahigh-resolution FT-ICR mass spectrometry characterization. Atmospheric Chemistry and Physics. 2012;12:5523–5536. [Google Scholar]
- Landolt PJ, Adams T, Rogg H. Trapping spotted wing drosophila, drosophila suzukii (matsumura) (diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. Journal of Applied Entomology. 2012;136:148–154. [Google Scholar]
- Lee A, Goldstein AH, Kroll JH, Ng NL, Varutbangkul V, Flagan RC, Seinfeld JH. Gas-phase products and secondary aerosol yields from the photooxidation of 16 different terpenes. Journal of Geophysical Research-Atmospheres. 2006;111:D17305. [Google Scholar]
- Loreto F, Schnitzler J. Abiotic stresses and induced BVOCs. Trends in Plant Science. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Loreto F, Bagnoli F, Fineschi S. One species, many terpenes: matching chemical and biological diversity. Trends in Plant Science. 2009;14:416–420. doi: 10.1016/j.tplants.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Veldink G, Vliegenthart J. Thermal-injury and ozone stress affect soybean lipoxygenases expression. FEBS Letters. 1992;309:225–230. doi: 10.1016/0014-5793(92)80778-f. [DOI] [PubMed] [Google Scholar]
- Mattson WJ, Haack RA. The role of drought in outbreaks of plant-eating insects. BioScience. 1987;37:110–118. [Google Scholar]
- McCormick AC, Unsicker SB, Gershenzon J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends in Plant Science. 2012;17:303–310. doi: 10.1016/j.tplants.2012.03.012. [DOI] [PubMed] [Google Scholar]
- McFrederick QS, Fuentes JD, Roulston T, Kathilankal JC, Lerdau M. Effects of air pollution on biogenic volatiles and ecological interactions. Oecologia. 2009;160:411–420. doi: 10.1007/s00442-009-1318-9. [DOI] [PubMed] [Google Scholar]
- McFrederick QS, Kathilankal JC, Fuentes JD. Air pollution modifies floral scent trails. Atmospheric Environment. 2008;42:2336–2348. [Google Scholar]
- Mentel Th.F., Kleist E, Andres S, Dal Maso M, Hohaus T, Kiendler-Scharr A, Rudich Y, Wildt J. Secondary aerosol formation from stress-induced biogenic emissions and possible climate feedbacks. Atmospheric Chemistry and Physics. 2013;13:8755–8770. [Google Scholar]
- Mittler R. Abiotic stress, the field environment and stress combination. Trends in Plant Science. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Monks PS. Gas-phase radical chemistry in the troposphere. Chemical Society Reviews. 2005;34:376–395. doi: 10.1039/b307982c. [DOI] [PubMed] [Google Scholar]
- Myers JA. Seedling carbohydrate storage, survival, and stress tolerance in a neotropical forest. University of Florida; 2005. [Google Scholar]
- Niinemets Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. Forest Ecology and Management. 2010;260:1623–1639. [Google Scholar]
- Niinemets Ü, Anten NPR. Packing the photosynthesis machinery: from leaf to canopy. In: Laisk A, Nedbal L, Govindjee, editors. Photosynthesis in silico: understanding complexity from molecules to ecosystems. Springer Verlag; Berlin: 2009. pp. 363–399. [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010a;7:2203–2223. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science. 2013;4:262. doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Peñuelas J. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Staudt M. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010b;7:1809–1832. [Google Scholar]
- Noe SM, Copolovici L, Niinemets Ü, Vaino E. Foliar limonene uptake scales positively with leaf lipid content: “non-emitting” species absorb and release monoterpenes. Plant Biology. 2008;10:129–137. doi: 10.1055/s-2007-965239. [DOI] [PubMed] [Google Scholar]
- Oikawa PY, Lerdau MT. Catabolism of volatile organic compounds influences plant survival. Trends in Plant Science. 2013 doi: 10.1016/j.tplants.2013.08.011. (in press) doi: 10.1016/j.tplants.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Oluwafemi S, Bruce TJA, Pickett JA, Ton J, Birkett MA. Behavioral responses of the leafhopper, Cicadulina storeyi China, a major vector of maize streak virus, to volatile cues from intact and leafhopper-damaged maize. Journal of Chemical Ecology. 2011;37:40–48. doi: 10.1007/s10886-010-9891-2. [DOI] [PubMed] [Google Scholar]
- Opriş O, Copaciu F, Soran ML, Ristoiu D, Niinemets Ü, Copolovici L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: leaf volatiles as a promising new tool to assess toxicity. Ecotoxicology and Environmental Safety. 2013;87:70–79. doi: 10.1016/j.ecoenv.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Pasqualini S, Reale L, Calderini O, Pagiotti R, Ederli L. Involvement of protein kinases and calcium in the NO-signalling cascade for defence-gene induction in ozonated tobacco plants. Journal of Experimental Botany. 2012;63:4485–4496. doi: 10.1093/jxb/ers133. [DOI] [PubMed] [Google Scholar]
- Parshintsev J, Nurmi J, Kilpeläinen I, Hartonen K, Kulmala M, Riekkola M-L. Preparation of β-caryophyllene oxidation products and their determination in ambient aerosol samples. Analytical and Bioanalytical Chemistry. 2008;390:913–919. doi: 10.1007/s00216-007-1755-4. [DOI] [PubMed] [Google Scholar]
- Pearse IS, Hughes K, Shiojiri K, Ishizaki S, Karban R. Interplant volatile signaling in willows: Revisiting the original talking trees. Oecologia. 2013;172:869–875. doi: 10.1007/s00442-013-2610-2. [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Cioni PL, Francini A, Lorenzini G, Nali C, Flamini G. Volatiles emission patterns in poplar clones varying in response to ozone. Journal of Chemical Ecology. 2012;38:924–932. doi: 10.1007/s10886-012-0162-2. [DOI] [PubMed] [Google Scholar]
- Peng J, van Loon JJA, Zheng S, Dicke M. Herbivore-induced volatiles of cabbage (Brassica oleracea) prime defence responses in neighbouring intact plants. Plant Biology. 2011;13:276–284. doi: 10.1111/j.1438-8677.2010.00364.x. [DOI] [PubMed] [Google Scholar]
- Peng J, Li Z, Xiang H, Huang J, Jia S, Miao X, Huang Y. Preliminary studies on differential defense responses induced during plant communication. Cell Research. 2005;15:187–192. doi: 10.1038/sj.cr.7290285. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Pinto DM, Blande JD, Nykanen R, Dong WX, Nerg AM, Holopainen JK. Ozone degrades common herbivore-induced plant volatiles: Does this affect herbivore prey location by predators and parasitoids? Journal of Chemical Ecology. 2007c;33:683–694. doi: 10.1007/s10886-007-9255-8. [DOI] [PubMed] [Google Scholar]
- Pinto DM, Blande JD, Souza SR, Nerg AM, Holopainen JK. Plant volatile organic compounds (VOCs) in ozone (O(3)) polluted atmospheres: The ecological effects. Journal of Chemical Ecology. 2010;36:22–34. doi: 10.1007/s10886-009-9732-3. [DOI] [PubMed] [Google Scholar]
- Pinto DM, Himanen SJ, Nissinen A, Nerg A-M, Holopainen JK. Host location behavior of Cotesia plutellae Kurdjumov (Hymenoptera: Braconidae) in ambient and moderately elevated ozone in field conditions. Environmental Pollution. 2008;156:227–231. doi: 10.1016/j.envpol.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Pinto DM, Nerg A-M, Holopainen JK. The role of ozone-reactive compounds, terpenes, and green leaf volatiles (GLVs), in the orientation of Cotesia plutellae. Journal of Chemical Ecology. 2007b;33:2218–2228. doi: 10.1007/s10886-007-9376-0. [DOI] [PubMed] [Google Scholar]
- Pinto DM, Tiiva P, Miettinen P, Joutsensaari J, Kokkola H, Nerg A-M, Laaksonen A, Holopainen JK. The effects of increasing atmospheric ozone on biogenic monoterpene profiles and the formation of secondary aerosols. Atmospheric Environment. 2007a;41:4877–4887. [Google Scholar]
- Ponzio C, Gols R, Pieterse CMJ, Dicke M. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Functional Ecology. 2013;27:587–598. [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. The Plant Journal. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rhoades D. Responses of alder and willow to attack by tent caterpillars and webworms - evidence for pheromonal sensitivity of willows. ACS Symposium Series. 1983;208:55–68. [Google Scholar]
- Richardson AD, Aikens M, Berlyn GP, Marshall P. Drought stress and paper birch (Betula papyrifera) seedlings: effects of an organic biostimulant on plant health and stress tolerance, and detection of stress effects with instrument-based, noninvasive methods. Journal of Arboriculture. 2004;30:52–61. [Google Scholar]
- Riederer M, Daiss A, Gilbert N, Kohle H. Semi-volatile organic compounds at the leaf/atmosphere interface: numerical simulation of dispersal and foliar uptake. Journal of Experimental Botany. 2002;53:1815–1823. doi: 10.1093/jxb/erf020. [DOI] [PubMed] [Google Scholar]
- Rissanen T, Hyotylainen T, Kallio M, Kronholm J, Kulmala M, Rikkola M. Characterization of organic compounds in aerosol particles from a coniferous forest by GC-MS. Chemosphere. 2006;64:1185–1195. doi: 10.1016/j.chemosphere.2005.11.079. [DOI] [PubMed] [Google Scholar]
- Ruther J, Furstenau B. Emission of herbivore-induced volatiles in absence of a herbivore - response of Zea mays to green leaf volatiles and terpenoids. Zeitschrift Fur Naturforschung C-a Journal of Biosciences. 2005;60:743–756. doi: 10.1515/znc-2005-9-1014. [DOI] [PubMed] [Google Scholar]
- Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A. Real-time monitoring of herbivore induced volatile emissions in the field. Physiologia Plantarum. 2010;138:123–133. doi: 10.1111/j.1399-3054.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Schoeneweiss DF. Drought predisposition to Cytospora canker in blue spruce. Plant Disease. 1983;67:383–385. [Google Scholar]
- Schuder I, Port G, Bennison J. The behavioural response of slugs and snails to novel molluscicides, irritants and repellents. Pest Management Science. 2004;60:1171–1177. doi: 10.1002/ps.942. [DOI] [PubMed] [Google Scholar]
- Semiz G, Blande JD, Heijari J, Işik K, Niinemets Ü, Holopainen JK. Manipulation of VOC emissions with methyl jasmonate and carrageenan in evergreen conifer Pinus sylvestris and evergreen broadleaf Quercus ilex. Plant Biology. 2012;14(Suppl 1):57–65. doi: 10.1111/j.1438-8677.2011.00485.x. [DOI] [PubMed] [Google Scholar]
- Semiz G, Heijari J, Isik K, Holopainen JK. Variation in needle terpenoids among Pinus sylvestris L. (Pinaceae) provenances from Turkey. Biochemical Systematics and Ecology. 2007;35:652–661. [Google Scholar]
- Sitch S, Cox PM, Collins WJ, Huntingford C. Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature. 2007;448:791–794. doi: 10.1038/nature06059. [DOI] [PubMed] [Google Scholar]
- Souza SR, Blande JD, Holopainen JK. Pre-exposure to nitric oxide modulates the effect of ozone on oxidative defenses and volatile emissions in lima bean. Environmental Pollution. 2013;179:111–119. doi: 10.1016/j.envpol.2013.03.065. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lhoutellier L. Monoterpene and sesquiterpene emissions from Quercus coccifera exhibit interacting responses to light and temperature. Biogeosciences. 2011;8:2757–2771. [Google Scholar]
- Steindel F, Beauchamp J, Hansel A, Kesselmeier J, Kleist E, Kuhn U, Wisthaler A, Wildt J. Stress induced VOC emissions from mildew infested oak. Geophysical Research Abstracts. 2005;7 EGU05-A-03010. [Google Scholar]
- Takabayashi J, Dicke M, Posthumus M. Volatile herbivore-induced terpenoids in plant mite interactions - variation caused by biotic and abiotic factors. Journal of Chemical Ecology. 1994;20:1329–1354. doi: 10.1007/BF02059811. [DOI] [PubMed] [Google Scholar]
- Tamogami S, Ralkwal R, Agrawal GK. Interplant communication: Airborne methyl jasmonate is essentially converted into JA and JA-lle activating jasmonate signalling pathway and VOCs emission. Biochemical and Biophysical Research Communications. 2008;376:723–727. doi: 10.1016/j.bbrc.2008.09.069. [DOI] [PubMed] [Google Scholar]
- Tani A, Hewitt CN. Uptake of aldehydes and ketones at typical indoor concentrations by houseplants. Environmental Science and Technology. 2009;43:8338–8343. doi: 10.1021/es9020316. [DOI] [PubMed] [Google Scholar]
- Tani A, Kato S, Kajii Y, Wilkinson M, Owen S, Hewitt N. A proton transfer reaction mass spectrometry based system for determining plant uptake of volatile organic compounds. Atmospheric Environment. 2007;41:1736–1746. [Google Scholar]
- Ton J, D’Allesandro M, Jourdie V, Jakab G, Karlen D, Held M, Mauch-Mani B, Turlings TCJ. Priming by airborne signals boosts direct and indirect resistance in maize. The Plant Journal. 2007;49:16–26. doi: 10.1111/j.1365-313X.2006.02935.x. [DOI] [PubMed] [Google Scholar]
- Tonneijck AEG. Effects of various ozone exposures on the susceptibility of bean leaves (Phaseolus vulgaris L.) to Botrytis cinerea. Environmental Pollution. 1994;85:59–65. doi: 10.1016/0269-7491(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- Trowbridge AM, Stoy PC. BVOC mediated plant-herbivore interactions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 21–46. [Google Scholar]
- Turlings T, Tumlinson J, Lewis W. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- Vahala J, Keinänen M, Schützendübel A, Polle A, Kangasjärvi J. Differential effects of elevated ozone on two hybrid aspen genotypes predisposed to chronic ozone fumigation. Role of ethylene and salicylic acid. Plant Physiology. 2003;132:196–205. doi: 10.1104/pp.102.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Fares S, Loreto F. Isoprene and nitric oxide reduce damages in leaves exposed to oxidative stress. Plant, Cell and Environment. 2008;31:1882–1894. doi: 10.1111/j.1365-3040.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- Vet L, Dicke M. Ecology of infochemical use by natural enemies in a tritrophic context. Annual Review of Entomology. 1992;37:141–172. [Google Scholar]
- Vuorinen T, Nerg AM, Holopainen JK. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but does not disturb tritrophic signalling. Environmental Pollution. 2004;131:305–311. doi: 10.1016/j.envpol.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Wargent JJ, Jordan BR. From ozone depletion to agriculture: Understanding the role of UV radiation in sustainable crop production. New Phytologist. 2013;197:1058–1076. doi: 10.1111/nph.12132. [DOI] [PubMed] [Google Scholar]
- Wargo PM. Consequences of environmental stress on oak: predisposition to pathogens. Annales des Sciences Forestieres. 1996;53:359–368. [Google Scholar]
- Webster B, Bruce T, Pickett J, Hardie J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Animal Behaviour. 2010;79:451–457. [Google Scholar]
- White TCR. The inadequate environment. Nitrogen and the abundance of animals. Springer-Verlag; Berlin, Germany: 1993. [Google Scholar]
- Wildt J, Kley D, Rockel A, Rockel P, Segschneider HJ. Emission of NO from several higher plant species. Journal of Geophysical Research. 1997;102:5919–5927. [Google Scholar]
- Yan Z, Wang C. Wound-induced green leaf volatiles cause the release of acetylated derivatives and a terpenoid in maize. Phytochemistry. 2006;67:34–42. doi: 10.1016/j.phytochem.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Yi H, Heil M, Adame-Alvarez RM, Ballhorn DJ, Ryu C. Airborne induction and priming of plant defenses against a bacterial pathogen. Plant Physiology. 2009;151:2152–2161. doi: 10.1104/pp.109.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakir A, Sadek MM, Bengtsson M, Hansson BS, Witzgall P, Anderson P. Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore. Journal of Ecology. 2013;101:410–417. [Google Scholar]
- Zhang P-J, Zheng S-J, van Loon JJA, Boland W, David A, Mumm R, Dicke M. Whiteflies interfere with indirect plant defense against spider mites in lima bean. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21202–21207. doi: 10.1073/pnas.0907890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kreisberg NM, Worton DR, Isaacman G, Weber RJ, Liu S, Goldstein AH. Insights into secondary organic aerosol formation mechanisms from measured Gas/Particle partitioning of specific organic tracer compounds. Environmental Science & Technology. 2013;47:3781–3787. doi: 10.1021/es304587x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang R, Sun X, He M, Wang H, Zhang Q, Ru M. Theoretical study on mechanism for O-3-initiated atmospheric oxidation reaction of beta-caryophyllene. Journal of Molecular Structure-Theochem. 2010;947:68–75. [Google Scholar]