Abstract
Biogenic volatile organic compound (BVOC) emissions are widely modeled as inputs to atmospheric chemistry simulations. However, BVOC may interact with cellular structures and neighboring leaves in a complex manner during volatile diffusion from the sites of release to leaf boundary layer and during turbulent transport to the atmospheric boundary layer. Furthermore, recent observations demonstrate that the BVOC emissions are bidirectional, and uptake and deposition of BVOC and their oxidation products are the rule rather than the exception. This review summarizes current knowledge of within-leaf reactions of synthesized volatiles with reactive oxygen species (ROS), uptake, deposition and storage of volatiles and their oxidation products as driven by adsorption on leaf surface and solubilization and enzymatic detoxification inside leaves. The available evidence indicates that due to reactions with ROS and enzymatic metabolism, the BVOC gross production rates are much larger than previously thought. The degree to which volatiles react within leaves and can be potentially taken up by vegetation depends on compound reactivity, physicochemical characteristics, as well as their participation in leaf metabolism. We argue that future models should be based on the concept of bidirectional BVOC exchange and consider modification of BVOC sink/source strengths by within-leaf metabolism and storage.
Keywords: catabolism, compound reactivity, compound breakdown, deposition, emission controls, reactive oxygen species, physicochemical characteristics, volatile uptake
INTRODUCTION
Biogenic volatile organic compound (BVOC) emissions play major roles in local and regional air quality by participating in ozone-formation reactions, and in regional to global climate by contributing to aerosol and cloud formation, resulting in large-scale biosphere-atmosphere feedbacks (Heald et al. 2008; Arneth et al. 2009; Hallquist et al. 2009; Guenther et al. 2012; Kulmala et al. 2013). Reliable model-based integrations of BVOC fluxes are needed as inputs for these large-scale models. Traditionally, BVOC emission has been considered as a unidirectional flux of inert (i.e., non-reactive) molecules from the site of synthesis to the ambient atmosphere, and emission models use this simplified concept to directly relate the rate of emission to environmental drivers (e.g., Guenther et al. 1995; Guenther et al. 2006; Monson et al. 2012). For BVOC produced de novo, it is generally assumed that the rate of emission is equal to the rate of production, while for those BVOC stored in specialized storage structures, the rate of emission is assumed to equal the rate of diffusion from the storage pools (for reviews see Niinemets et al. 2010c; Monson et al. 2012; Grote, Monson & Niinemets 2013). These assumptions likely reflect the circumstance that most of the interest in BVOC has to date been oriented towards non-oxygenated volatile isoprenoids (isoprene, monoterpenes, and sesquiterpenes), and less research has dealt with oxygenated volatile compounds (OVOC) such as short-chained volatiles, e.g., acetaldehyde, formaldehyde, acetone, methanol, ethanol, and formic and acetic acids, and the oxidation products of isoprenoids. In contrast to the volatile isoprenoids, OVOC can be rapidly metabolized enzymatically and directly integrated into the central carbon and energy metabolism of plants.
Recent experimental and modeling work demonstrates that the assumption that BVOC emission rate always equals the rate of production at the site of synthesis or rate of vaporization at the site of emission is problematic, especially for OVOC. First, for several compounds, in particular those which partition strongly into the aqueous or lipid phases of the leaf, there can be significant time-lags between changes in BVOC production (or emission from storage pools) and emission from the leaf (Niinemets & Reichstein 2003b; Niinemets & Reichstein 2003a; Noe, Niinemets & Schnitzler 2010; Harley 2013). Second, a unidirectional emission flux can only be supported when ambient gas-phase concentrations are lower than within-leaf concentrations. While this can be the case when the rate of compound production is high such as in strong constitutive isoprenoid emitters, emitting species typically grow intermixed with non-emitting species that have inherently low leaf isoprenoid concentrations and can potentially take up emitted volatiles from the ambient air (Noe et al. 2008; Himanen et al. 2010), and this process can be sustained if there is a chemical sink (metabolization) within the plants (section Chemical sink heterogeneity). In addition, the rates of constitutive isoprene and monoterpene emissions in emitting species vary over several orders of magnitude in dependence on light conditions in the canopy (Harley, Guenther & Zimmerman 1996; Harley, Guenther & Zimmerman 1997; Niinemets et al. 2002a; Grote 2007; Niinemets, Copolovici & Hüve 2010b), suggesting that in turbulently mixed canopy air, low-emitting leaves could also constitute a sink of volatiles.
It has long been known that inorganic species in the atmosphere such as ammonium (Farquhar, Wetselaar & Firth 1979; Farquhar et al. 1980), ozone (Laisk, Kull & Moldau 1989) and SO2 (Pfanz et al. 1987) can be rapidly deposited to vegetation (Wesely 1989). Laboratory experiments on individual leaves clearly illustrate the potential for bidirectional fluxes of a variety of BVOC, including short-chained aldehydes (Kesselmeier 2001; Rottenberger et al. 2004; Karl et al. 2005; Rottenberger et al. 2005) and organic acids (Kesselmeier 2001; Kuhn et al. 2002), but uptake of larger alkenes such as monoterpenes (Copolovici et al. 2005; Noe et al. 2008) has also been observed. Furthermore, large canopy-scale BVOC deposition fluxes have recently been highlighted (Karl et al. 2010; Jardine et al. 2011; Park et al. 2013a; Park et al. 2013b), implying that the bidirectional exchange of volatiles is much more important than generally thought.
Recent evidence further suggests that the assumption of a non-reactive diffusion pathway from the site of synthesis and/or volatilization to the ambient atmosphere is not necessarily valid. Under oxidative stress, a large proportion of reactive isoprenoids might be oxidized along the diffusion pathway generating volatile molecules previously thought to be found only in the atmosphere as the result of oxidative reactions (Jardine et al. 2012b; Jardine et al. 2013). Thus, in addition to the rate of synthesis, within-leaf redox environment may importantly modulate the emission flux of reactive molecules.
This review analyzes the recent evidence on within-leaf reactivity of volatiles, bidirectional emission controls and the significance of the “non-inert” performance of BVOC at different scales of biological organization from cells to ecosystems. First, we provide the basic framework of biophysical and physiological emission flux controls of different volatile compounds, and further analyze the importance of within-leaf redox environment on BVOC within-leaf reactivity. We then review the existing evidence on bidirectional emissions, the bidirectional emissions controls and finally evaluate the significance of such controls at scales ranging from leaf to ecosystems. Overall, we emphasize the need to revise the concept of BVOC emission to include the enzymatic and non-enzymatic reactions of BVOC in planta and to include physicochemical controls on emission, storage, uptake and deposition. This review emphasizes the fundamentally different set of controls operating on the emission and uptake of non-oxygenated lipophilic volatiles, and oxygenated water-soluble volatiles, but also underscores that within-leaf and within-canopy reactions can oxidize a large part of non-oxygenated volatiles before they reach the leaf, canopy and atmospheric boundary layers.
BASICS OF EMISSION OF VOLATILES: FROM UNIDIRECTIONAL TO BIDIRECTIONAL FLUX
Steady-state emission responses
Except for those BVOC that are stored in surface glands or trichomes and may escape directly to the atmosphere, exchange of plant-produced volatiles with the atmosphere occurs through the stomata (Fall & Monson 1992; Loreto et al. 1996; Städler & Reifenrath 2009). As cuticular conductances are low for most volatiles, cuticular emission flux can be neglected apart from conditions when stomata are closed (see also section Deposition on surface for the discussion on surface deposition flux). The exchange of BVOC between plants and the atmosphere will therefore obey Fick’s first law, and the flux of a given BVOC (Ji, mol m−2 s−1) is directly proportional to the difference in BVOC gas-phase concentration (or partial pressure or mole fraction) between the intercellular air space of the leaf (in the sub-stomatal cavity) and the air outside the leaf boundary layer, and inversely proportional to the sum of the resistances between them. Assuming little or no flux across the leaf cuticle, and assuming a low resistance across the leaf boundary layer, a reasonable assumption even at moderate wind speeds, this resistance pathway is dominated by the stomata, and
| (1) |
where Xl,i is the mole fraction of the given BVOC i in the intercellular air space of the leaf and Xa,i that in the ambient air (both in mol mol−1), and gs,i is the stomatal conductance of the given volatile (mol m−2 s−1), while the inverse, rs,i, is the stomatal resistance to the BVOC. For simplicity, Eq. 1 ignores the effect of mass flow of water vapor on the exchange flux (e.g., Ball 1987). The mass flow effect is generally relatively small and we refer to past studies for a full treatment of fluxes (e.g., Niinemets & Reichstein 2003b; Niinemets & Reichstein 2003a; Niinemets et al. 2011).
The stomatal conductance of any BVOC molecule can be conveniently linked to the stomatal conductance of water vapor, gw, as:
| (2) |
where Ds,w is the binary (air/water vapor) diffusion coefficient for water vapor and Ds,i is that for the given volatile. Diffusivities of different volatiles are tabulated in chemical reference databases (e.g., Lugg 1968; Marrero & Mason 1972), but are still lacking for many plant volatiles. However, diffusivities at different temperatures can be predicted with minor errors using structure-function relationships, e.g., based on predicted molar volumes (e.g., Niinemets & Reichstein 2003b). In fact, the ratio Ds,i/Ds,w varies little with temperature for many volatiles (e.g., for isoprene see Singsaas et al. 1997; Sun et al. 2013).
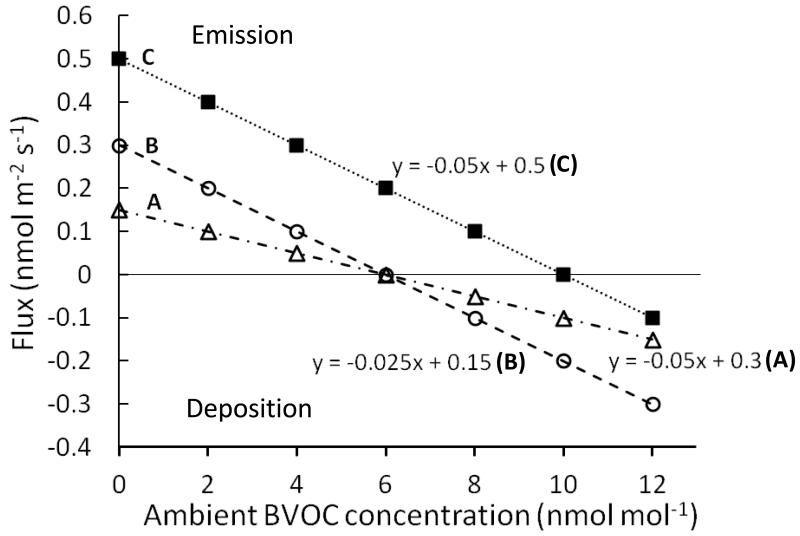
Whether or not a given BVOC is emitted or taken up at any given moment of time depends on the direction of the concentration gradient, Xl,i – Xa,i, between the leaf intercellular air space and the ambient air. When Xl,i = Xa,i, there is no net flux and this concentration is known as the compensation point (Xcomp, Fig. 1). If the atmospheric concentration exceeds Xcomp, uptake will occur, while when the within-leaf concentration is above the compensation point, emission results (Fig. 1).
Figure 1.
Schematic illustration of the potential for bidirectional flux in three hypothetical situations. Curves (A) and (B) represent two leaves with similar rates of BVOC production but different values of stomatal conductance, indicated by the different slopes. The balance between production and catabolism is such that they can maintain a gas-phase concentration of 6 nmol mol−1. When the BVOC ambient concentration exceeds 6 nmol mol−1, the BVOC will be taken up by the leaf, and when the ambient concentration falls below 6 nmol mol−1, emission will occur. The value of 6 nmol mol−1, at which there is no net flux, is referred to as the compensation point. Curve (C) represents a case in which the stomatal conductance is identical to Curve (A) but the net production of BVOC is much higher, resulting in a higher compensation point and a greater likelihood of emission over deposition.
In the literature, there often seems to be some misunderstanding about stomatal controls on volatile exchange. Stomata can sometimes control the emission rate under non-steady-state conditions (section Stomatal controls on emission), but we want to emphasize that when uptake occurs through the stomata, stomata will always control the deposition flux, even if the internal concentration Xl,i is zero. Such a dependence on stomatal conductance has been demonstrated for numerous volatiles including formaldehyde (Filella, Peñuelas & Llusià 2006; Seco, Peñuelas & Filella 2008), acetone, acetonitrile, acrolein, methyl ethyl ketone (MEK), isobutyl methyl ketone (IBMK), chloroform, and benzene (Omasa et al. 2000), acetaldehyde (Cojocariu, Kreuzwieser & Rennenberg 2004; Karl et al. 2005), methyl vinyl ketone (MVK), methacrolein (MACR), and croton aldehyde (Tani, Tobe & Shimizu 2010).
The strength of the stomatal control on uptake of different compounds depends on the diffusivity of the given compound according to Eq. 2. When there is no apparent stomatal control over uptake, the emissions may be driven by deposition on leaf surfaces rather than by stomatal uptake (section Deposition on surface). As pointed out below, stomatal control on emission is more complex and is driven by different mechanisms.
Chemical sources and sinks
Equation 1 indicates that at a given ambient BVOC concentration, the direction and the magnitude of the exchange is driven by BVOC source strength, which is given by the net rate of BVOC production within the leaf (i.e., the rate of production less the rate of further metabolism). For some compounds, chemical conversion of the given compound into other metabolites has long been recognized, such as foliar conversion of ethanol transported from roots of flooded plants to acetaldehyde (Kreuzwieser, Scheerer & Rennenberg 1999; Kreuzwieser et al. 2001; Kreuzwieser & Rennenberg 2013). However, for some other compounds such as isoprene, the rate of metabolism has been considered negligible until recently (Jardine et al. 2012b; Jardine et al. 2013). In light of new evidence, within-leaf reactivity can have a major effect on Xl,i and thus the source strength of most volatiles. Furthermore, any atmospheric volatile that does not have a source within the leaf, including many anthropogenic volatiles and the whole range of products arising from BVOC oxidation, can be taken up by the leaves provided the compound can be metabolized (or go into solution). Understanding the capacity for BVOC degradation, metabolization and/or storage in the apoplast and in the symplast is highly relevant as this capacity is a key factor determining the BVOC compensation point.
Because the emission of a given BVOC has essentially no effect on the atmospheric concentration outside the leaf boundary layer, at least in turbulent conditions and over the time-scale of minutes to hours, the emission can be sustained as long as the production continues and the within-leaf gas-phase concentration exceeds the atmospheric concentration. Uptake, on the other hand, will cease as soon as an equilibrium is reached and the concentrations inside and outside the leaf reach the same value (Xcomp). The fact that continuous uptake is frequently observed (e.g., Copolovici et al. 2005; Fowler et al. 2009; Stimler et al. 2010; Jardine et al. 2011; Stimler et al. 2011; Sandoval-Soto et al. 2012; Park et al. 2013a; Park et al. 2013b) therefore suggests the presence of significant sinks for BVOC within the leaves. In the case of inorganic atmospheric constituents such as O3 which can diffuse through the stomata, it is clear that this highly reactive molecule is rapidly reduced upon contact with cellular metabolites or surface structures such that the concentration of O3 within the leaf can be close to zero (Laisk et al. 1989), or be very low when atmospheric concentrations are high (Moldau & Bichele 2002; Loreto & Fares 2007). When the internal concentration is zero or very low, the rate of uptake is controlled primarily by stomatal conductance and the ambient concentration.
Until recently, all models of BVOC deposition assumed that, unlike O3, BVOC were largely non-reactive in plants. However, new evidence is accumulating that there are chemical BVOC sinks inside leaves such that, after entering the leaf, the compounds are rapidly metabolized, thereby maintaining a concentration gradient sufficient to drive the deposition flux (e.g., Copolovici et al. 2005; Stimler et al. 2010; Stimler et al. 2011; Sandoval-Soto et al. 2012). The nature and magnitude of these enzymatic and non-enzymatic BVOC sinks within the leaf will vary for a given BVOC and with changing environment, e.g., light, temperature and stress level (see section Heterogeneity of biochemical sources and sinks and implications for the emission controls).
Stomatal controls on emission
While the compound uptake rate always depends on stomatal conductance, equation 1 seems to suggest that the emission rate is also proportional to stomatal conductance. However, stomata can only control the emission flux when the within-leaf gas-phase volatile mole fraction Xl,i, is independent of stomatal conductance. When stomatal conductance decreases, Xl,i – Xa,i increases, balancing the reduction in gs,i such that in the steady state, stomata cannot limit the emission flux, provided the build-up of the volatile concentration does not inhibit its own synthesis rate (Niinemets & Reichstein 2003b; Harley 2013). It has been demonstrated that emission rates of isoprene (Fall & Monson 1992) and monoterpenes (Loreto et al. 1996) are independent of stomatal conductance. However, the rate of emission of oxidized compounds such as methanol (Nemecek-Marshall et al. 1995; Harley et al. 2007; Hüve et al. 2007) is reduced by stomatal closure. This enigmatic behavior has been explained by the differences in compound physicochemical characteristics (Niinemets & Reichstein 2003b; Harley 2013), in particular, by differences in compound equilibrium gas/liquid-phase partition coefficient, the Henry’s law constant (Hpc, m3 Pa mol−1) (Staudinger & Roberts 2001). The equilibrium gas-phase concentration of a given compound is given as:
| (3) |
where Cw is the compound aqueous-phase concentration (mol m−3) and p is the air pressure (Pa). Compounds with a high Hpc support high gas-phase concentration at a given liquid-phase concentration, while compounds with high solubility (low Hpc), support a low gas-phase concentration. Gas-phase concentration of isoprene with a high Hpc of 7780 Pa m3 mol−1 at 25 °C (Niinemets & Reichstein 2003b), i.e., with a low solubility, increases essentially immediately, within seconds, after a change in stomatal conductance and as a result, increases in Xl,i – Xa,i fully balance any change in stomatal conductance, maintaining the flux. However, methanol with a Hpc of only 0.46 Pa m3 mol−1 has four orders of magnitude greater aqueous-phase solubility, and thus, gas-phase concentrations increase much more slowly after reductions in stomatal conductance, resulting in temporary limitations of the emission flux that may last up to several hours (Niinemets & Reichstein 2003b; Harley 2013). Thus, dissolution of compounds in the leaf water effectively uncouples the rate of emission of highly soluble compounds from their rate of production, and allows stomata to control their flux in non-steady-state situations. In addition to methanol (Nemecek-Marshall et al. 1995; Geron et al. 2006; Harley et al. 2007; Hüve et al. 2007), such transient reductions of emission rates have been observed for a number of oxygenated compounds with low Hpc such as acetic acid (Gabriel et al. 1999), formaldehyde (Seco et al. 2008), linalool (Niinemets et al. 2002b), and methylbutenol (Harley unpublished data).
However, it is important to recognize that even if stomata can limit the emission rate, the emission control is shared between the stomata and the rates of compound production and consumption (Jardine et al. 2008 for non-stomatal controls on acetaldehyde emission). Under exceptional circumstances such as in ozone-fumigated leaves, stomata actually close, but huge bursts of methanol are mainly driven by a rapid increase in methanol synthesis rate as the result of stress-dependent activation of pectin methylesterases (Beauchamp et al. 2005). Thus, temporal dynamics in the emissions of oxygenated volatiles need to be carefully examined, ideally using dynamic models, to gain insight into the stomatal and non-stomatal factors altering the emission rate.
Physicochemical sinks and sources
From the perspective of compound uptake, presence of a certain capacity for compound within-leaf storage is a physicochemical sink helping to sustain lower values of Xl,i, and thus reducing the rate with which Xl,i – Xa,i approaches 0. This way deposition flux can be maintained when the compound ambient concentrations are relatively high (Omasa et al. 2000; Trapp & Karlson 2001; Copolovici et al. 2005). Thus, although the diffusion gradient, Xl,i – Xa,i, ultimately will reach zero for compounds that are not metabolized within the leaf, this can take a very long time, depending on the effective size of the “physicochemical” storage pool.
In addition to the leaf liquid phase that can store water-soluble compounds, hydrophobic compounds can be sequestered in the leaf lipid phase (e.g., Niinemets & Reichstein 2002; Copolovici et al. 2005; Noe et al. 2010) with similar impacts on bidirectional fluxes. For example, Noe et al. (2008) demonstrated that several species of plants exposed to high concentrations of limonene were able to take up limonene from the surrounding air. The magnitude of the uptake was strongly correlated with the lipid content of the leaves, suggesting that limonene was being removed via dissolution into the lipid phase.
The capacity of the compound storage in the leaf lipophilic phase is often characterized by the compound octanol/water partition coefficient, Kow, that is typically low for OVOC and high for non-substituted isoprenoids, although isoprene has a relatively low Kow value (e.g., Copolovici & Niinemets 2005). Ultimately, the effective size of the storage depends on the characteristics of the compound (Hpc and Kow) and the size of the leaf gas-, liquid- and lipid-phase pools (Kirschbaum et al. 2007 for pertinent calculations; Niinemets et al. 2011).
Historically, Wesely (1989) recognized that dissolution of volatiles could represent a significant sink and lead to sustained deposition fluxes. However, he was concerned primarily with liquid water on surfaces or extracellular water in the sub-stomatal cavity. Furthermore, studies investigating the uptake of volatile and semi-volatile pollutants have defined the leaf bioconcentration factor, Fb, (leaf/gas-phase partition coefficient) as the ratio of the compound concentration in the leaves (CL) relative to that in the gas phase [mol m−3 leaf (mol m−3 air)−1] (Simonich & Hltes 1994; Hiatt 1998; Hiatt 1999; Bakker 2000; Steyaert et al. 2009). Thus, analogously to Eq. 3, gas-phase concentration can be expressed as:
| (4) |
The bioconcentration of a given compound depends on its partitioning between leaf lipid and aqueous fractions, i.e., on compound Kow and Hpc values and corresponding leaf volume fractions (Trapp et al. 1990). Typically, bioconcentration of persistent volatile and semivolatile pollutants is strongly driven by partitioning to lipid phase such that there is a strong correlation between Fb and Kow (Simonich & Hltes 1994; McLachlan 1996; Hiatt 1998; Hiatt 1999; Bakker 2000; Tao & Hornbuckle 2001) as well as correlation of Fb with leaf lipid fraction (Simonich & Hltes 1994; McLachlan 1996; Bakker 2000). Bioconcentration factors for many important volatile and semivolatile pollutants have been determined and used in predicting the deposition flux (Hiatt 1998; Hiatt 1999; Bakker 2000 for a review; Steyaert et al. 2009), but we argue that the concept coming from pollution research is of value for predicting emission and depositions kinetics of key BVOC as well.
In practice, whether the uptake flux is maintained due to the presence of a chemical sink or a physicochemical sink is not always obvious as the effect of a large physicochemical sink on the emission kinetics can be similar to that of a chemical sink. In fact, for a large physicochemical sink, the condition Xl,i – Xa,i might be reached so slowly that there is no experimental evidence of reaching an equilibrium during the measurements. For instance, it has been demonstrated that for some persistent chlorinated hydrocarbons, it can take years to reach an equilibrium with leaves (Jensen et al. 1992). However, the principal difference between a chemical and physicochemical sink is that physicochemically stored volatiles might be re-released upon increases in temperature (or increases in stomatal conductance for some compounds), while this is not the case of a chemical sink that permanently consumes molecules.
Another important difference among chemical and physicochemical sinks is how the sink strength changes with environmental conditions. While the strength of a biochemical sink can depend on any environmental driver that can modify the pathway flux rates, physicochemical sink strength depends on compound physicochemical properties that determine compound volatility (partitioning coefficients) and diffusivity. Both the partitioning coefficients and diffusivity depend on temperature. For dissociable compounds such as organic acids, the physicochemical sink strength also depends on pH in the given cellular or extracellular compartment. This is because for the liquid/gas-phase equilibrium only the concentration of the non-dissociated form matters and accordingly, the apparent Henry’s law constant decreases exponentially with increasing pH (Niinemets et al. 2011). Although temperature can affect the strength of both the physicochemical and chemical sinks, the effects are different. Temperature typically increases the rate of enzymatic processes up to the optimum temperature, and the rates decrease thereafter. In contrast, the rate of non-enzymatic, free-radical processes increases monotonically with increasing temperature. Analogously, compound volatility increases monotonically with increasing temperature and thus, the physicochemical sink strength decreases with increasing temperature. These differences among temperature responses of biochemical and chemical and physicochemical processes can provide important insight in interpreting the processes driving bidirectional exchange in the field.
Deposition onto and release from leaf surface
Although stomatal uptake is a major sink for BVOC in leaves, hydrophobic BVOC and especially hydrophobic semivolatiles can adsorb directly onto the cuticle as a result of gas-phase (dry) deposition (Bakker 2000; Riederer et al. 2002; Keyte et al. 2009; Burkhardt & Pariyar 2014). Diffusion of volatile and semivolatile BVOC molecules into the cuticle and through the cuticle into the leaf can also occur (Bakker 2000; Keyte et al. 2009). However, the rate of diffusion into the cuticle is typically slow, especially for large BVOC and semivolatile molecules and when the crystalline phase of the cuticle is high (Bakker 2000). Nevertheless, when stomatal conductance is low, uptake of hydrophobic compounds through the cuticle can be significant (Riederer et al. 2002; Keyte et al. 2009), and the rate of cuticular diffusion can be importantly enhanced by cuticular damage or modifications, e.g., by salty aerosols (Burkhardt & Pariyar 2014).
What are the implications of potential cuticular exchange for plant BVOC? Characteristic plant hydrophobic BVOC such as mono- and sesquiterpenes can significantly adsorb onto and diffuse into cuticles and dissolve in the amorphous cuticle phase (Schmid, Steinbrecher & Ziegler 1992). In fact, analysis of cuticles has demonstrated presence of many BVOC including mono- and sesquiterpenes and their derivatives (Estell et al. 1994a; Estell et al. 1994b). Recently, Hiemanen et al. (2010) demonstrated that leaves of birch (Betula) can take up and release semivolatiles from neighboring plants and suggested that this process is driven by cuticular deposition. Thus, the cuticular exchange of hydrophobic BVOC seems to be a general phenomenon and clearly needs further experimental work to assess its importance.
Although predominantly hydrophobic, there can be hydrophilic regions on the leaf surface. In particular, aqueous cuticular pores can occur over anticlinal cell walls, in cuticular ledges and at the base of trichomes (Schönherr 2006). Furthermore, the outer guard cell surface is often hydrophilic, especially once subject to damage by abrasion or acidic deposition (Burkhardt & Eiden 1990; Burkhardt et al. 1999)(Burkhardt & Pariyar 2014). Water-soluble volatiles can be exchanged through the aqueous pores and can also dissolve in water condensed on hydrophilic leaf regions. In fact, for short-chained OVOC, it has been demonstrated that cuticular deposition might be important during the dry season when stomatal conductance is low (Rottenberger et al. 2004).
However, dissolution of BVOC on leaf surface cannot serve as a long-term sink for BVOC. Compounds dissolved in the surface water will inevitably re-volatilize with the evaporation of dew or rain water, and the initiation of vertical transport driven by wind turbulence. Such “outgassing” of volatiles from liquid water on leaf surface has been suggested to be responsible for rapid increase in emission of oxygenated volatiles such as methanol in the morning (MacDonald & Fall 1993; Nemecek-Marshall et al. 1995), although the enhanced emission of these volatiles is also compatible with relaxation of stomatal controls on emissions in the morning when stomata open (Niinemets & Reichstein 2003b; Harley et al. 2007; Harley 2013). On the other hand, there is a plethora of epiphytic organisms living on leaf surface with bacteria being the most abundant group (Hirano & Upper 2000; Papen et al. 2002; Lindow & Brandl 2003; Redford et al. 2010). Methylotrophic bacteria on leaves are known to metabolize methanol produced by the host (Abanda-Nkpwatt et al. 2006). From a BVOC exchange point of view, these bacteria serve as a sink for methanol in the atmosphere, sustaining deposition to surfaces (Fall & Benson 1996). Depending on bacterial populations on the leaves, many additional BVOC molecules could be consumed by foliar bacteria, potentially importantly contributing to BVOC exchange.
Overall, deposition and release of compounds from leaf surfaces are expected to be governed mainly by compound and cuticle physicochemical characteristics, and cuticular structure as well as by temperature. Thus, modeling such transfer processes might seem simple. However, there is a large variation in cuticular structural and chemical characteristics among species (Schreiber & Riederer 1996b; Schreiber & Riederer 1996a) introducing important complications in modeling surface exchange. Furthermore, potential involvement of bacteria in surface exchange would imply that biologic control mechanism could exert an important control on surface BVOC exchange as well.
HETEROGENEITY OF BIOCHEMICAL SOURCES AND SINKS AND IMPLICATIONS FOR THE EMISSION CONTROLS
The previous section highlighted the fundamentals of BVOC emission and deposition fluxes in dependence on ambient and within-leaf concentrations, stomatal openness and strengths of sources and sinks. In particular, evidence for these sinks, resulting from BVOC metabolism or BVOC dissolution into the aqueous or lipid phases has accumulated in recent years. Although simple principles govern volatile exchange between plants and atmosphere, complicated emission patterns can result from the huge physicochemical diversity of plant-emitted volatiles as well as from pathway heterogeneities that determine what compounds are being formed, when the various metabolic routes are activated, to which degree they are activated and whether and when they are deactivated (Niinemets, Loreto & Reichstein 2004; Grote et al. 2013). These variations determine the strengths of sinks and sources, compound residence times within the leaves and probabilities of compound reaction within the leaves at different times during the day and in different locations in vegetation (Figs. 2-3).
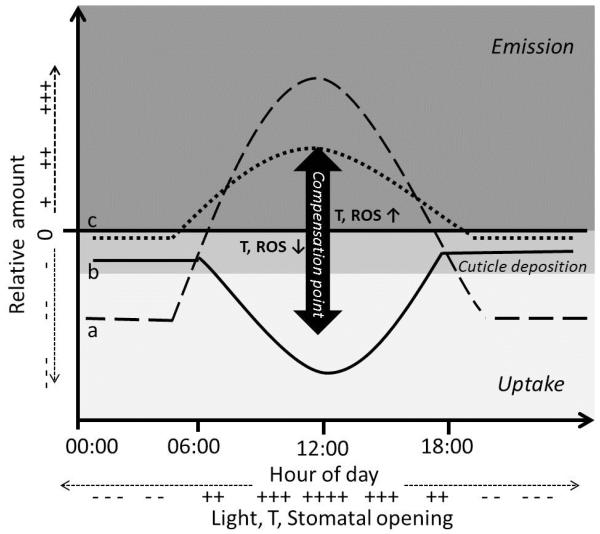
Figure 2.
Three typical BVOC flux dynamics: case a (dashed lines) shows BVOC prevalently produced and emitted in response to temperature, light and stomatal opening. During night hours, non-stomatal uptake takes place, and BVOC are metabolized or stored inside leaves; case b (solid line) shows flux dynamics of BVOC not produced by the vegetation, but undergoing cuticular deposition in the night hours and uptake through stomata during the light hours; case c (dotted line) shows flux dynamics of BVOC which are both produced and metabolized by plants, thus, the net flux depends on the compensation point which increases in response to oxidative stress. Ambient concentrations above or below the compensation point may turn leaves from a sink to a source, respectively.
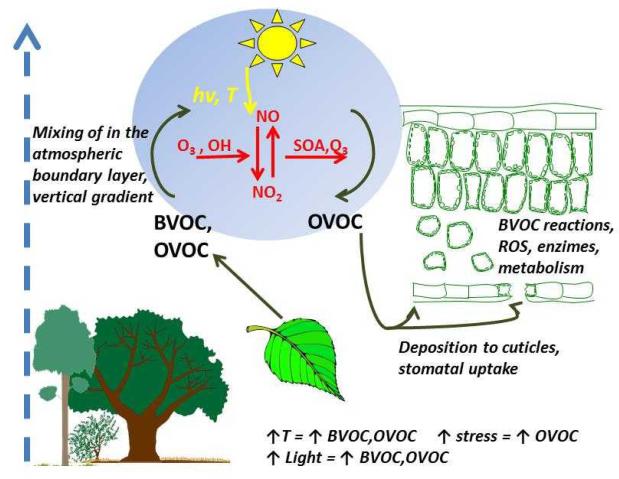
Figure 3.
Biogenic volatile (BVOC) and oxygenated volatile (OVOC) atmosphere-ecosystem exchange dynamics. The vegetation/atmosphere exchange is highly dynamic and is driven by BVOC and OVOC formation in the leaves, BVOC emission, oxidation inside the leaves and in the atmosphere and by foliar uptake (dry deposition). Primary OVOC can be produced by plants by primary metabolic reactions. Secondary OVOC can be produced by oxidation of non-oxygenated volatiles, and in atmospheric reactions involving primary plant/produced compounds. This dynamic exchange further leads to important feedbacks between secondary organic aerosol formation and atmospheric reactivity that alters the concentrations of volatiles in the atmosphere (e.g., Kulmala et al. 2013 for the discussion of the feedbacks).
Biochemical source heterogeneity
Different pathways with contrasting regulatory mechanisms are responsible for the production of different classes of volatiles (for recent reviews on volatile production see Li & Sharkey 2013; Monson 2013; Rajabi Memari, Pazouki & Niinemets 2013). The rate of volatile isoprenoid synthesis in constitutively emitting species is often tightly linked to photosynthetic metabolism, and therefore the emissions primarily respond to light and temperature, similarly to photosynthesis (for reviews see Niinemets et al. 2010c; Grote et al. 2013; Li & Sharkey 2013; Niinemets et al. 2013a). The emissions also respond to CO2 concentration, although often differently from photosynthesis (Niinemets et al. 2010c; Sun et al. 2012b; Grote et al. 2013; Li & Sharkey 2013). However, the rates of synthesis and emissions of monoterpenes and sesquiterpenes stored in specialized storage organs are typically uncoupled and not linked to photosynthetic metabolism (Kesselmeier & Staudt 1999; Niinemets et al. 2004; Niinemets et al. 2010c), although there may be significant plasticity of environmental responses of storage emissions, for example, during the season (Tarvainen et al. 2005; Holzinger et al. 2006). Finally, all plant species emit inducible volatile isoprenoids under stress conditions, often in light-and temperature dependent manner (Paré & Tumlinson 1997; Hansen & Seufert 2003; Kask, Kännaste & Niinemets 2013; Niinemets, Kännaste & Copolovici 2013b).
Oxygenated volatiles are produced by multiple metabolic pathways. Only oxygenated isoprenoids such as methylbutenol that is constitutively emitted similarly to isoprene in several pine species (e.g., Harley et al. 1998; Gray, Lerdau & Goldstein 2003; Gray et al. 2011) and oxygenated monoterpenes such as linalool and 1,8-cineole that are classical components of induced emission mixtures (Paré & Tumlinson 1997; Niinemets et al. 2002b; Niinemets et al. 2010a) can be directly associated with photosynthetic metabolism. In fact, oxygenated volatiles are often by-products of central metabolic processes such as degradation of cell walls and turnover of cellular metabolites (for a review see Seco, Peñuelas & Filella 2007). The rates of synthesis of these OVOC can dramatically increase during specific developmental stages. For example, methanol emissions from cell walls in growing leaves reflect chemical transformations during relaxation and rigidification of cell walls in expanding tissues (Fall 2003; Hüve et al. 2007).
OVOC production can also drastically increase in response to a variety of stresses. Emissions of some stress-induced OVOC are associated with specific stresses. For example, acetaldehyde, ethanol, and acetic acid emissions in flooded trees are indicative of cytosolic fermentation processes primarily in roots and the biosynthesis of acetyl-CoA (Jardine et al. 2010b; Jardine et al. 2012a; Kreuzwieser & Rennenberg 2013). In general, this alternate route of acetyl-CoA production in various plant organs in response to different environmental cues is therefore tightly linked with the enzymatic production and consumption of these OVOC (Jardine et al. 2010b; Jardine et al. 2012a).
Several ubiquitous stress pathways, associated with ROS formation, in particular the oxylipin pathway (Box 1), further lead to emission of a large number of OVOC (Seco et al. 2007 for a review of OVOC). Fatty acid peroxidation reactions produce a large array of volatile oxylipins that can be classified into distinct structural groups including alkanals (e.g., propanal, butanal, pentanal, hexanal), 2-alkenals (e.g., 2-propenal, 2-butenal, 2-pentenal, 2-hexenal), furans and furanones (e.g., tetrahydrofuran, 2-ethyl furan), and dicarbonyls (e.g., malondialdehyde, glyoxal, methyl glyoxal, and diacetyl) (Matsui et al. 2010). In addition, the enzymatic peroxidation of plant fatty acids by lipoxygenase enzymes can lead to the formation and emission of characteristic oxidation products known as green leaf volatiles (GLV) via the lipoxygenase pathway (Hatanaka 1993; Fall et al. 1999; Loreto & Schnitzler 2010). In this pathway, the formation of the classic C6 GLV in plants is initiated by the ubiquitous type 2 lipoxygenase enzymes (13-LOX) in chloroplasts which catalyze the oxygenation of α-linolenic acid (the dominant fatty acid in the aerial tissues of most plants) to form 13-hydroperoxy linolenic acid (HPLA) (Andreou & Feussner 2009). HPLA can be degraded by hydroperoxide lyase to form the primary GLV (Z)-3-hexenal which is then reduced and acetylated to form the corresponding alcohol (Z)-3-hexen-1-ol and acetate ester (Z)-3-hexen-1-yl acetate (D’Auria et al. 2007).
Box 1. Lipids and their oxidation products in plants.
Lipids constitute a heterogeneous class of molecules characterized by high hydrophobicity. They serve numerous critical functions in plants including structural functions in membranes, participation in the light reactions of photosynthesis, as well as participation in antioxidant and signaling processes (e.g., Ohlrogge & Browse 1995). Saturated lipids are extremely resistant to oxidation within both plants and the environment, and plant alkanes with ages greater than one billion years have been detected in lake sediments (Oro et al. 1965). In contrast, unsaturated lipids including isoprenoids and fatty acids are highly susceptible to oxidation with their pools rapidly turned over under oxidizing conditions. Moreover, the oxidative power of the lower atmosphere is strongly influenced by the emission of unsaturated volatile lipids from vegetation, especially isoprenoids and other reactive volatile lipids which are emitted from many plants at high rates and which fuel atmospheric chemistry through photooxidation reactions.
Reactive oxygen species (ROS) including singlet oxygen (1O2), superoxide anion (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (OH) are continuously generated in plants by the incomplete reduction of oxygen (O2). While, ROS concentrations within the plants are generally kept low by ROS quenching and scavenging systems, excessive ROS accumulation can result in extensive oxidation of plant lipids (Apel & Hirt 2004; Jardine et al. 2010a). While traditionally described at the “oxygen paradox” where ROS are simply a toxic byproduct of aerobic metabolism (Davies 1995), ROS-lipid signaling is now recognized as an integral component of plant response to abiotic and biotic stress as well as regulation of growth, development, and programmed cell death (Mittler et al. 2011; Suzuki et al. 2011).
Furthermore, oxidation products of volatiles themselves can be highly reactive. In particular, oxygenated volatiles (OVOC) that contain an α-β unsaturated carbonyl group are classified as reactive electrophile species (RES). RES are biologically very active due to their extremely high chemical reactivity with nucleic acids, proteins, and metabolites (Farmer & Davoine 2007). Therefore, oxidative stress generated by RES can lead to gene expression patterns at all levels (Farmer & Davoine 2007).
ROS-initiated lipid peroxidation
Like all radical reactions, lipid peroxidation reactions involve an initiation, propagation, and termination stage (Wheatley 2000). Lipid peroxidation is initiated when a ROS-lipid encounter leads to the formation of a lipid radical which then combines with molecular oxygen to form a lipid peroxy radical. The propagation stage is characterized by a chain reaction where lipid peroxy radicals reacts with other nearby lipids to form additional lipid radicals. Finally, the termination stage occurs when lipid radicals react with other lipid radicals or when they reacts with antioxidants that can terminate lipid peroxidation processes by converting lipid peroxy radicals into more benign lipid hydroperoxides. Given the typical close physical proximity of lipids in biological structures including membranes, storage vesicles, and glands, the rapid termination of lipid peroxidation is critical to avoid massive oxidative damage, loss of biological functions, and cell death.
Plant oxylipins
The oxidation of plant fatty acids via non-enzymatic (Durand et al. 2009; Mene-Saffrane et al. 2009) and enzymatic (Heiden et al. 2003; Andreou & Feussner 2009; Gigot et al. 2010) mechanisms produces a broad range of oxidation product biomarkers termed oxylipins. The accumulation of ROS in plant tissues initiates fatty acid (e.g., α-linolenic acid) peroxidation, yielding a large array of ‘oxidative stress’ biomarkers (Table 1). Lipid peroxidation generates a number of products which have been extensively used as quantitative indicators of oxidative damage in plants (Gutteridge 1995; Shulaev & Oliver 2006). For example, 4-hydroxy-2-nonenal (HNE), 4-hydroxy-2-hexenal (HHE), and malondialdehyde are widely used as biomarkers of non-enzymatic lipid peroxidation (Halliwell & Gutteridge 1999; Hartley et al. 1999; Long & Picklo 2010). However, the extraction from plant tissues, derivatization, and compound-specific analysis (GC-MS or HPLC) of these reactive carbonyl compounds remains a challenge due to their trace abundances, high reactivity, water solubility, and volatility (Shibamoto 2006). Nonetheless, a number of classes of lipid peroxidation products have been identified including hydrocarbons, ketones, furans, alkanals, 2-alkenals, 2,4-alkadienals, 2-hydroxyalkanals, 4-hydroxy-2-alkenals, and dicarbonyls (Frankel, Hu & Tappel 1989; Mark et al. 1997; Nielsen et al. 1997; Moseley et al. 2003; Shibamoto 2006; Steeghs et al. 2006; Kawai, Takeda & Terao 2007).
Table 1.
Example of isoprene (red text) and fatty acid (black text) peroxidation biomarkers from plants under oxidative stress
| Parent lipid | Class | Oxidation Biomarkers |
|---|---|---|
| isoprene | isoprene | methacrolein, methyl vinyl ketone |
| isoprene | isoprene | 3-methyl furan, 2-methyl-3-buten-2-ol |
| fatty acids | GLVs | 3-hexenal, 3-hexen-1-ol, 3-hexen-1-yl acetate |
| fatty acids | furans and furanones | tetrahydrofuran, 2-ethyl furan, 5-ethyl 2(5H)-furanone |
| fatty acids | alkanes | propane, butane, pentane…undecane |
| fatty acids | 2-alkenes | 2-propene, 2-butene, 2-pentene…2-undecene |
| fatty acids | alkanals | propanal, butanal, pentanal…undecanal |
| fatty acids | 2-alkenals | 2-propenal, 2-butenal, 2-pentenal…2-undecenal |
| fatty acids | 2,4-alkadienals | 2,4-hexadienal, 2,4-heptadienal, 2,4-octadienal |
| fatty acids | 2-ketones | 2-butanone, 2-pentanone… 2-undecanone |
| fatty acids | alkenones | 1-hexen-3-one, 1-penten-3-one, 1-octen-3-one, 6-methyl-5-hepten-2-one |
| fatty acids | 4-hydroxy 2-alkenals | 4-hydroxy-2-hexenal, 4-hydroxy-2-nonenal |
| fatty acids | dicarbonyls | malondialdehyde, gyloxal, methyl glyoxal, diacetyl |
Release of multiple classes of OVOC such as GLV, methanol, acetaldehyde and acetone from plants has been documented during processes known to be associated with ROS accumulation including programmed cell death during senescence (de Gouw et al. 1999; Holopainen et al. 2010; Sun, Copolovici & Niinemets 2012a), and a wide variety of biotic and abiotic stresses including pathogen attack (Jansen et al. 2009; Toome et al. 2010), high ambient ozone concentrations (Heiden et al. 2003; Beauchamp et al. 2005; Cojocariu et al. 2005), herbivory (Arimura, Matsui & Takabayashi 2009; Copolovici et al. 2011), desiccation (de Gouw et al. 2000), high light and temperature (Loreto et al. 2006; Copolovici et al. 2012), mechanical wounding (Fall et al. 1999; Karl et al. 2001; Loreto et al. 2006; Brilli et al. 2011), and freeze-thaw events (Fall et al. 2001). In addition, acids like formic and acetic acid can also be emitted as a result of intercellular and often non-enzymatic degradations of carbohydrate and fats (Kesselmeier et al. 1998; Kesselmeier & Staudt 1999; Staudt, Wolf & Kesselmeier 2000). Both enzymatic and non-enzymatic mechanisms can lead to the formation of characteristic fatty acid peroxidation biomarkers that may be detectable as gas-phase emissions from plant tissue under stress (Box 1).
Differently from compounds produced in situ, the source strength of compounds transported from other organs can also be driven by the rate of compound transport. Such effects are important for flooding-driven emission when ethanol produced in the roots is transported to the leaves and oxidized to the more volatile acetaldehyde (Kreuzwieser & Rennenberg 2013). Thus, acetaldehyde source strength in flooded trees is often driven by ethanol transport rate (Kreuzwieser et al. 1999; Kreuzwieser et al. 2001; Cojocariu et al. 2004). Within-leaf production of acetaldehyde has also been recently discussed in the context of a pyruvate overflow hypothesis supporting acetyl-CoA production as a part of the pyruvate dehydrogenase bypass system (Jardine et al. 2010b; Jardine et al. 2012a).
This huge heterogeneity of emission pathways including distinct tissue localization has important implications for the bidirectional emission controls. In particular, synchronization or non-synchronization of the timing of physiological changes in stomatal conductance and rates of formation of volatiles affects the pool size of volatiles. Differences in pool size imply modification in the compound residence times that in turn will alter the rate of secondary within-leaf chemical reactions. In addition, changes in BVOC sink/source strengths also imply modifications in the compensation point of the volatiles. Complex kinetics of volatiles including oscillations and emission bursts have been observed, reflecting asynchronous rates of various key physiological processes (e.g., Beauchamp et al. 2005; Harley et al. 2007; Hüve et al. 2007). There might be an urge to link these emission kinetics immediately to the rate of compound synthesis. However, under inherently non-steady-state conditions, dynamic emission models are needed to deconvolute different physiological and physicochemical emission controls (Niinemets et al. 2004).
Chemical sink heterogeneity
The nature of the chemical sinks for oxygenated volatiles
Once produced or taken up, many oxygenated volatiles can enter into catabolic pathways potentially leading to their complete oxidation to CO2 (Oikawa & Lerdau 2013), but they may also be integrated into anabolic pathways involved in biosynthesis of biologically relevant molecules. Although not all catabolic and anabolic pathways for different volatiles have been fully resolved, metabolism of volatile biogenic molecules can be an important carbon source for the plant such that evolution of novel biochemical pathways for consumption of volatiles clearly does contribute to plant survival (Oikawa & Lerdau 2013).
The metabolism including C2 OVOC is well understood, and pathways involving ethanol, acetaldehyde and acetic acid are constitutively active in leaves and are further upregulated under certain stress conditions such as flooding (Kreuzwieser et al. 1999; Kreuzwieser & Rennenberg 2013). Ultimately, C2 OVOC can be converted to acetyl-CoA and enter primary metabolism or be catabolized to CO2. In contrast, the pathways responsible for C1 OVOC consumption, in particular methanol reactions are less well understood. It was recently shown that methanol can be directly acetylated by acetyl CoA to produce the OVOC methyl acetate (Jardine et al. 2014). Formic acid can participate in the transfer of methyl and hydroxymethyl groups for the synthesis of other molecules (Kesselmeier & Staudt 1999; Kuhn et al. 2002) or oxidized to CO2 in mitochondria (Igamberdiev, Bykova & Kleczkowski 1999; Hanson & Roje 2001). Achkor et al. (2003) reported detoxification of formaldehyde by glutathione-dependent formaldehyde dehydrogenase (FALDH) in Arabidopsis.
Carbonyl sulfide (COS), a C1 molecule, although oxygen-containing, is seldom considered together with other OVOC, but there is a strong chemical sink of COS inside leaves. This sink is dominated by carbonic anhydrase driven reactions and to a lesser extent by Ribulose 1,5-carboxylase/oxygenase (Rubisco) driven reactions (Stimler et al. 2010) such that the atmospheric compensation point for COS is often very low (Kesselemier & Merk 1993; Xu, Bingemer & Schmidt 2002; Stimler et al. 2010; Stimler et al. 2011; Sandoval-Soto et al. 2012).
Involvement of cytochrome P-450 dependent oxidases and glutathione-S-transferases (GSTS) have been postulated in detoxification of a variety of oxygenated and non-oxygenated BVOC molecules (Trapp & Karlson 2001). In addition, aldehyde dehydrogenase (ALDH) protein superfamily of nicotinamide adenine dinucleotide phosphate (NADP+)–dependent enzymes, together with peroxidase families can oxidize a wide range of aldehydes by oxidizing these to their corresponding carboxylic acids (Kirch et al. 2004). Non-enzymatic reactions involving reactive oxygen species (Box 1) can also play a role for conversion of OVOC species with double bonds. Thus, a large number of metabolic and oxidative pathways can serve as important chemical sinks within the leaves.
Furthermore, the activity of oxidative pathways can be strongly enhanced under oxidative stress conditions induced by exposure to pollutants like ozone or exposure to high temperatures (Karl et al. 2010). In fact, OVOC is taken up from the ambient atmosphere both under non-stressed and stressed conditions, but both apoplastic and symplastic concentrations of OVOC, in particular, the concentrations of oxidation products of isoprene, methyl vinyl ketone (MVK) and methacrolein (MACR), are strongly reduced under stress (Karl et al. 2010). Similar behavior is expected for oxidation products of monoterpenes and OVOC like hydroxyacetone, glycolaldehyde, C5 carbonyls, and nopinone.
As noted above, chemical sinks and physicochemical storage (in leaf lipid and aqueous phases) cannot always be conclusively separated as leaves can support large equilibrium pools for many oxygenated volatiles (Niinemets & Reichstein 2003b; Niinemets & Reichstein 2003a). Thus, the pertinent question is to what extent physicochemical sinks contribute to the uptake of OVOC. In fact, within-leaf OVOC concentrations have seldom been estimated. Tani and Hewitt (2009) found that the total foliar uptake of aldehydes and ketones by a range of houseplants was 30-100 times greater than the amount dissolved in the leaves, suggesting that fast scavenging of these aldehydes is the primary sink maintaining the uptake flux. In another study, Tani et al. (2010) observed that MACR is taken up at a faster rate than MVK, and intercellular MACR concentration is lower than that for MVK. Given that the Henry’s law constant is actually larger for MACR (15.6 Pa m3 mol−1 at 25 °C) than for MVK (2.5 Pa m3 mol−1 at 25 °C) (Iraci et al. 1999), this evidence suggests that the differences in the uptake rates are driven by greater within-leaf chemical scavenging of MACR rather than by differences in physicochemical sink strength.
Chemical sinks for non-oxygenated volatiles
Due to its high vapor pressure, isoprene exists as a gas under normal physiological conditions and can therefore escape plants and enter the surrounding atmosphere. This process occurs to such a large magnitude that terrestrial isoprene emissions accounts for an estimated one third of global volatile organic compound emissions from all natural and human sources combined (Guenther et al. 1995; Guenther et al. 2012). Although past research has highlighted the role of isoprene in the protection of photosynthesis during abiotic stress, the mechanisms still remain unclear. Nonetheless, a growing body of evidence suggests that isoprene safeguards plants under stress by functioning as an effective antioxidant (Vickers et al. 2009). In particular, isoprene protects the leaves against volatile oxidants from ambient air such as ozone (Loreto et al. 2001), and quenches nitrogen oxide (NO) in leaves followed by a decrease in isoprene emission and modification of the NO compensation point (Velikova, Fares & Loreto 2008). It has been argued for long that the oxidation of BVOC in leaves, especially under stress conditions, involves reactions with reactive oxygen species (ROS) generated by oxidative stress (Box 1, Loreto & Velikova 2001; Velikova, Edreva & Loreto 2004; Velikova et al. 2008). Recently, the first direct evidence that isoprene functions as an antioxidant by directly reacting with ROS was obtained by quantifying gas-phase emissions of isoprene and its oxidation products methyl vinyl ketone, methacrolein, and 3-methyl furan from tropical (Jardine et al. 2012b; Jardine et al. 2013) and desert (Jardine et al. 2010a) plants under abiotic stress. Increased emissions of isoprene oxidation products (Iox) relative to isoprene (I) were stimulated by high temperature stress at the branch and ecosystem levels. 13CO2 and pyruvate-2-13C leaf-labeling showed a rapid incorporation of 13C into both isoprene and Iox, providing additional evidence for their production from isoprene peroxidation (Jardine et al. 2012b).
The evidence of chemical reactions of monoterpenes is scarce, but in Quercus ilex with fosmidomycin-inhibited monoterpene emission, the uptake of the reactive monoterpene α-pinene increased with increasing temperature, while the uptake of the non-reactive α-terpineol decreased with increasing temperature (Copolovici et al. 2005). Because the physicochemical gradient from ambient air to leaves decreases with increasing temperature due to greater volatility of terpenes, the increased α-pinene uptake also suggests the presence of a chemical sink within the leaves (Copolovici et al. 2005).
In a similar fashion, exposure of photosynthetic microorganisms and vascular plants to photooxidative conditions resulted in the emission of volatile oxidation products of β-carotene, including the aldehyde β-cyclocitral and the ketone β-ionone (Walsh, Jones & Dunstan 1998; Ramel et al. 2012). Similarly to reactive electrophile species which mediate gene responses to ROS, including initiating the expression of an array of defense genes (Box 1), the biological activity of these isoprenoid peroxidation products is high (Havaux 2013). However, unlike β-carotene which is tightly associated with pigment-binding complexes in photosynthetic membranes, isoprene and likely other volatile isoprenoids such as mono- and sesquiterpenes can freely diffuse through cell membranes where they can terminate fatty acid peroxidation chain reactions by oxidizing, diffusing away from the membrane and either being emitted as an oxidation product to the atmosphere or being further metabolized (Box 1).
What is the implication of the findings of chemical sinks within the leaves for widespread volatiles such as isoprene which is produced at high rates between 20 - 100 nmol m−2 s−1 in isoprene-emitting plants? In emitting species, the concentrations in the intercellular air space (typically a few μmol mol−1) far exceed the atmospheric concentrations (typically a few nmol mol−1) and although a compensation point presumable exists, it is orders of magnitude above the ambient levels. Thus, the net flux of isoprene, at least during daytime, is from the leaf to the atmosphere in isoprene-emitting species. However, in the case of “non-emitting” species, possible metabolism of isoprene within the leaf suggests that a deposition flux may be sustained, the rate of uptake being jointly determined by ambient concentrations, the rate of metabolism and the stomatal conductance.
The other important implication of oxidation of volatiles is that novel oxidized molecules with different physicochemical characteristics are generated that can exhibit different exchange characteristics than the non-oxidized parent molecules. In particular, the emission rates of these volatiles can be modified by changes in stomatal conductance. Although the level of stomatal control over emissions is expected to be relatively weak for MACR and MVK due to their moderate solubility, changes in stomatal conductance will still importantly alter the residence time of these compounds inside the leaves, thereby enhancing the probability of within-leaf metabolism. As several of the oxidation products of volatile isoprenoids are known to be toxic (Kai et al. 2012), a capacity for metabolization of these compounds is physiologically highly relevant.
Variation of the BVOC compensation points: role of sinks, sources and environmental conditions
Compensation point is an important concept of bidirectional exchange in atmospheric science, and is widely employed in bidirectional exchange modeling as it allows for a convenient switch between emission and deposition (e.g., Pleim & Ran 2011). However, for plant/atmosphere exchange, there is a large uncertainty in compensation point values not only for different compounds, but even for the very same compounds. For any given compound, variable compensation points for different plant species and for the same species under different conditions have been demonstrated in the literature. For example, Rottenberger et al. (2004) reported compensation points on the order of 0.6 nmol mol−1 for acetaldehyde and formaldehyde for different Amazonian species, while Seco et al. (2008), reported compensation points for formaldehyde of 20 nmol mol−1 for the Mediterranean species Quercus ilex and Pinus halepensis. Jardine et al. (2008) found different compensation points for acetaldehyde during light and dark periods for Populus deltoides and Quercus ilex (Jardine et al. 2008) with the compensation points being much larger in light than in dark. In Rottenberger et al. (2004), there was a higher compensation point during dry vs. wet season in tropical trees.
The reasons for such vast differences have been seldom addressed, and we argue that multiple factors can be responsible for changes in the compensation point (Figs. 1-2). For ammonium, it has been demonstrated that differences in compensation points can reflect both physiological and physicochemical factors, but physicochemical factors, in particular, pH and temperature-dependent changes in its Henry’s law constant dominate modifications in the compensation point (Husted & Schjoerring 1996; Wang et al. 2013). For acetaldehyde emissions in Pinus taeda, Karl et al. (2005) observed that the compensation point increased exponentially with increasing leaf temperature. Temperature-dependent increase in the compensation point likely indicates enhanced within-leaf acetaldehyde source strength perhaps from cytosolic pyruvate (Karl et al. 2002a), and is also consistent with reduced physicochemical sink as acetaldehyde becomes increasingly volatile at higher temperatures. The evidence of light-dependent increase in the acetaldehyde compensation point in the study of Jardine et al. (2008) further suggests that light-dependent changes in the source strength were driving the modifications in the compensation point (see Fig. 1 for theoretical calculations). On the other hand, in the Karl et al. (2005) study, the compensation point decreased after ozone fumigation, suggesting activation of an acetaldehyde chemical sink (e.g., activation of an aldehyde dehydrogenase) in this species. Interpretation of the seasonal changes and species differences in the compensation point is less clearcut, although these differences might indicate environmentally-driven seasonal and species differences in the sink/source strengths.
The evidence of a strong dependence of the compensation points on the sink strength highlights a major limitation of the compensation point concept as a basis for simulating bidirectional exchange. Due to strong variations in sink/source strengths, compensation points will vary and assuming a fixed compensation point will lead to major bias in predicted emissions. In fact, using the available evidence of sink/source strength variations in dependence on environmental conditions within a simple modeling framework (Eq. 1), it becomes evident that the compensation points and the slopes of the relationships linking the ambient concentration to the flux rate should also vary during the day (Figs. 1-2). In fact, fitting daily and longer-term relationships of compound flux vs. ambient concentration often results in highly scattered relationships (Cojocariu et al. 2004; Rottenberger et al. 2004; Karl et al. 2005; Rottenberger et al. 2008; Seco et al. 2008; Graus et al. 2013). Thus, compensation points cannot be precisely derived from such relationships pooling multiple sources of variance. Therefore, compensation point estimates from regressions linking ambient concentration and flux rates over periods of time with fluctuating environmental conditions are potentially associated with large errors and inherent variability. These large variations likely reflect the key limitation that the compensation point is importantly driven by sink and source strengths that can vary due to inherent physiological regulation mechanisms characteristic to a given compound as well as due to non-enzymatic reactions and environmental controls on compound physicochemical characteristics. Although the use of compensation points has been successful for simulating bidirectional exchange kinetics for some compounds such as ammonium (Farquhar et al. 1980; Schjoerring, Husted & Mattsson 1998; Fowler et al. 2009), we argue that it might be more appropriate to simulate the dynamics of BVOC sinks and sources rather than apply a given value of a compensation point in flux simulations. On the other hand, bidirectional exchange models coupled to detailed measurements under different environmental conditions (e.g., Karl et al. 2005; Jardine et al. 2008) could be inverted to estimate the sink and source activities corresponding to any given value of the compensation point.
BIDIRECTIONAL EXCHANGE AT THE CANOPY SCALE
How do canopies differ from leaves?
Although the same principal chemical and physiological mechanisms operate at the leaf and canopy scales, canopies are characterized by strong variations in environmental characteristics (e.g., Niinemets 2007), differences in the proportion of constitutively emitting and non-emitting species, differences in the stress status of the trees and foliage at different positions in the canopy and variations in the concentrations of volatiles and oxidants (e.g., Noe et al. 2012). Thus, BVOC production rates and BVOC compensation points of leaves at different canopy positions may be expected to vary widely, complicating attempts to apply the compensation point methodology at the canopy scale. Indeed, the rate of BVOC exchange (Cojocariu et al. 2004; Niinemets et al. 2010b) and even the flux direction for oxidized volatiles (Cojocariu et al. 2004; Karl et al. 2004) can depend on the foliage position within the canopy. Until recently, discrepancies between observed canopy fluxes and leaf-level fluxes have been attributed to within-canopy gas-phase oxidation of leaf-released volatiles by atmospheric oxidants. However, evidence of within-leaf oxidation reactions of many volatiles has recently accumulated (see the section Chemical sink heterogeneity), raising the possibility that some volatiles released within the canopy may be taken up and oxidized by other leaves before exiting the canopy air space. Furthermore, oxidation reactions can take place in liquid and wax phases on the leaf surface. Although oxidation reactions may prove important both within and outside the leaf, differences in the suite of oxidizing species, physical phase (lipid, liquid, gas) of oxidization reactions and degree of volatile mixing imply that within-canopy and within-leaf oxidation processes may be very different.
Thus, the pertinent question is how increased understanding of leaf-level deposition processes can be used to improve our ability to understand and model canopy-level processes. Unraveling the controls at the canopy scale is further complicated by relatively sparse data sets and infrequent temporal data coverage, conventionally 30 min. for micrometeorological techniques used for flux estimation (Karl et al. 2002b; Fares et al. 2012). Furthermore, flux measurements made at night, when deposition processes may predominate, are frequently unreliable because assumptions underlying the micrometeorological flux techniques are violated.
In fact, estimates of canopy-scale fluxes made by up-scaling fluxes measured with enclosures at leaf level often fail to agree with fluxes measured at ecosystem level with micrometeorological techniques. Bouvier-Brown et al. (2009) and Ciccioli et al. (1999) detected much lower fluxes of sesquiterpenes above plant canopies than predicted from branch enclosure measurements, revealing significant within-canopy losses of certain isoprenoids due to their high reactivity.
The situation is further complicated by the circumstance that canopy processes can differ for non-oxidized and oxidized molecules. In the ambient atmosphere, isoprenoids can react with ozone, and NO3 and OH radicals (Atkinson & Arey 2003), with compound lifetimes ranging from a few seconds for some very reactive mono- and sesquiterpenes to a few hours for less reactive isoprenoids such as isoprene and α-pinene (for calculation of chemical lifetimes see e.g., Arneth & Niinemets 2010; Holopainen, Nerg & Blande 2013). The reactive compounds can notably influence the oxidizing capacity and the ozone-forming potential of the atmosphere and the formation of secondary organic aerosol and contribute to the atmospheric source of OVOC (Andreae & Crutzen 1997; Fuentes et al. 2000). OVOC have generally a longer lifetime in the atmosphere than non-saturated non-oxygenated volatiles, up to several days (e.g., acetone life-time is 15 days). This means that they are retained longer and can be transported to greater distances from the emission source, where they can take part in photochemical reactions or be deposited (Goldstein & Galbally 2007).
Canopy deposition velocity framework
As discussed above, the magnitude and direction of BVOC flux depends on the size and direction of the concentration gradient between the leaf intercellular air space and the ambient air. The concentration of a given BVOC in the ambient air outside the leaf and the capacity of plants to produce/metabolize that BVOC determine its leaf compensation point. In contrast to the compensation point formulation used in studies of bidirectional exchange in individual leaves, studies of canopy-level deposition flux, F (mol m−2 s−1), have traditionally employed the concept of deposition velocity, using an analogy with Ohm’s law:
| (5) |
where νd is the deposition velocity (m s−1) and Ca is the compound ambient concentration (mol m−3). Deposition velocity is given as the inverse of the sum of three resistances in series:
| (6) |
In the present context, Ra (the sum of aerodynamic resistances) and Rb (the quasi-laminar sublayer resistance) can be ignored, and the so-called surface resistance (Rc) is the major determinant of whether or not a given volatile species is deposited. Rc describes the effective resistance to uptake by surface elements, including vegetation, and incorporates a variety of factors, including stomatal resistance and a mesophyll resistance terms that include, among other things, the magnitude of both the within-plant source and within-plant sink for the volatile of interest. Wesely (1989) defined a reactivity factor, f0, which describes the oxidation rate of the volatile within the plant. The factor f0 ranges from 1 for extremely reactive compounds like O3 to 0 for non-reactive compounds. Those considered slightly reactive are assigned a value of 0.1.
The traditional deposition velocity framework (Wesely 1989) continues to be widely used in regional air quality models (e.g., Zhang et al. 2002; Pleim & Ran 2011). In application of the algorithm, the compound concentration within the leaf is assumed to be very small and the flux is therefore a function of the ambient concentration and modeled stomatal conductance (Eq. 5). This generally works well for extremely reactive species like O3 and SO2 (Pleim & Ran 2011). While in principle the same formulation can be applied to organic species, very few experimentally determined estimates of f0 exist for such compounds, and recent evidence (Karl et al. 2010) suggests that the previously assumed values may be too low, significantly underestimating within-plant rates of destruction, and thus underestimating deposition velocities as well. For example, initially (Wesely 1989), values of f0 of 0 were assigned to several BVOC (e.g., formaldehyde, acetaldehyde, and formic acid) which have subsequently been shown to be taken up by vegetation at both the leaf and canopy scales. This underscores the need to re-evaluate the canopy resistance term for a wide range of BVOC, as we learn more about their metabolism within the plant. Thus, one approach to improving our ability to predict deposition rates would be to conduct detailed canopy-scale deposition studies (comparing deposition velocities to that of ozone for example) for a variety of BVOC to estimate values of f0. However, difficulties associated with micrometeorological flux studies during the night, when deposition fluxes may dominate, pose a technical obstacle to such a strategy.
Bidirectional fluxes of BVOC at the ecosystem scale
It is important to distinguish between BVOC species that are produced within the plant and others that can originate from BVOC oxidation processes in the ambient atmosphere. If there is no source for a given BVOC within the plant, deposition is the only possibility, and the rate of deposition is jointly determined by the atmospheric concentration, the rate at which the compound is broken down within the plant and the stomatal conductance. For those BVOC which are produced by the plant, the situation is far more complicated, since bidirectional fluxes are possible, and the rate of production also contributes strongly to both the size and direction of the flux.
The atmospheric concentration of a given BVOC can fluctuate over a wide range during the diurnal cycle, and it plays a major role in determining whether leaves are a net sink or net source of BVOC, since only when the atmospheric concentration falls below the compensation point, leaves can emit. Factors contributing to the diurnal variation in atmospheric concentration include the emission source strength for compounds produced within the plant, the atmospheric removal rate, and the diurnal changes in boundary layer thickness. Thus, the expansion of the atmospheric boundary layer during the central hours of the day can lead to a decrease in atmospheric concentration of BVOC, while the night-time collapse of the boundary layer can concentrate many BVOC within a much smaller volume, increasing the likelihood of leaf uptake (Fuentes et al. 2000; Betts, Ball & McCaughey 2001). For those oxygenated BVOC which arise from oxidation processes in the atmosphere, both the emission rates of their precursors and the rates of atmospheric oxidation affect the concentration. For some OVOC, such as acetone or formaldehyde, there may be sources both within the plant and atmosphere. These photochemical processes which are maximized during periods of high light and warm temperature can significantly increase the atmospheric concentration of OVOC (Seco et al. 2007). When ambient air OVOC concentrations build up, the troposphere can represent a source of OVOC and generate a positive gradient, thus promoting deposition. This process has been indeed inferred to dominate the removal of BVOC from the atmosphere (Hallquist et al. 2009).
All of these interactions make it exceedingly difficult to predict the magnitude and direction of flux at any given time. In the tropical forest of Amazonia, the uptake of formic and acetic acids at the branch level was found to be controlled largely by changes in atmospheric concentration, as the measured compensation points were low and stayed within a fairly narrow range (0.16-0.30 nmol mol−1) well below the usually observed atmospheric concentrations (Kuhn et al. 2002). In a tropical rain forest in Costa Rica, bidirectional exchange of methanol, acetaldehyde and acetone was observed, with emissions during the day and deposition at night (Karl et al. 2004). Although stomata have traditionally been assumed to close at night, there is now convincing evidence (Dawson et al. 2007) that many woody species maintain significant stomatal conductance at night, thus facilitating deposition processes for those compounds whose production in planta is dependent on light and/or temperature. Although above-canopy emissions were generally observed during the day, within-canopy concentration profiles indicated that upper canopy leaves appeared to be a net source of acetaldehyde, while shade leaves within the canopy served as a sink (Karl et al. 2004). Deposition velocities were far higher than traditional deposition models predict, implying much lower values of Rc (and f0) than previously thought. In two studies in orange (Citrus aurantium) orchards, high concentrations in ambient air were associated with OVOC deposition to orange canopies (Staudt et al. 2000; Fares et al. 2012).
Apart from leaves, soil with litter and roots can constitute an important source (Hayward et al. 2001; Asensio et al. 2007a; Leff & Fierer 2008; Aaltonen et al. 2011; Greenberg et al. 2012; Horvath et al. 2012) or chemical and/or physicochemical sink (Minnich & Schumacher 1993; Pegoraro et al. 2006; Asensio et al. 2007a; Asensio et al. 2007b; Reid & Jaffé 2013) of different BVOC. Although this review primarily focuses on plant emissions, we note that consideration of soil processes might be important to close the ecosystem BVOC balance.
Recently, ecosystem-level compensation points for organic acids (1.4 nmol mol−1 for formic acid and 2.1 nmol mol−1 for acetic acid) in a central Amazonian forest were reported (Jardine et al. 2011). A seasonal switch between formic and acetic acid net emission in the wet season and net deposition during the dry season was observed and largely attributed to changes in atmospheric concentrations due to seasonal biomass burning pollution (Jardine et al. 2011). Although the flux vs. ambient concentration relationships were scattered as is often the case in estimations of leaf-level compensation points (Jardine et al. 2011), these results further underscore the importance of considering BVOC bidirectional exchange at the ecosystem level.
From a limited set of compounds to a full spectrum of atmospheric BVOC and OVOC
The vast majority of studies to date have focused on BVOC emissions from plant canopies, because of their importance in atmospheric processes. Much fewer studies have considered BVOC deposition, and even these few studies have focused on a narrow range of plant-produced and bidirectionally-exchanged short-chained OVOC such as methanol, acetone, acetaldehyde, formic and acetic acids. As a result, little is known about deposition rates for the large number of BVOC oxidation products formed in the atmosphere, although the limited available evidence points to rates of deposition of most OVOC at rates that significant that can alter the composition of the atmosphere in ways not captured by existing models. Attempts to further our understanding of deposition processes have profited greatly from new advancements in BVOC measurement technology. The recently developed proton transfer reaction – time of flight – mass spectrometer (PTR-TOF-MS), for instance, can measure an unprecedented number of BVOC simultaneously with very high time resolution (Jordan et al. 2009). Recent eddy covariance applications revealed the presence of high concentrations and bidirectional fluxes of BVOC and their oxidation products (Park et al. 2013a; Park et al. 2013b). Although parent ions and their fragments could not be distinguished, more than 555 ions were measured in an orange (Citrus aurantium) orchard using a unique method that enabled quantification of bidirectional exchange even for the multitude of compounds found at very low atmospheric concentrations (Park et al. 2013a; Park et al. 2013b). On a carbon basis, estimated emission from the sum of 484 observed ions was 2.8 times as large as the sum of the ten major compounds traditionally measured, supporting the idea that many previously undetected BVOC exist in the atmosphere and are actively exchanged within ecosystems as hypothesized by Goldstein and Galbally (2007) and Hallquist et al. (2009). Moreover, the heavier compounds showed net deposition, suggesting that heavier molecules with lower vapor pressure deposit more efficiently to plant canopies. Among the deposited compounds, OVOC were predominant, in agreement with the idea that less-oxygenated BVOC are emitted from the ecosystem, whereas secondary compounds produced through atmospheric photochemical process and containing more oxygen atoms are preferentially removed by deposition. The daily dynamics of fluxes also showed that deposition mainly occurred in morning and afternoon hours, while the mid-day fluxes were dominated by emission from the ecosystem and/or within-canopy photochemical production when both temperature and light intensity reached the maximum values (Park et al. 2013a; Park et al. 2013b).
Implications for emission/deposition models
Recent evidence indicating presence of significant chemical and physicochemical sinks for BVOC and OVOC within the leaves that can lead to overall complex bidirectional exchange dynamics calls for rethinking of how to best simulate trace gas exchange between plants and atmosphere (Figs. 2-3). There is a growing consensus in atmospheric science that unidirectional flux modeling such as separate consideration of dry deposition and emissions should be replaced by bidirectional flux modeling (Pleim & Ran 2011), and we argue that plant trace gas exchange should also follow this path. Current BVOC emission models such as the Biogenic Emission Inventory System (BEIS, Pierce et al. 1998; Schwede, Pouliot & Pierce 2005; Hogrefe et al. 2011) and the Model of Emissions of Gases and Aerosols from Nature (MEGAN, Guenther et al. 2006) still do not consider many BVOC species which could be important in order to achieve an accurate estimate of ozone and SOA formation through regional photochemistry, although MEGAN2.1 (Guenther et al. 2012) deals explicitly with 138 different compounds. MEGAN2.1 recognizes the occurrence of bidirectional fluxes and includes a separate category for the short-chain oxygenated compounds ethanol, acetaldehyde, formaldehyde, acetic and formic acids. Millet et al. (2010) used a version of MEGAN2.1 to estimate terrestrial acetaldehyde flux, and recognized the bidirectional nature of that flux. Their approach was fairly crude, incorporating a Leaf Area Index factor that resulted in emissions from upper canopy leaves and deposition to lower leaves, consistent with the observations of Karl et al. (2004) and Jardine et al. (2008). Clearly, a key implication of within-canopy variations in emission and deposition processes is that the canopy itself, in particular, leaf area and leaf property distributions, should be better represented in the models.
Recent studies have shown that small changes in BVOC emission potential parameterization can lead to considerable changes of the prediction of physical and chemical properties of the atmosphere in the Mediterranean climate (Fares et al. 2013; Richards et al. 2013). The presence of still poorly explored BVOC in the atmosphere may also account for a sizable amount of the missing OH chemical reactivity and ozone chemical loss observed in plant environments (e.g., Di Carlo et al. 2004; Nölscher et al. 2012). Moreover, global chemistry and transport models should be finely parameterized to account for deposition of BVOC. Karl et al. (2010) demonstrated that altering the deposition scheme of the chemical transport model of Emoons et al. (2010) by incorporating the fast metabolic conversion of OVOC within leaves leads to substantially higher estimates of OVOC deposition, thus reducing OVOC concentrations in the lower troposphere and leading to changes in model predictions of SOA and tropospheric OH and ozone concentrations (Fig. 3). These pieces of evidence collectively indicate that next generation modeling schemes clearly provide more realistic temporal predictions, resulting in major improvements in simulations of biosphere-atmosphere exchange processes.
OUTLOOK FOR FUTURE RESEARCH
Depending on the frequency of observations (minutes, hours, days), a plant ecosystem can behave as a BVOC source or sink. Based on recent results, and considering the complexity of plant-atmosphere interactions (Fig. 3), more leaf-level investigations to determine the compensation point for a wider range of BVOC species than examined to date are clearly called for. In addition, studies to determine how compensation points vary in response to, among other factors, light, temperature and imposition of various stresses will be crucial. Studies on the deposition rate of a wide range of atmospheric organic constituents which have no source within leaves should also be examined, to determine whether their uptake is relatively slow, as older models predict, or rapid, implying much more rapid degradation processes within the leaf. If they are taken up at rates similar to ozone, for example, characterizing their deposition rates would be much more straightforward, depending largely on stomatal conductance and ambient concentrations.
While clearly useful and important for enhancing our understanding of the underlying physiological processes involved, it will be exceedingly difficult to apply such information at the canopy or ecosystem scale. Additional field studies, ideally at sites already equipped for micrometeorological flux measurements of CO2 and H2O, should be performed in order to capture the large suite of compounds exchanged with the atmosphere, and perhaps determine canopy-scale compensation points and examine how they vary with environmental conditions. Given that the spectrum of compounds emitted and their exchange characteristics vary considerably across different vegetation types as a result of phenology and atmospheric variability (Keenan et al. 2009; Fares et al. 2010; Fares et al. 2012), long-term studies are likely particularly fruitful in bringing bidirectional BVOC modeling forward.
Acknowledgements
ÜN’s research on BVOC is funded by the Estonian Ministry of Science and Education (institutional grant IUT-8-3), Estonian Science Foundation (grant 9253), and the European Commission through the European Regional Fund (Center of Excellence in Environmental Adaptation) and the European Research Council (advanced grant 322603, SIP-VOL+). KJ’s work on BVOC is supported by the Office of Biological and Environmental Research of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231 as part of their Terrestrial Ecosystem Science Program. We thank the reviewers and Francesco Loreto for insightful comments on the manuscript.
References
- Aaltonen H, Pumpanen J, Pihlatie M, Hakola H, Hellen H, Kulmala L, Vesala T, Bäck J. Boreal pine forest floor biogenic volatile organic compound emissions peak in early summer and autumn. Agricultural and Forest Meteorology. 2011;151:682–691. [Google Scholar]
- Abanda-Nkpwatt D, Müsch M, Tschiersch J, Boettner M, Schwab W. Molecular interaction between Methylobacterium extorquens and seedlings: growth promotion, methanol consumption, and localization of the methanol emission site. Journal of Experimental Botany. 2006;57:4025–4032. doi: 10.1093/jxb/erl173. [DOI] [PubMed] [Google Scholar]
- Achkor H, Diaz M, Fernandez MR, Biosca JA, Pares X, Martinez MC. Enhanced formaldehyde detoxification by overexpression of glutathione-dependent formaldehyde dehydrogenase from Arabidopsis. Plant Physiology. 2003;132 doi: 10.1104/pp.103.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae MO, Crutzen PJ. Atmospheric aerosols: biogeochemical sources and role in atmospheric chemistry. Science. 1997;276:1052–1058. [Google Scholar]
- Andreou A, Feussner I. Lipoxygenases - structure and reaction mechanism. Phytochemistry. 2009;70:1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Arimura G-I, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant and Cell Physiology. 2009;50:911–923. doi: 10.1093/pcp/pcp030. [DOI] [PubMed] [Google Scholar]
- Arneth A, Niinemets Ü. Induced BVOCs: how to bug our models? Trends in Plant Science. 2010;15:118–125. doi: 10.1016/j.tplants.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Arneth A, Unger N, Kulmala M, Andreae MO. Clean the air, heat the planet? Science. 2009;326:672–673. doi: 10.1126/science.1181568. [DOI] [PubMed] [Google Scholar]
- Asensio D, Peñuelas J, Llusià J, Ogaya R, Filella I. Interannual and interseasonal soil CO2 efflux and VOC exchange rates in a Mediterranean holm oak forest in response to experimental drought. Soil Biology & Biochemistry. 2007a;39:2471–2484. [Google Scholar]
- Asensio D, Peñuelas J, Ogaya R, Llusià J. Seasonal soil VOC exchange rates in a Mediterranean holm oak forest and their responses to drought conditions. Atmospheric Environment. 2007b;41:2456–2466. [Google Scholar]
- Atkinson R, Arey J. Atmospheric degradation of volatile organic compounds. Chemical Reviews. 2003;103:4605–4638. doi: 10.1021/cr0206420. [DOI] [PubMed] [Google Scholar]
- Bakker MI. Atmospheric deposition of semivolatile organic compounds to plants. Utercht University; 2000. [Google Scholar]
- Ball JT. Calculations related to gas exchange. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal function. Stanford University Press; Stanford: 1987. pp. 445–476. [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relations between ozone uptake and emission of LOX products. Plant, Cell and Environment. 2005;28:1334–1343. [Google Scholar]
- Betts AK, Ball JH, McCaughey JH. Near-surface climate in the boreal forest. Journal of Geophysical Research. 2001;106:33529–33541. [Google Scholar]
- Bouvier-Brown NC, Goldstein AH, Gilman JB, Kuster WC, de Gouw JA. In-situ ambient quantification of monoterpenes, sesquiterpenes, and related oxygenated compounds during BEARPEX 2007: implications for gas- and particle-phase chemistry. Atmospheric Chemistry and Physics. 2009;9:5505–5518. [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “time-of-flight” mass spectrometry (PTR-TOF) PloS ONE. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J, Eiden R. The ion concentration of dew condensed on Norway spruce (Picea abies (L.) Karst.) and Scots pine (Pinus sylvestris L.) needles. Trees: Structure and Function. 1990;4:22–26. [Google Scholar]
- Burkhardt J, Kaiser H, Goldbach H, Kappen L. Measurements of electrical leaf surface conductance reveal recondensation of transpired water vapour on leaf surfaces. Plant Cell and Environment. 1999;22:189–196. [Google Scholar]
- Burkhardt J, Pariyar S. Particulate pollutants are capable to ‘degrade’ epicuticular waxes and to decrease the drought tolerance of Scots pine (Pinus sylvestris L.) Environmental Pollution. 2014;184:659–667. doi: 10.1016/j.envpol.2013.04.041. [DOI] [PubMed] [Google Scholar]
- Ciccioli P, Brancaleoni E, Frattoni M, Di Palo V, Valentini R, Tirone G, Seufert G, Bertin N, Hansen U, Csiky O, Lenz R, Sharma M. Emission of reactive terpene compounds from orange orchards and their removal by within-canopy processes. Journal of Geophysical Research. 1999;104:8077–8094. [Google Scholar]
- Cojocariu C, Escher P, Häberle KH, Matyssek R, Rennenberg H, Kreuzwieser J. The effect of ozone on the emission of carbonyls from leaves of adult Fagus sylvatica. Plant, Cell and Environment. 2005;28:603–611. [Google Scholar]
- Cojocariu C, Kreuzwieser J, Rennenberg H. Correlation of short-chained carbonyls emitted from Picea abies with physiological and environmental parameters. The New Phytologist. 2004;162:717–727. doi: 10.1111/j.1469-8137.2004.01061.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Pazouki L, Niinemets Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. Journal of Plant Physiology. 2012;169:664–672. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutinosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici LO, Filella I, Llusià J, Niinemets Ü, Peñuelas J. The capacity for thermal protection of photosynthetic electron transport varies for different monoterpenes in Quercus ilex. Plant Physiology. 2005;139:485–496. doi: 10.1104/pp.105.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici LO, Niinemets Ü. Temperature dependencies of Henry’s law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere. 2005;61:1390–1400. doi: 10.1016/j.chemosphere.2005.05.003. [DOI] [PubMed] [Google Scholar]
- D’Auria JC, Pichersky E, Schaub A, Hansel A, Gershenzon J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. The Plant Journal. 2007;49:194–207. doi: 10.1111/j.1365-313X.2006.02946.x. [DOI] [PubMed] [Google Scholar]
- Davies KJA. Oxidative stress: the paradox of aerobic life. Biochemical Society Symposium. 1995:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Burgess SO, Tu KP, Oliveira RS, Santiago LS, Fisher JB, Simonin KA, Ambrose AR. Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiology. 2007;27:561–575. doi: 10.1093/treephys/27.4.561. [DOI] [PubMed] [Google Scholar]
- de Gouw JA, Howard CJ, Custer TG, Baker BM, Fall R. Proton-transfer chemical-ionization mass spectrometry allows real-time analysis of volatile organic compounds released from cutting and drying of crops. Environmental Science & Technology. 2000;34:2640–2648. [Google Scholar]
- de Gouw JA, Howard CJ, Custer TG, Fall R. Emissions of volatile organic compounds from cut grass and clover are enhanced during the drying process. Geophysical Research Letters. 1999;26:811–814. [Google Scholar]
- Di Carlo P, Brune WH, Martinez M, Harder H, Lesher R, Ren X, Thornberry T, Carroll MA, Young V, Shepson PB, Riemer D, Apel E, Campbell C. Missing OH reactivity in a forest: evidence for unknown reactive biogenic VOCs. Science. 2004;304:722–724. doi: 10.1126/science.1094392. [DOI] [PubMed] [Google Scholar]
- Durand T, Bultel-Ponce V, Guy A, Berger S, Mueller MJ, Galano JM. New bioactive oxylipins formed by non-enzymatic free-radical-catalyzed pathways: the phytoprostanes. Lipids. 2009;44:875–888. doi: 10.1007/s11745-009-3351-1. [DOI] [PubMed] [Google Scholar]
- Emmons LK, Walters S, Hess PG, Lamarque J-F, Pfister GG, Fillmore D, Granier C, Guenther A, Kinnison D, Laepple T, Orlando J, Tie X, Tyndall G, Wiedinmyer C, Baughcum SL, Kloster S. Description and evaluation of the Model for Ozone and Related chemical Tracers, version 4 (MOZART-4) Geoscientific Model Development. 2010;3:43–67. [Google Scholar]
- Estell RE, Fredrickson EL, Anderson DM, Mueller WF, Remmenga MD. Relationship of tarbush leaf surface secondary chemistry to livestock herbivory. Journal of Range Management. 1994a;47:424–428. [Google Scholar]
- Estell RE, Havstad KM, Fredrickson EL, Gardea-Torresdey JL. Secondary chemistry of the leaf surface of Flourensia cernua. Biochemical Systematics and Ecology. 1994b;22:73–77. [Google Scholar]
- Fall R. Abundant oxygenates in the atmosphere: a biochemical perspective. Chemical Reviews. 2003;103:4941–4952. doi: 10.1021/cr0206521. [DOI] [PubMed] [Google Scholar]
- Fall R, Benson AA. Leaf methanol - the simplest natural product from plants. Trends in Plant Science. 1996;1:296–301. [Google Scholar]
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. Journal of Geophysical Research. 1999;104:15963–15974. [Google Scholar]
- Fall R, Karl T, Jordan A, Lindinger W. Biogenic C5 VOCs: release from leaves after freeze-thaw wounding and occurrence in air at a high mountain observatory. Atmospheric Environment. 2001;35:3905–3916. [Google Scholar]
- Fall R, Monson RK. Isoprene emission rate and intercellular isoprene concentration as influenced by stomatal distribution and conductance. Plant Physiology. 1992;100:987–992. doi: 10.1104/pp.100.2.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares S, McKay M, Holzinger R, Goldstein AH. Ozone fluxes in a Pinus ponderosa ecosystem are dominated by non-stomatal processes: evidence from long-term continuous measurements. Agricultural and Forest Meteorology. 2010;150:420–431. [Google Scholar]
- Fares S, Park J-H, Gentner DR, Weber R, Ormeño E, Karlik J, Goldstein AH. Seasonal cycles of biogenic volatile organic compound fluxes and concentrations in a California citrus orchard. Atmospheric Chemistry and Physics. 2012;12:9865–9880. [Google Scholar]
- Fares S, Schnitzhofer R, Jiang X, Guenther A, Hansel A, Loreto F. Observations of diurnal to weekly variations of monoterpene-dominated fluxes of volatile organic compounds from Mediterranean forests: implications for regional modeling. Environmental Science & Technology. 2013;47:11073–11082. doi: 10.1021/es4022156. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Davoine C. Reactive electrophile species. Current Opinion in Plant Biology. 2007;10:380–386. doi: 10.1016/j.pbi.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Firth PM, Wetselaar R, Weir B. On the gaseous exchange of ammonia between leaves and the environment: determination of the ammonia compensation point. Plant Physiology. 1980;66:710–714. doi: 10.1104/pp.66.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Wetselaar R, Firth PM. Ammonia volatilization from senescing leaves of maize. Science. 1979;203:1257–1258. doi: 10.1126/science.203.4386.1257. [DOI] [PubMed] [Google Scholar]
- Filella I, Peñuelas J, Llusià J. Dynamics of the enhanced emissions of monoterpenes and methyl salicylate, and decreased uptake of formaldehyde, by Quercus ilex leaves after application of jasmonic acid. The New Phytologist. 2006;169:135–144. doi: 10.1111/j.1469-8137.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Fowler D, Pilegaard K, Sutton MA, et al. Atmospheric composition change: ecosystems - atmosphere interactions. Atmospheric Environment. 2009;43:5193–5267. [Google Scholar]
- Frankel EN, Hu ML, Tappel AL. Rapid headspace gas-chromatography of hexanal as a measure of lipid-peroxidation in biological samples. Lipids. 1989;24:976–981. doi: 10.1007/BF02544544. [DOI] [PubMed] [Google Scholar]
- Fuentes JD, Lerdau M, Atkinson R, Baldocchi D, Bottenheim JW, Ciccioli P, Lamb B, Geron C, Gu L, Guenther A, Sharkey TD, Stockwell W. Biogenic hydrocarbons in the atmospheric boundary layer: A review. Bulletin of the American Meteorological Society. 2000;81:1537–1575. [Google Scholar]
- Gabriel R, Schäfer L, Gerlach C, Rausch T, Kesselmeier J. Factors controlling the emissions of volatile organic acids from leaves of Quercus ilex L. (holm oak) Atmospheric Environment. 1999;33:1347–1355. [Google Scholar]
- Geron C, Guenther AB, Greenberg J, Karl T, Rasmussen R. Biogenic volatile organic compound emissions from desert vegetation of the southwestern US. Atmospheric Environment. 2006;40:1645–1660. [Google Scholar]
- Gigot C, Ongena M, Fauconnier ML, Wathelet JP, Du Jardin P, Thonart P. The lipoxygenase metabolic pathway in plants: potential for industrial production of natural green leaf volatiles. Biotechnologie Agronomie Societe Et Environnement. 2010;14:451–460. [Google Scholar]
- Goldstein AH, Galbally IE. Known and unexplored organic constituents in the Earth’s atmosphere. Environmental Science & Technology. 2007;41:1514–1521. doi: 10.1021/es072476p. [DOI] [PubMed] [Google Scholar]
- Graus M, Eller ASD, Fall R, Yuan B, Qian Y, Westra P, de Gouw J, Warneke C. Biosphere-atmosphere exchange of volatile organic compounds over C4 biofuel crops. Atmospheric Environment. 2013;66:161–168. [Google Scholar]
- Gray DW, Breneman SR, Topper LA, Sharkey TD. Biochemical characterization and homology modeling of methylbutenol synthase and implications for understanding hemiterpene synthase evolution in plants. The Journal of Biological Chemistry. 2011;286:20582–20590. doi: 10.1074/jbc.M111.237438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DW, Lerdau MT, Goldstein AH. Influences of temperature history, water stress, and needle age on methylbutenol emissions. Ecology. 2003;84:765–776. [Google Scholar]
- Greenberg JP, Asensio D, Turnipseed A, Guenther AB, Karl T, Gochis D. Contribution of leaf and needle litter to whole ecosystem BVOC fluxes. Atmospheric Environment. 2012;59:302–311. [Google Scholar]
- Grote R. Sensitivity of volatile monoterpene emission to changes in canopy structure: a model-based exercise with a process-based emission model. The New Phytologist. 2007;173:550–561. doi: 10.1111/j.1469-8137.2006.01946.x. [DOI] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 315–355. [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P. A global model of natural volatile compound emissions. Journal of Geophysical Research. 1995;100:8873–8892. [Google Scholar]
- Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature) Atmospheric Chemistry and Physics. 2006;6:3181–3210. [Google Scholar]
- Guenther AB, Jiang X, Heald CL, Sakulyanontvittaya T, Duhl T, Emmons LK, Wang X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model. Dev. 2012;5:1471–1492. [Google Scholar]
- Gutteridge JMC. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clinical Chemistry. 1995;41:1819–1828. [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd ed. Oxford University Press; Oxford, New York: 1999. [Google Scholar]
- Hallquist M, Wenger JC, Baltensperger U, et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmospheric Chemistry and Physics. 2009;9:5155–5236. [Google Scholar]
- Hansen U, Seufert G. Temperature and light dependence of β-caryophyllene emission rates. Journal of Geophysical Research - Atmospheres. 2003;108:4801. [Google Scholar]
- Hanson AD, Roje S. One-carbon metabolism in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:119–137. doi: 10.1146/annurev.arplant.52.1.119. [DOI] [PubMed] [Google Scholar]
- Harley P, Fridd-Stroud V, Greenberg J, Guenther A, Vasconcellos P. Emission of 2-methyl-3-buten-2-ol by pines: a potentially large natural source of reactive carbon to the atmosphere. Journal of Geophysical Research. 1998;103:25479–25486. [Google Scholar]
- Harley P, Greenberg J, Niinemets Ü, Guenther A. Environmental controls over methanol emission from leaves. Biogeosciences. 2007;4:1083–1099. [Google Scholar]
- Harley P, Guenther A, Zimmerman P. Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves. Tree Physiology. 1996;16:25–32. doi: 10.1093/treephys/16.1-2.25. [DOI] [PubMed] [Google Scholar]
- Harley P, Guenther A, Zimmerman P. Environmental controls over isoprene emission in deciduous oak canopies. Tree Physiology. 1997;17:705–714. doi: 10.1093/treephys/17.11.705. [DOI] [PubMed] [Google Scholar]
- Harley PC. The roles of stomatal conductance and compound volatility in controlling the emission of volatile organic compounds from leaves. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 181–208. [Google Scholar]
- Hartley DP, Kolaja KL, Reichard J, Petersen DR. 4-hydroxynonenal and malondialdehyde hepatic protein adducts in rats treated with carbon tetrachloride: immunochemical detection and lobular localization. Toxicology and Applied Pharmacology. 1999;161:23–33. doi: 10.1006/taap.1999.8788. [DOI] [PubMed] [Google Scholar]
- Hatanaka A. The biogeneration of green odour by green leaves. Biochemistry. 1993;37:1201–1218. [Google Scholar]
- Havaux M. Carotenoid oxidation products as stress signals in plants. The Plant Journal. 2013 doi: 10.1111/tpj.12386. [DOI] [PubMed] [Google Scholar]
- Hayward S, Muncey RJ, James AE, Halsall CJ, Hewitt CN. Monoterpene emissions from soil in a Sitka spruce forest. Atmospheric Environment. 2001;35:4081–4087. [Google Scholar]
- Heald CL, Henze DK, Horowitz LW, Feddema J, Lamarque J-F, Guenther A, Hess PG, Vitt F, Seinfeld JH, Goldstein AH, Fung I. Predicted change in global secondary organic aerosol concentrations in response to future climate, emissions, and land use change. Journal of Geophysical Research - Atmospheres. 2008;113:D05211. [Google Scholar]
- Heiden AC, Kobel K, Langebartels C, Schuh-Thomas G, Wildt J. Emissions of oxygenated volatile organic compounds from plants. Part I: Emissions from lipoxygenase activity. Journal of Atmospheric Chemistry. 2003;45:143–172. [Google Scholar]
- Hiatt MH. Bioconcentration factors for volatile organic compounds in vegetation. Analytical Chemistry. 1998;70:851–856. doi: 10.1021/ac971167m. [DOI] [PubMed] [Google Scholar]
- Hiatt MH. Leaves as an indicator of exposure to airborne volatile organic compounds. Environmental Science and Technology. 1999;33:4126–4133. [Google Scholar]
- Himanen SJ, Blande JD, Klemola T, Pulkkinen J, Heijari J, Holopainen JK. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants - a mechanism for associational herbivore resistance? The New Phytologist. 2010;186:722–732. doi: 10.1111/j.1469-8137.2010.03220.x. [DOI] [PubMed] [Google Scholar]
- Hirano SS, Upper CD. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae - a pathogen, ice nucleus, and epiphyte. Microbiology and Molecular Biology Reviews. 2000;64:624–653. doi: 10.1128/mmbr.64.3.624-653.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe C, Isukapalli SS, Tang X, Georgopoulos PG, He S, Zalewsky EE, Hao W, Ku J-Y, Key T, Sistla G. Impact of biogenic emission uncertainties on the simulated response of ozone and fine particulate matter to anthropogenic emission reductions. Journal of the Air & Waste Management Association. 2011;61:92–108. doi: 10.3155/1047-3289.61.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen JK, Heijari J, Oksanen E, Alessio GA. Leaf volatile emissions of Betula pendula during autumn coloration and leaf fall. Journal of Chemical Ecology. 2010;36:1068–1075. doi: 10.1007/s10886-010-9857-4. [DOI] [PubMed] [Google Scholar]
- Holopainen JK, Nerg A-M, Blande JD. Multitrophic signalling in polluted atmospheres. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 285–314. [Google Scholar]
- Holzinger R, Lee A, McKay M, Goldstein AH. Seasonal variability of monoterpene emission factors for a Ponderosa pine plantation in California. Atmospheric Chemistry and Physics. 2006;6:1267–1274. [Google Scholar]
- Horvath E, Hoffer A, Sebok F, Dobolyi C, Szoboszlay S, Kriszt B, Gelencser A. Experimental evidence for direct sesquiterpene emission from soils. Journal of Geophysical Research - Atmospheres. 2012;117:D15304. [Google Scholar]
- Husted S, Schjoerring JK. Ammonia flux between oilseed rape plants and the atmosphere in response to changes in leaf temperature, light intensity, and air humidity. Interactions with leaf conductance and apoplastic NH +4 and H+ concentrations. Plant Physiology. 1996;112:67–74. doi: 10.1104/pp.112.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüve K, Christ MM, Kleist E, Uerlings R, Niinemets Ü, Walter A, Wildt J. Simultaneous growth and emission measurements demonstrate an interactive control of methanol release by leaf expansion and stomata. Journal of Experimental Botany. 2007;58:1783–1793. doi: 10.1093/jxb/erm038. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Bykova N, Kleczkowski LA. Origins and metabolism of formate in higher plants. Plant Physiology and Biochemistry. 1999;37:503–513. [Google Scholar]
- Iraci LT, Baker BM, Tyndall GS, Orlando JJ. Measurements of the Henry’s law coefficients of 2-methyl-3-buten-2-ol, methacrolein, and methylvinyl ketone. Journal of Atmospheric Chemistry. 1999;33:321–330. [Google Scholar]
- Jansen RMC, Miebach M, Kleist E, van Henten EJ, Wildt J. Release of lipoxygenase products and monoterpenes by tomato plants as an indicator of Botrytis cinerea-induced stress. Plant Biology. 2009;11:859–868. doi: 10.1111/j.1438-8677.2008.00183.x. [DOI] [PubMed] [Google Scholar]
- Jardine K, Abrell L, Kurc SA, Huxman T, Ortega J, Guenther A. Volatile organic compound emissions from Larrea tridentata (creosotebush) Atmospheric Chemistry and Physics. 2010a;10:12191–12206. [Google Scholar]
- Jardine K, Barron-Gafford GA, Norman JP, Abrell L, Monson RK, Meyers KT, Pavao-Zuckerman M, Dontsova K, Kleist E, Werner C, Huxman TE. Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light-dark transitions. Photosynthesis Research. 2012a;113:321–333. doi: 10.1007/s11120-012-9746-5. [DOI] [PubMed] [Google Scholar]
- Jardine K, Harley P, Karl T, Guenther A, Lerdau M, Mak JE. Plant physiological and environmental controls over the exchange of acetaldehyde between forest canopies and the atmosphere. Biogeosciences. 2008;5:1559–1157. [Google Scholar]
- Jardine K, Wegener F, Abrell L, van Haren J, Werner C. Phytogenic biosynthesis and emission of methyl acetate. Plant, Cell and Environment. 2014;37:414–424. doi: 10.1111/pce.12164. [DOI] [PubMed] [Google Scholar]
- Jardine K, Yañez Serrano A, Arneth A, Abrell L, Jardine A, Artaxo P, Alves E, Kesselmeier J, Taylor T, Saleska S, Huxman T. Ecosystem-scale compensation points of formic and acetic acid in the central Amazon. Biogeosciences. 2011;8:3709–3720. [Google Scholar]
- Jardine KJ, Meyers K, Abrell L, Alves EG, Serrano AMY, Kesselmeier J, Karl T, Guenther A, Vickers C, Chambers JQ. Emissions of putative isoprene oxidation products from mango branches under abiotic stress. Journal of Experimental Botany. 2013;64:3669–3679. doi: 10.1093/jxb/ert202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine KJ, Monson RK, Abrell L, Saleska SR, Arneth A, Jardine A, Ishida FY, Maria A, Serrano Y, Artaxo P, Karl T, Fares S, Goldstein A, Loreto F, Huxman T. Within-plant isoprene oxidation confirmed by direct emissions of oxidation products methyl vinyl ketone and methacrolein. Global Change Biology. 2012b;18:973–984. [Google Scholar]
- Jardine KJ, Sommer E, Saleska SR, Huxman TE, Harley PC, Abrell L. Gas phase measurements of pyruvic acid and its volatile metabolites. Environmental Science and Technology. 2010b;44:2454–2460. doi: 10.1021/es903544p. [DOI] [PubMed] [Google Scholar]
- Jensen S, Eriksson G, Kylin H, Strachan WMJ. Atmospheric pollution by persistent organic compounds: monitoring with pine needles. Chemosphere. 1992;24:229–245. [Google Scholar]
- Jordan A, Haidacher S, Hanel G, Hartungen E, Märk L, Seehauser H, Schottkowsky R, Sulzer P, Märk TD. A high resolution and high sensitivity proton-transfer-reaction time-offlight mass spectrometer (PTR-TOF-MS) International Journal of Mass Spectrometry. 2009;286:122–128. [Google Scholar]
- Kai H, Hirashima K, Matsuda O, Ikegami H, Winkelmann T, Nakahara T, Iba K. Thermotoleramt cyclamen with reduced acrolein and methyl vinyl ketone. Journal of Experimental Botany. 2012;63:4143–4150. doi: 10.1093/jxb/ers110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Curtis AJ, Rosenstiel TN, Monson RK, Fall R. Transient releases of acetaldehyde from tree leaves - products of a pyruvate overflow mechanism. Plant, Cell and Environment. 2002a;25:1121–1131. [Google Scholar]
- Karl T, Guenther A, Jordan A, Fall R, Lindinger W. Eddy covariance measurement of biogenic oxygenated VOC emissions from hay harvesting. Atmospheric Environment. 2001;35:491–495. [Google Scholar]
- Karl T, Harley P, Emmons L, Thornton B, Guenther A, Basu C, Turnipseed A, Jardine K. Efficient atmospheric cleansing of oxidized organic trace gases by vegetation. Science. 2010;330:816–819. doi: 10.1126/science.1192534. [DOI] [PubMed] [Google Scholar]
- Karl T, Harley PC, Guenther A, Rasmussen R, Baker B, Jardine K, Nemitz E. The bi-directional exchange of oxygenated VOCs between a loblolly pine (Pinus taeda) plantation and the atmosphere. Atmospheric Chemistry and Physics. 2005;5:3015–3031. [Google Scholar]
- Karl T, Potosnak M, Guenther A, Clark D, Walker J, Herrick JD, Geron C. Exchange processes of volatile organic compounds above a tropical rain forest: Implications for modeling tropospheric chemistry above dense vegetation. Journal of Geophysical Research - Atmospheres. 2004;109:D18306. [Google Scholar]
- Karl TG, Spirig C, Rinne J, Stroud C, Prevost P, Greenberg J, Fall R, Guenther A. Virtual disjunct eddy covariance measurements of organic compound fluxes from a subalpine forest using proton transfer reaction mass spectrometry. Atmospheric Chemistry and Physics. 2002b;2:279–291. [Google Scholar]
- Kask K, Kännaste A, Niinemets Ü. Emission of volatile organic compounds as a signal of plant stress. Scientific Bulletin of ESCORENA. 2013;8:79–93. [Google Scholar]
- Kawai Y, Takeda S, Terao J. Lipidomic analysis for lipid peroxidation-derived aldehydes using gas chromatography-mass spectrometry. Chemical Research in Toxicology. 2007;20:99–107. doi: 10.1021/tx060199e. [DOI] [PubMed] [Google Scholar]
- Keenan T, Niinemets Ü, Sabate S, Gracia C, Peñuelas J. Seasonality of monoterpene emission potentials in Quercus ilex and Pinus pinea: Implications for regional BVOC emissions modelling. Journal of Geophysical Research - Atmospheres. 2009;114:D22202. [Google Scholar]
- Kesselemier J, Merk L. Exchange of carbonyl sulfide (COS) between agricultural plants and the atmosphere: studies on the deposition of COS to peas, corn and rapeseeds. Biogeochemistry. 1993;23:47–59. [Google Scholar]
- Kesselmeier J. Exchange of short-chain oxygenated volatile organic compounds (VOCs) between plants and the atmosphere: a compilation of field and laboratory studies. Journal of Atmospheric Chemistry. 2001;39:219–233. [Google Scholar]
- Kesselmeier J, Bode K, Gerlach C, Jork EM. Exchange of atmospheric formic and acetic acids with trees and crop plants under controlled chamber and purified air conditions. Atmospheric Environment. 1998;32:1765–1775. [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. Journal of Atmospheric Chemistry. 1999;33:23–88. [Google Scholar]
- Keyte I, Wild E, Dent J, Jones KC. Investigating the foliar uptake and within-leaf migration of phenanthrene by moss (Hypnum cupressiforme) using two-photon excitation microscopy with autofluorescence. Environmental Science and Technology. 2009;43:5755–5761. doi: 10.1021/es900305c. [DOI] [PubMed] [Google Scholar]
- Kirch H-H, Bartels D, Wei Y, Schnable PS, Wood AJ. The ALDH gene superfamily of Arabidopsis. Trends in Plant Science. 2004;9:371–377. doi: 10.1016/j.tplants.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum MUF, Niinemets Ü, Bruhn D, Winters AJ. How important is aerobic methane release by plants? Functional Plant Science and Biotechnology. 2007;1:138–145. [Google Scholar]
- Kreuzwieser J, Harren FJM, Laarhoven LJJ, Boamfa I, te Lintel HS, Scheerer U, Huglin C, Rennenberg H. Acetaldehyde emission by the leaves of trees - correlation with physiological and environmental parameters. Physiologia Plantarum. 2001;113:41–49. [Google Scholar]
- Kreuzwieser J, Rennenberg H. Flooding-driven emissions from trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 237–252. [Google Scholar]
- Kreuzwieser J, Scheerer U, Rennenberg H. Metabolic origin of acetaldehyde emitted by poplar (Populus tremula x P. alba) trees. Journal of Experimental Botany. 1999;50:757–765. [Google Scholar]
- Kuhn U, Rottenberger S, Biesenthal T, Ammann C, Wolf A, Schebeske G, Oliva ST, Tavares TM, Kesselmeier J. Exchange of short-chain monocarboxylic acids by vegetation at a remote tropical forest site in Amazonia. Journal of Geophysical Research. 2002;D107:8069. [Google Scholar]
- Kulmala M, Nieminen T, Chellapermal R, Makkonen R, Bäck J, Kerminen V-M. Climate feedbacks linking the increasing atmospheric CO2 concentration, BVOC emissions, aerosols and clouds in forest ecosystems. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 489–508. [Google Scholar]
- Laisk A, Kull O, Moldau H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiology. 1989;90:1163–1167. doi: 10.1104/pp.90.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff JW, Fierer N. Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biology & Biochemistry. 2008;40:1629–1636. [Google Scholar]
- Li Z, Sharkey TD. Molecular and pathway controls on biogenic volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 119–151. [Google Scholar]
- Lindow SE, Brandl MT. Microbiology of the phyllosphere. Applied and Environmental Microbiology. 2003;69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EK, Picklo MJ. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: make some room HNE. Free Radical Biology and Medicine. 2010;49:1–8. doi: 10.1016/j.freeradbiomed.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Loreto F, Barta C, Brilli F, Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell and Environment. 2006;29:1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Fabozzi C, Tricoli D. Evidence of the photosynthetic origin of monoterpenes emitted by Quercus ilex L. leaves by 13C labeling. Plant Physiology. 1996;110:1317–1322. doi: 10.1104/pp.110.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Fares S. Is ozone flux inside leaves only a damage indicator? Clues from volatile isoprenoid studies. Plant Physiology. 2007;143:1096–1100. doi: 10.1104/pp.106.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S. Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiology. 2001;126:993–1000. doi: 10.1104/pp.126.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends in Plant Science. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]
- Lugg GA. Diffusion coefficients of some organic and other vapors in air. Analytical Chemistry. 1968;40:1072–1077. [Google Scholar]
- MacDonald RC, Fall R. Detection of substancial emissions of methanol from plants to the atmosphere. Atmospheric Environment. 1993;27A:1709–1713. [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. Journal of Neurochemistry. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Marrero TR, Mason EA. Gaseous diffusion coefficients. Journal of Physical and Chemical Reference Data. 1972;1:3–118. [Google Scholar]
- Matsui K, Sugimoto K, Kakumyan P, Khorobrykh SA, Mano J.i. Volatile oxylipins and related compounds formed under stress in plants. In: Armstrong D, editor. Lipidomics. Humana Press; New York: 2010. pp. 17–28. [DOI] [PubMed] [Google Scholar]
- McLachlan MS. Bioaccumulation of hydrophobic chemicals in agricultural food chains. Environmental Science & Technology. 1996;30:252–259. [Google Scholar]
- Mene-Saffrane L, Dubugnon L, Chetelat A, Stolz S, Gouhier-Darimont C, Farmer EE. Nonenzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. The Journal of Biological Chemistry. 2009;284:1702–1708. doi: 10.1074/jbc.M807114200. [DOI] [PubMed] [Google Scholar]
- Millet DB, Guenther A, Siegel DA, Nelson NB, Singh HB, de Gouw JA, Warneke C, Williams J, Eerdekens G, Sinha V, Karl T, Flocke F, Apel E, Riemer DD, Palmer PI, Barkley M. Global atmospheric budget of acetaldehyde: 3-D model analysis and constraints from in-situ and satellite observations. Atmospheric Chemistry and Physics. 2010;10:3405–3425. [Google Scholar]
- Minnich M, Schumacher B. Behavior and determination of volatile organic compounds in soil: a literature review. EPA/600/R-93/140. Office of Research and Development, United States Enironmental Protection Agency; Washington, DC: 1993. [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends in Plant Science. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Moldau H, Bichele I. Plasmalemma protection by the apoplast as assessed from above-zero ozone concentrations in leaf intercellular air spaces. Planta. 2002;214:484–487. doi: 10.1007/s00425-001-0678-0. [DOI] [PubMed] [Google Scholar]
- Monson RK. Metabolic and gene expression controls on the production of biogenic volatile organic compounds. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 153–179. [Google Scholar]
- Monson RK, Grote R, Niinemets Ü, Schnitzler J-P. Tansley review. Modeling the isoprene emission rate from leaves. The New Phytologist. 2012;195:541–559. doi: 10.1111/j.1469-8137.2012.04204.x. [DOI] [PubMed] [Google Scholar]
- Moseley R, Hilton JR, Stephens P, Waddington RJ, Thomas DW. Evaluation of oxidative stress biomarkers in chronic inflammatory disease prognoses. Journal of Dental Research. 2003;82:545–545. [Google Scholar]
- Nemecek-Marshall M, MacDonald RC, Franzen JJ, Wojciechowski CL, Fall R. Methanol emission from leaves. Enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development. Plant Physiology. 1995;108:1359–1368. doi: 10.1104/pp.108.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clinical Chemistry. 1997;43:1209–1214. [PubMed] [Google Scholar]
- Niinemets Ü. Photosynthesis and resource distribution through plant canopies. Plant, Cell and Environment. 2007;30:1052–1071. doi: 10.1111/j.1365-3040.2007.01683.x. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010a;7:2203–2223. [Google Scholar]
- Niinemets Ü, Ciccioli P, Noe SM, Reichstein M. Scaling BVOC emissions from leaf to canopy and landscape: how different are predictions based on contrasting emission algorithms? In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013a. pp. 357–390. [Google Scholar]
- Niinemets Ü, Copolovici L, Hüve K. High within-canopy variation in isoprene emission potentials in temperate trees: implications for predicting canopy-scale isoprene fluxes. Journal of Geophysical Research - Biogeosciences. 2010b;115:G04029. [Google Scholar]
- Niinemets Ü, Hauff K, Bertin N, Tenhunen JD, Steinbrecher R, Seufert G. Monoterpene emissions in relation to foliar photosynthetic and structural variables in Mediterranean evergreen Quercus species. The New Phytologist. 2002a;153:243–256. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science. Frontiers in Plant-Microbe Interaction. 2013b;4:262. doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, Lerdau MT, Monson RK, Peñuelas J. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in Plant Science. 2004;9:180–186. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Noe SM, Peñuelas J, Staudt M. The leaf-level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences. 2010c;7:1809–1832. [Google Scholar]
- Niinemets Ü, Reichstein M. A model analysis of the effects of nonspecific monoterpenoid storage in leaf tissues on emission kinetics and composition in Mediterranean sclerophyllous Quercus species. Global Biogeochemical Cycles. 2002;16:1110. DOI 1110.1029/2002GB001927. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: a sensitivity analysis. Journal of Geophysical Research - Atmospheres. 2003a;108:4211. doi:4210.1029/2002JD002626. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: sensitivity or insensitivity of the emission rates to stomatal closure explained. Journal of Geophysical Research - Atmospheres. 2003b;108:4208. doi:4210.1029/2002JD002620. [Google Scholar]
- Niinemets Ü, Reichstein M, Staudt M, Seufert G, Tenhunen JD. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiology. 2002b;130:1371–1385. doi: 10.1104/pp.009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe SM, Copolovici L, Niinemets Ü, Vaino E. Foliar limonene uptake scales positively with leaf lipid content: “non-emitting” species absorb and release monoterpenes. Plant Biology. 2008;10:129–137. doi: 10.1055/s-2007-965239. [DOI] [PubMed] [Google Scholar]
- Noe SM, Hüve K, Niinemets Ü, Copolovici L. Seasonal variation in vertical volatile compounds air concentrations within a remote hemiboreal mixed forest. Atmospheric Chemistry and Physics. 2012;12:3909–3926. [Google Scholar]
- Noe SM, Niinemets Ü, Schnitzler J-P. Modeling the temporal dynamics of monoterpene emission by isotopic labeling in Quercus ilex leaves. Atmospheric Environment. 2010;44:392–399. [Google Scholar]
- Nölscher AC, Williams J, Sinha V, et al. Summertime total OH reactivity measurements from boreal forest during HUMPPA-COPEC 2010. Atmospheric Chemistry and Physics. 2012;12:8257–8270. [Google Scholar]
- Ohlrogge J, Browse J. Lipid biosynthesis. The Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa PY, Lerdau MT. Catabolism of volatile organic compounds influences plant survival. Trends in Plant Science. 2013;18:695–703. doi: 10.1016/j.tplants.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Omasa K, Tobe K, Hosomi M, Kobayashi M. Absorption of ozone and seven organic pollutants by Populus nigra and Camellia sasanqua. Environmental Science & Technology. 2000;34:2498–2500. [Google Scholar]
- Oro J, Nooner DW, Zlatkis A, Wikström SA, Barghoor ES. Hydrocarbons of biological origin in sediments about two billion tears old. Science. 1965;148:77–79. doi: 10.1126/science.148.3666.77. [DOI] [PubMed] [Google Scholar]
- Papen H, Geßler A, Zumbusch E, Rennenberg H. Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Current Microbiology. 2002;44:56–60. doi: 10.1007/s00284-001-0074-9. [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiology. 1997;114:1161–1167. doi: 10.1104/pp.114.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-H, Goldstein AH, Timkovsky J, Fares S, Weber R, Karlik J, Holzinger R. Active atmosphere-ecosystem exchange of the vast majority of detected volatile organic compounds. Science. 2013a;341:643–647. doi: 10.1126/science.1235053. [DOI] [PubMed] [Google Scholar]
- Park J-H, Goldstein AH, Timkovsky J, Fares S, Weber R, Karlik J, Holzinger R. Eddy covariance emission and deposition flux measurements using proton transfer reaction - time of flight - mass spectrometry (PTR-TOF-MS): comparison with PTR-MS measured vertical gradients and fluxes. Atmospheric Chemistry and Physics. 2013b;13:1439–1456. [Google Scholar]
- Pegoraro E, Rey A, van Haren J, Lin G-G. Drought effect on isoprene production and consumption in Biosphere 2 tropical rainforest. Global Change Biology. 2006;12:456–469. [Google Scholar]
- Pfanz H, Martinoia E, Lange O-L, Heber U. Mesophyll resistances to SO2 fluxes into leaves. Plant Physiology. 1987;85:922–927. doi: 10.1104/pp.85.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce T, Geron C, Bender L, Dennis R, Tonnesen G, Guenther A. Influence of increased isoprene emissions on regional ozone modeling. Journal of Geophysical Research - Atmospheres. 1998;103:25611–25629. [Google Scholar]
- Pleim J, Ran L. Surface flux modeling for air quality applications. Atmosphere. 2011;2:271–302. [Google Scholar]
- Rajabi Memari H, Pazouki L, Niinemets Ü. The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Springer; Berlin: 2013. pp. 47–93. [Google Scholar]
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylides C, Havaux M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N. The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Journal of Experimental Botany. 2010;12:2885–2893. doi: 10.1111/j.1462-2920.2010.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MC, Jaffé PR. A push-pull test to measure root uptake of volatile chemicals from wetland soils. Environmental Science & Technology. 2013;47:3190–3198. doi: 10.1021/es304748r. [DOI] [PubMed] [Google Scholar]
- Richards NAD, Arnold SR, Chipperfield MP, Miles G, Rap A, Siddans R, Monks SA, Hollaway MJ. The Mediterranean summertime ozone maximum: global emission sensitivities and radiative impacts. Atmospheric Chemistry and Physics. 2013;13:2331–2345. [Google Scholar]
- Riederer M, Daiß A, Gilbert N, Köhle H. Semi-volatile organic compounds at the leaf/atmosphere interface: numerical simulation of dispersal and foliar uptake. Journal of Experimental Botany. 2002;53:1815–1823. doi: 10.1093/jxb/erf020. [DOI] [PubMed] [Google Scholar]
- Rottenberger S, Kleiss B, Kuhn U, Wolf A, Piedade MTF, Junk W, Kesselmeier J. The effect of flooding on the exchange of the volatile C2-compounds ethanol, acetaldehyde and acetic acid between leaves of Amazonian floodplain tree species and the atmosphere. Biogeosciences. 2008;5:1085–1100. [Google Scholar]
- Rottenberger S, Kuhn U, Wolf A, Schebeske G, Oliva ST, Tavares TM, Kesselmeier J. Formaldehyde and acetaldehyde exchange during leaf development of the Amazonian deciduous tree species Hymenaea courbaril. Atmospheric Environment. 2005;39:2275–2279. [Google Scholar]
- Rottenberger S, Kuhn U, Wolf A, Schebeske G, Olivia ST, Tavares TM, Kesselmeier J. Exchange of short-chain aldehydes between Amazonian vegetation and the atmosphere. Ecological Applications. 2004;14:S247–S262. [Google Scholar]
- Sandoval-Soto L, Kesselmeier M, Schmitt V, Wild A, Kesselmeier J. Observations of the uptake of carbonyl sulfide (COS) by trees under elevated atmospheric carbon dioxide concentrations. Biogeosciences. 2012;9:2935–2945. [Google Scholar]
- Schjoerring JK, Husted S, Mattsson M. Physiological parameters controlling plant-atmosphere ammonia exchange. Atmospheric Environment. 1998;32:491–498. [Google Scholar]
- Schmid C, Steinbrecher R, Ziegler H. Partition coefficients of plant cuticles for monoterpenes. Trees: Structure and Function. 1992;6:32–36. [Google Scholar]
- Schönherr J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. Journal of Experimental Botany. 2006;57:2471–2491. doi: 10.1093/jxb/erj217. [DOI] [PubMed] [Google Scholar]
- Schreiber L, Riederer M. Determination of diffusion coefficients of octadecanoic acid in isolated cuticular waxes and their relationship to cuticular water permeabilities. Plant, Cell and Environment. 1996a;19:1075–1082. [Google Scholar]
- Schreiber L, Riederer M. Ecophysiology of cuticular transpiration: comparative investigation of cuticular water permeability of plant species from different habitats. Oecologia. 1996b;107:426–432. doi: 10.1007/BF00333931. [DOI] [PubMed] [Google Scholar]
- Schwede D, Pouliot G, Pierce T. Changes to the biogenic emissions inventory system version 3 (BEIS3); Paper presented at the 4th Annual CMAS Models-3 User’s Conference; Chapel Hill, NC. September 26 - 28, 2005.2005. [Google Scholar]
- Seco R, Peñuelas J, Filella I. Short-chain oxygenated VOCs: emission and uptake by plants and atmospheric sources, sinks, and concentrations. Atmospheric Environment. 2007;41:2477–2499. [Google Scholar]
- Seco R, Peñuelas J, Filella I. Formaldehyde emission and uptake by Mediterranean trees Quercus ilex and Pinus halepensis. Atmospheric Environment. 2008;42:7907–7914. [Google Scholar]
- Shibamoto T. Analytical methods for trace levels of reactive carbonyl compounds formed in lipid peroxidation systems. Journal of Pharmaceutical and Biomedical Analysis. 2006;41:12–25. doi: 10.1016/j.jpba.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Shulaev V, Oliver DJ. Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research. Plant Physiology. 2006;141:367–372. doi: 10.1104/pp.106.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonich SL, Hltes RA. Vegetation-atmosphere partitioning of polycyclic aromatic hydrocarbons. Environmental Science and Technology. 1994;28:939–943. doi: 10.1021/es00054a028. [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD. Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiology. 1997;115:1413–1420. doi: 10.1104/pp.115.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Städler E, Reifenrath K. Glucosinolates on the leaf surface perceived by insect herbivores: review of ambiguous results and new investigations. Phytochemistry Reviews. 2009;8:207–225. [Google Scholar]
- Staudinger J, Roberts PV. A critical compilation of Henry’s law constant temperature dependence relations for organic compounds in dilute aqueous solutions. Chemosphere. 2001;44:561–576. doi: 10.1016/s0045-6535(00)00505-1. [DOI] [PubMed] [Google Scholar]
- Staudt M, Wolf A, Kesselmeier J. Influence of environmental factors on the emissions of gaseous formic and acetic acids from orange (Citrus sinensis L.) foliage. Biogeochemistry. 2000;48:199–216. [Google Scholar]
- Steeghs MML, Moeskops BAWM, van Swam K, Cristescu SM, Scheepers PTJ, Harren FJM. On-line monitoring of UV-induced lipid peroxidation products from human skin in vivo using proton-transfer reaction mass spectrometry. International Journal of Mass Spectrometry. 2006;253:58–64. [Google Scholar]
- Steyaert NLL, Hauck M, Van Hulle SWH, Hendriks AJ. Modelling bioaccumulation of semi-volatile organic compounds (SOCs) from air in plants based on allometric principles. Chemosphere. 2009;77:727–732. doi: 10.1016/j.chemosphere.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Stimler K, Berry JA, Montzka SA, Yakir D. Association between carbonyl sulfide uptake and 18Δ during gas exchange in C3 and C4 leaves. Plant Physiology. 2011;157:509–517. doi: 10.1104/pp.111.176578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimler K, Montzka SA, Berry JA, Rudich Y, Yakir D. Relationships between carbonyl sulfide (COS) and CO2 during leaf gas exchange. The New Phytologist. 2010;186:869–878. doi: 10.1111/j.1469-8137.2010.03218.x. [DOI] [PubMed] [Google Scholar]
- Sun Z, Copolovici L, Niinemets Ü. Can the capacity for isoprene emissions acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? Journal of Plant Research. 2012a;125:263–274. doi: 10.1007/s10265-011-0429-7. [DOI] [PubMed] [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Noe SM, Rasulov B, Copolovici L, Vislap V. Enhanced isoprene emission capacity and altered light responsiveness in aspen grown under elevated atmospheric CO2 concentration. Global Change Biology. 2012b;18:3423–3440. [Google Scholar]
- Sun Z, Niinemets Ü, Hüve K, Rasulov B, Noe SM. Elevated atmospheric CO2 concentration leads to increased whole-plant isoprene emission in hybrid aspen (Populus tremula x Populus tremuloides) The New Phytologist. 2013;198:788–800. doi: 10.1111/nph.12200. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Tani A, Hewitt CN. Uptake of aldehydes and ketones at typical indoor concentrations by houseplants. Environmental Science and Technology. 2009;43:8338–8343. doi: 10.1021/es9020316. [DOI] [PubMed] [Google Scholar]
- Tani A, Tobe S, Shimizu S. Uptake of methacrolein and methyl vinyl ketone by tree saplings and implications for forest atmosphere. Environmental Science & Technology. 2010;44:7096–7101. doi: 10.1021/es1017569. [DOI] [PubMed] [Google Scholar]
- Tao Z, Hornbuckle KC. Uptake of polycyclic aromatic hydrocarbons (PAHS) by broad leaves: analysis of kinetic limitations. Water, Air, and Soil Pollution. 2001;1:275–283. [Google Scholar]
- Tarvainen V, Hakola H, Hellén H, Bäck J, Hari P, Kulmala M. Temperature and light dependence of the VOC emissions of Scots pine. Atmospheric Chemistry and Physics. 2005;5:989–998. [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- Trapp S, Karlson U. Aspects of phytoremediation of organic pollutants. Journal of Soils and Sediments. 2001;1:37–43. [Google Scholar]
- Trapp S, Matthies M, Scheunert I, Topp EM. Modeling the bioconcentration of organic chemicals in plants. Environmental Science and Technology. 1990;24:1246–1252. [Google Scholar]
- Velikova V, Edreva A, Loreto F. Endogenous isoprene protects Phragmites australis leaves against singlet oxygen. Physiologia Plantarum. 2004;122:219–225. [Google Scholar]
- Velikova V, Fares S, Loreto F. Isoprene and nitric oxide reduce damages in leaves exposed to oxidative stress. Plant Cell and Environment. 2008;31:1882–1894. doi: 10.1111/j.1365-3040.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nature Chemical Biology. 2009;5:283–291. doi: 10.1038/nchembio.158. [DOI] [PubMed] [Google Scholar]
- Walsh K, Jones GJ, Dunstan RH. Effect of high irradiance and iron on volatile odour compounds in the cyanobacterium Microcystis aeruginosa. Phytochemistry. 1998;49:1227–1239. doi: 10.1016/s0031-9422(97)00943-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Pedas P, Eriksson D, Schjoerring JK. Elevated atmospheric CO2 decreases the ammonia compensation point of barley plants. Journal of Experimental Botany. 2013;64:2713–2724. doi: 10.1093/jxb/ert117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesely ML. Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical models. Atmospheric Environment. 1989;23:1293–1304. [Google Scholar]
- Wheatley RA. Some recent trends in the analytical chemistry of lipid peroxidation. Trac-Trends in Analytical Chemistry. 2000;19:617–628. [Google Scholar]
- Xu X, Bingemer HM, Schmidt U. The flux of carbonyl sulfide and carbon disulfide between the atmosphere and a spurce forest. Atmospheric Chemistry and Physics Discussions. 2002;2:171–181. [Google Scholar]
- Zhang L, Moran MD, Makar PA, Brook JR, Gong S. Modelling gaseous dry deposition in AURAMS: a unified regional air-quality modelling system. Atmospheric Environment. 2002;36:537–560. [Google Scholar]