Abstract
Objectives
To summarise the evidence from studies of acute kidney injury (AKI) with regard to the effect of pre-AKI renal function and post-AKI renal function recovery on long-term mortality and renal outcomes, and to assess whether these factors should be taken into account in future prognostic studies.
Design/Setting
A systematic review of observational studies listed in Medline and EMBASE from 1990 to October 2012.
Participants
All AKI studies in adults with data on baseline kidney function to identify AKI; with outcomes either stratified by pre-AKI and/or post-AKI kidney function, or described by the timing of the outcomes.
Outcomes
Long-term mortality and worsening chronic kidney disease (CKD).
Results
Of 7385 citations, few studies met inclusion criteria, reported baseline kidney function and stratified by pre-AKI or post-AKI function. For mortality outcomes, three studies compared patients by pre-AKI renal function and six by post-AKI function. For CKD outcomes, two studies compared patients by pre-AKI function and two by post-AKI function. The presence of CKD pre-AKI (compared with AKI alone) was associated with doubling of mortality and a fourfold to fivefold increase in CKD outcomes. Non-recovery of kidney function was associated with greater mortality and CKD outcomes in some studies, but findings were inconsistent varying with study design. Two studies also reported that risk of poor outcome reduced over time post-AKI. Meta-analysis was precluded by variations in definitions for AKI, CKD and recovery.
Conclusions
The long-term prognosis after AKI varies depending on cause and clinical setting, but it may also, in part, be explained by underlying pre-AKI and post-AKI renal function rather than the AKI episode itself. While carefully considered in clinical practice, few studies address these factors and with inconsistent study design. Future AKI studies should report pre-AKI and post-AKI function consistently as additional factors that may modify AKI prognosis.
Keywords: EPIDEMIOLOGY, PUBLIC HEALTH
Strengths and limitations of this study.
This systematic review followed strict inclusion, exclusion and quality assessment criteria to summarise the available evidence regarding the role of pre-acute kidney injury (AKI) baseline and post-AKI recovery of renal function in long-term AKI outcomes.
Few studies reported long-term AKI outcomes stratified by pre-AKI and post-AKI factors. Quantitative meta-analysis was precluded by heterogeneity in design of included studies.
The review includes papers from Medline and EMBASE up to October 2012. It may potentially have missed studies published in 2013–2014 or available in other databases.
Introduction
Acute kidney injury (AKI) affects an estimated 13–18% of hospitalised patients,1 frequently under the care of specialties other than nephrology. With the advent of an internationally agreed definition based on changes in serum creatinine and urine output,2 there is now increasing awareness of the poor outcomes suffered by such patients and this has been accompanied by an emphasis on early detection in an effort to improve patient safety and outcomes.1 Some of the poor outcomes (including increased mortality2 3 and development of chronic kidney disease (CKD)4–6) are increasingly described after hospital discharge, but this is variable and factors associated with long-term prognosis are poorly understood.1 Recent guidelines from the National Institute for Health and Care Excellence (NICE) call for more studies into AKI, with non-AKI comparators, but without specifying which factors should be included in study design.
AKI occurs in many different situations and the context is likely to be important when studying onset of AKI and assessing future outcome risk. In particular, clear knowledge of prior kidney (pre-AKI baseline) function is essential to distinguish AKI from CKD. This ‘baseline function’ is typically a creatinine measurement prior to hospitalisation, but may not be available. This can be solved in clinical practice using good clinical judgement, but in epidemiological studies patients with missing baseline values are either assumed to be normal, estimated from other results or excluded. Baseline function is required for grading AKI severity; as a reference for establishing if recovery is complete; and as a means of stratifying patients with and without pre-AKI CKD. It is intuitive that AKI in patients with advanced baseline pre-AKI CKD could present and behave differently,7 thus studies without baseline function are at risk of selection bias and misclassification of CKD as AKI.
After an episode of AKI (for instance at hospital discharge or clinic review), the physician also has an opportunity to consider a patient's most recent post-AKI kidney function. Accounting for post-AKI recovery from AKI (eg, return of creatinine to within 20% pre-AKI creatinine2) may assist future risk assessment. However, the timing of this post-AKI assessment may also influence its predictive ability, since clinical course may vary, with recovery and deterioration possible and not necessarily at a constant rate.8
Thus, while there is evidence that AKI may have a poor overall prognosis, in this systematic review we seek to evaluate whether previous observational studies have demonstrated the modifying contribution of pre-AKI baseline function, post-AKI recovery and the timing of outcomes. We also assess whether stratification by these factors should be necessary in future prognostic studies and might provide valuable information to the physician making a risk assessment.
Methods
A systematic review of observational studies was undertaken in accordance with guidelines for meta-analysis of observational studies in epidemiology (MOOSE).9 Medline and EMBASE were searched from January 1990 through October 2012 using Medline subject heading (MeSH) terms and free text for AKI (acute renal failure, acute kidney disease, acute kidney injury, acute dialysis, RIFLE, AKIN) and prognosis (prognosis, survival, mortality, follow up, progression, chronic kidney disease, renal replacement therapy, chronic dialysis, end-stage kidney disease; see online supplementary material). Reference lists of relevant studies and review articles were also searched. There were no language restrictions.
Studies were included if AKI was defined with: creatinine changes within a specified time interval, hospital episode coding for AKI, or the initiation of acute renal replacement therapy (RRT). Studies had to report either mortality or a CKD outcome (development of CKD, progression of CKD severity, or end-stage kidney disease (ESKD)). Studies were included that reported adults (age over 18 years), had at least 50 participants with AKI surviving to hospital discharge, and had follow-up of at least 1 year. Studies of those with only specialised conditions (eg, cancer, chemotherapy, transplantation) were excluded.
Two reviewers independently screened titles, abstracts and full papers against the inclusion and exclusion criteria. Where disagreement could not be resolved by discussion, a third author was available, but was not required. One researcher extracted information from the included studies into a data extraction proforma, with confirmation by a second reviewer.
Quality assessment
Six quality criteria (based on guidelines for assessing the quality of prognostic studies10) with a specific focus on the key areas of potential bias and heterogeneity in studies identifying AKI and subsequent outcomes, were used to assess the included studies (figure 1).
Figure 1.
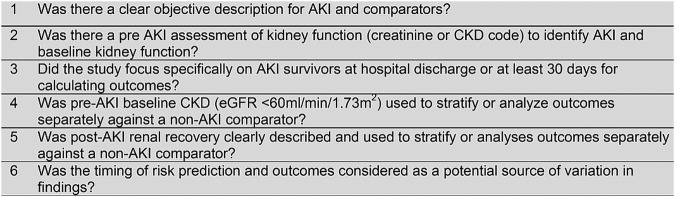
Quality assessment criteria (AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate).
Analysis
Study characteristics and quality assessment information were tabulated and described. Multiple papers from the same study data set were presented together with findings reported from the most complete paper.
Quality criteria 1–3 limited analysis to studies that accurately identified patients with AKI and restricted to those who survived the acute event, without significant potential for misclassification of patients with CKD. If criteria 1–3 were not met, the study was excluded from further analysis.
Further analysis was limited to studies that analysed patients separately according to one of quality criteria 4–6 (analysis by pre-AKI baseline, post-AKI recovery, or timing of outcomes).
The remaining studies, satisfying criteria 1–3 and one of 4–6, were then summarised in two tables for mortality and CKD outcomes. Clinical setting of AKI was described for each table as follows: intensive care (intensive therapy unit (ITU): patients admitted to ITU, regardless of RRT use), postoperative, cardiac (patients presenting following a myocardial infarction or coronary angiogram), unselected (studies of hospital admissions where the clinical settings above did not describe all patients). Retrospective or prospective design was also reported.
Mortality and CKD outcomes were presented separately, reporting study characteristics, population, exposure, comparators, follow-up and measure of outcome. Multivariate adjusted risk ratios for survival and CKD outcomes were displayed graphically if available. If the timing of outcome was considered in a study this was indicated and described.
Results
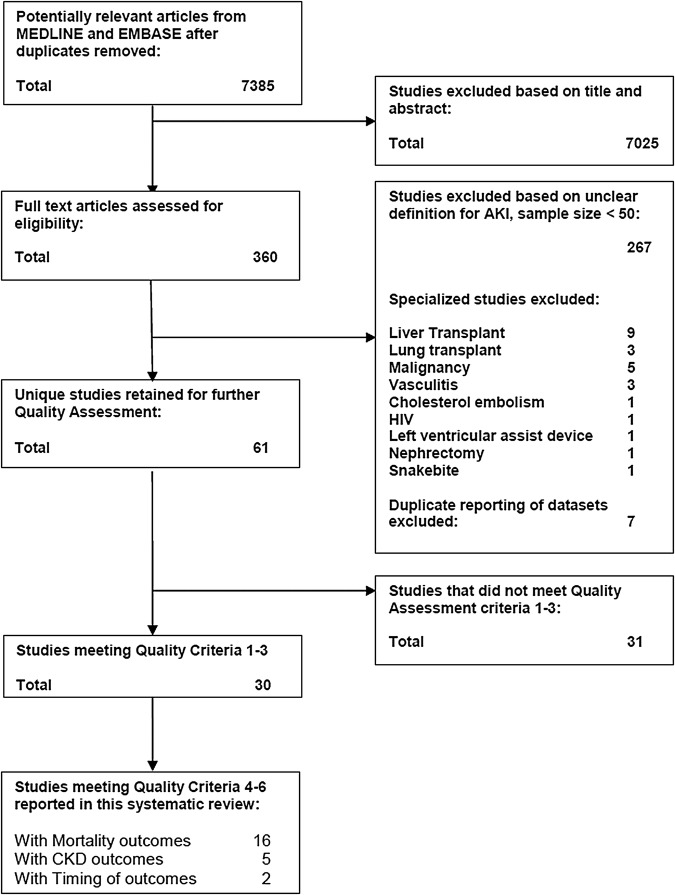
The literature search identified 7385 citations. Following review of titles, abstracts and full texts (figure 2), 68 papers describing 61 unique studies were identified (further details summarised in online supplementary material). Study size varied, ranging from 61 to 82 711 individuals with follow-up from 12 to 142 months. Thirty of 61 studies satisfied quality criteria 1–3, reported either mortality or CKD outcomes (and these studies are summarised in table 1).11–39 Notably, 28/61 studies had insufficient pre-AKI data to avoid misclassification of CKD as AKI. Of 30 remaining studies stratification or separation by exclusion of pre-AKI or post-AKI function subgroups was performed in 16/30 mortality studies with 14/30 not conducting separate analyses. It was also performed in 5/30 CKD outcome studies with 25/30 not conducting separate analyses. As noted in table 2, most studies satisfied quality criteria 1–3 by excluding patient groups rather than by stratifying by pre-AKI or post-AKI function. Only three mortality studies stratified by pre-AKI function and six by post-AKI function. Two CKD studies stratified by pre-AKI and two post-AKI function. Only two mortality studies8 19 and one CKD outcome study8 addressed whether the risk of outcomes changed with increasing time from AKI.
Figure 2.
Study selection and quality assessment (AKI, acute kidney injury; CKD, chronic kidney disease).
Table 1.
Description and application of quality criteria 4–6 in studies satisfying criteria 1–3
| Mortality outcomes reported |
CKD outcomes reported |
For either outcome | |||||
|---|---|---|---|---|---|---|---|
| Paper | N | Clinical setting | By pre-AKI baseline | By post-AKI recovery | By pre-AKI function | By post-AKI recovery | Timing considered |
| Hoste et al27 | 82 | ITU | N | N | – | – | – |
| Lopes et al28 | 61 | ITU | N | N | – | – | – |
| Manns et al 29 | 66 | ITU | N | N | – | – | – |
| Schiffl and Fischer11 | 226 | ITU | CKD excluded | Y | – | – | – |
| Triverio et al17 | 95 | ITU | Y | Y | – | – | – |
| Bucaloiu et al12 | 1610 | Unselected | CKD excluded | Recovered only | CKD excluded | Recovered only | – |
| Hsu et al13 | 782 | Unselected | CKD only | N | N | N | – |
| Ishani et al18 | 7197 | Unselected | Y by code alone | N | Y by code alone | N | – |
| Jones et al14 | 719 | Unselected | CKD excluded | Recovered only | CKD excluded | Recovered only | – |
| Lafrance and Miller19 | 82 711 | Unselected | Y | Y | – | – | Sensitivity analysis excluding first 6 months |
| Lo et al30 | 343 | Unselected | N (excluded only if eGFR <45) | N | – | – | – |
| Ng et al31 | 262 | Unselected | N | N | – | – | – |
| Ponte et al21 | 177 | Unselected | N (Cr >1.4 excluded) | Y | – | – | – |
| Wald et al32 | 41 327 | Unselected | N | N | N | N | – |
| Gupta et al33 | 143 | Cardiac | N | N | – | – | – |
| Kimura et al34 | 81 | Cardiac | N | N | – | – | – |
| Lindsay et al35 | 179 | Cardiac | Y | N | – | – | – |
| Maioli et al15 | 167 | Cardiac | CKD only | Y | – | – | – |
| Rihal et al36 | 185 | Cardiac | N | N | – | – | – |
| Roghi et al37 | 106 | Cardiac | N | N | – | – | – |
| Brown et al22 | 1886 | Postoperative | N | Y | – | – | – |
| Coca et al23 | 6257 | Postoperative | N | Y | – | – | – |
| Ishani et al8 | 4053* | Postoperative | N | N | N | N | Changing HRs for mortality and CKD outcomes in time intervals |
| Kheterpal et al16 | 101 | Postoperative | CKD excluded | N | – | – | – |
| Loef et al24 | 145 | Postoperative | N | Y | – | – | – |
| Luckraz et al38 | 53 | Postoperative | N | N | – | – | – |
| Mehta et al25 | 2083 | Postoperative | N (excluded only Cr>2) | Y | – | – | – |
| Swaminathan et al39 | 1113 | Postoperative | N | N† | – | – | – |
| Van Kuijk et al26 | 120 | Postoperative | N | N | CKD excluded | Y | – |
| Wu et al20 | 4393 | Postoperative | Y by GFR>/<45 | Y | Y by GFR>/<45 | Y | – |
*19 779 in total, 4053 of which had >50% cr rise.
†Outcomes by rate of fall of cr over a 24 h rather than a recovery variable.
–, Outcome not addressed by study; AKI, acute kidney injury; CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; ITU, intensive therapy unit;; N, outcome addressed but not per quality criterion.
Table 2.
Studies of mortality outcomes in AKI by pre-AKI and post-AKI kidney function
| Mortality | N | Clinical setting | Design | AKI exposure | Comparator | Follow-up | Pre-AKI baseline separation | Post-AKI recovery separation | Recovery definition | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Schiffl and Fischer11 | 226 | ITU | Cohort | Acute RRT | None | 5 years | CKD excluded | Y | Within 10% baseline | Mortality 83% without recovery, 33% with recovery HR without vs with recovery 4.1† (no non-AKI comparator) |
| Triverio et al17 | 95 | ITU | Cohort | Acute RRT | None | 3 years | Y | Y | eGFR>60 | Mortality 50% if baseline CKD, 29% if eGFR <60 at discharge, 18% if no renal impairment before or after AKI |
| Bucaloiu et al12 | 1610 | Unselected | Cohort | Cr rise >50% | No AKI | 3.3 years* | CKD excluded | Recovered only | Within 10% baseline | HR Mortality 1.48† |
| Hsu et al13 | 782 | Unselected | Cohort | Acute RRT | CKD with no RRT | 4 years | CKD only (eGFR<45) | N | – | HR composite end point of ESKD or mortality 1.3† |
| Ishani et al18 | 7197 | Unselected | Cohort | Code | No AKI or CKD code | 2 years | Y by code alone | N | – | HR mortality vs no AKI or CKD AKI and CKD 3.24†, AKI 2.48†, CKD 1.45† |
| Jones et al14 | 719 | Unselected | Cohort | Code | No AKI | 2.5 years* | CKD excluded | Recovered only | Within 10% baseline | HR Mortality 1.08 |
| Lafrance et al19 | 82 711 | Unselected | Cohort | Cr rise >50% | No AKI | 2.34 years* | Y | Recovered only in a subanalysis | Within 10% baseline | HR mortality for AKI vs no AKI in 90-day survivors 1.41† Subgroup of 6-month survivors 1.13† Subgroup with recovery 1.47† |
| Ponte et al21 | 177 | Unselected | Cohort | Cr rise from<1.4 mg/dL to >2 | None | 7.2 years* | CKD excluded (Cr >1.4 mg/dL) | Y | Cr<1.4 mg/dL | 10-year mortality 40% with recovery, 57% without recovery |
| Lindsay et al35 | 179 | Cardiac | Cohort | Cr rise 50% from<1.2 mg/dL | No AKI | 1 year | CKD excluded (cr >1.2 mg/dL) | N | – | 1-year mortality 9.5% AKI, 2.7% no-AKI |
| Maioli et al15 | 167 | Cardiac | Cohort | Cr rise (0.5 mg/dL by 3 days) | No AKI | 3.8 years* | CKD only (eGFR <60) | Y | Within 25% baseline at 3 months | HR mortality for AKI with recovery1.3†, without recovery 2.3† |
| Brown et al22 | 1886 | Postoperative | Cohort | Cr rise 0.3 mg/dL or 50% | No AKI | 2.6 years* | N | Y | Number of days AKI definition met | HR mortality for AKI vs no AKI by AKI duration 1–2 days 1.51†, 3–6 days 1.74†, >7 days 3.45†, persistent 5.75† |
| Coca et al23 | 6257 | Postoperative | Cohort | Cr rise 0.3 mg/dL or 50% | No AKI | 3.8 years* | N | Y | Number of days AKI definition met | HR mortality for AKI vs no AKI by AKI duration <2 days 1.15†, 3–6 days 1.5†, >7 days any duration with RRT 2.10† |
| Kheterpal et al16 | 101 | Postoperative | Cohort | Cr clearance fall to<50 | No AKI | 1 year | CKD excluded | N | – | 1-year mortality 12% AKI, 9% no AKI |
| Loef et al24 | 145 | Postoperative | Cohort | Cr rise 25% | No AKI | 100 months | N | Y | “Improved to or below the preoperative level” | HR mortality for AKI 1.63†, AKI with recovery 1.66†, AKI without recovery 1.72†, non-recovery vs recovery 1.22 (not significant) |
| Mehta et al25 | 2083 | Postoperative | Cohort | Cr rise 50% or 0.7 mg/dL | No AKI | 7 years* | N | Y | AKI definition no longer met at 7 days | HR mortality for AKI with recovery 1.21†, without recovery 1.47† |
| Wu et al20 | 4393 | Postoperative | Cohort | Cr rise >50% | No AKI and no CKD | 4.76 years* | Y by GFR>/<45 | Y | Within 50% baseline | HR mortality for AKI only 1.94†, CKD only 2.64†, AKI and CKD 3.28† Stratified by recovery AKI and CKD with recovery 3.0†, without recovery 4.59† AKI only with recovery 1.96†, without recovery 2.18† CKD without AKI 2.59† |
*Mean/median.
†Statistically significant p<0.05.
–, Outcome not addressed by study; AKI, acute kidney injury; CKD, chronic kidney disease; Cr, serum creatinine; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease (dialysis >90 days); ITU, intensive therapy unit; N, outcome addressed but not per quality criterion; RRT, renal replacement therapy.
Among these studies, definitions varied: AKI was defined by acute RRT,13 different thresholds for creatinine rise,15 23–26 or hospital episode code.14 18 CKD was defined by hospital codes,18 low estimated glomerular filtration rate (eGFR) for CKD cut-off (<45 mL/min/1.73 m2)13 20 conventional eGFR for CKD (<60 mL/min/1.73 m2),15 varying thresholds based on serum creatinine21 35 or hospital episode code.18 Definitions of renal recovery also varied: with definitions of its timing ranging from 3 days26 to 3 months;15 and its extent from within 50% of baseline20 to within 10% of baseline,14 or below a pre-AKI value.24
Mortality findings
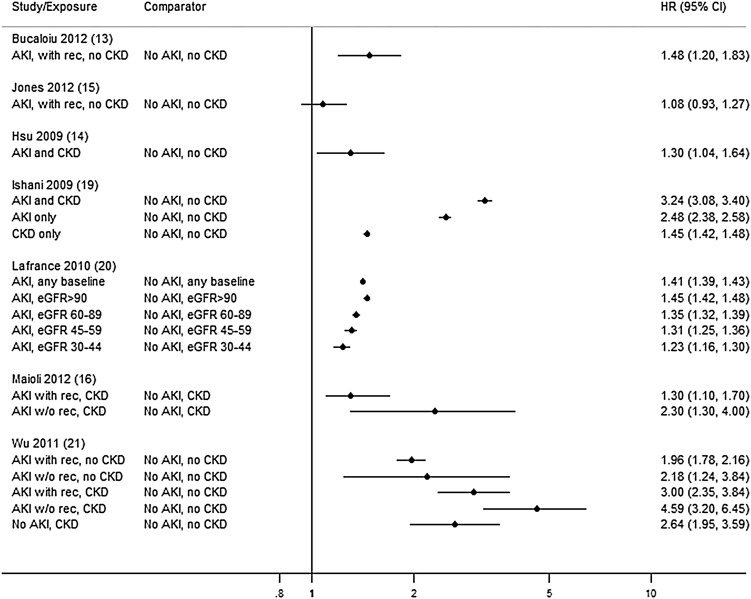
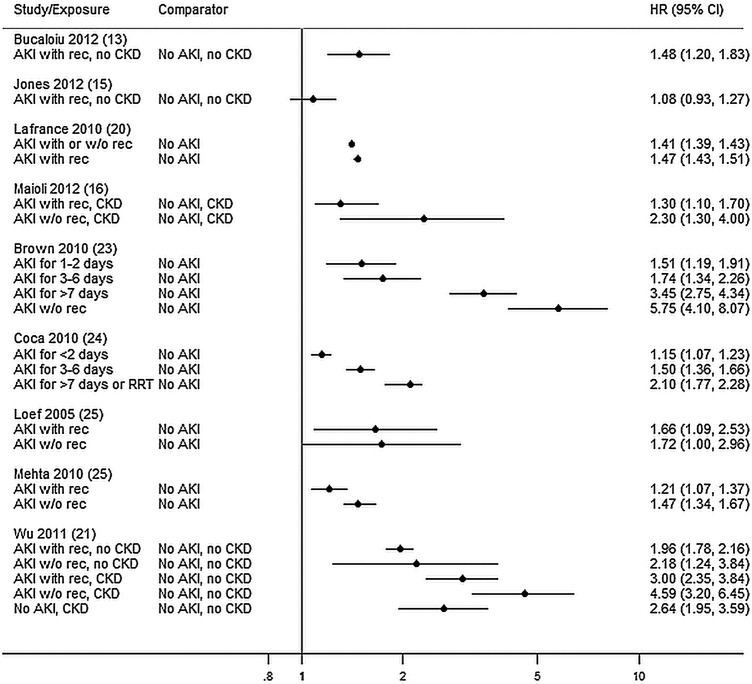
There were 16 mortality studies (table 2). Follow-up ranged from 1 to 7 years and mortality up to 83% for patients with AKI at 5 years. AKI was associated with increased mortality in all but one study14 regardless of pre-AKI baseline (HR 1.08 to 4.59; figure 3) or recovery of renal function (HR 1.08 to 5.75; figure 4). Five studies did not report non-AKI comparators11 17 21 or risk ratios.16 35
Figure 3.
Mortality—by pre-AKI baseline (AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; w/o rec, without recovery; with rec, with recovery).
Figure 4.
Mortality—by post AKI recovery (AKI, acute kidney injury; CKD, chronic kidney disease; w/o rec, without recovery; with rec, with recovery).
Of the three studies stratified by pre-AKI baseline function, in two the overall prognosis was worse in patients with AKI with prior CKD with doubling of HRs with respect to a non-AKI non-CKD comparator.15 20 However, in a third study, where comparators were also stratified by eGFR, the independent mortality risk from AKI diminished with advancing CKD.19 Six studies compared mortality stratifying either by post-AKI recovery (four studies)15 20 24 25 or by AKI duration (two studies).22 23 All but one15 were postoperative studies with different thresholds for defining recovery. Risk from incomplete recovery varied from minimal with overlapping CIs15 20 24 25 to greater than double when AKI lasted more than 1 week.22 23 Only one study combined stratification by pre-AKI baseline and by post-AKI recovery (defining recovery to 50% baseline, CKD as eGFR<45 mL/min/1.73 m2) with non-recovery as well as baseline CKD adding to mortality risk.20
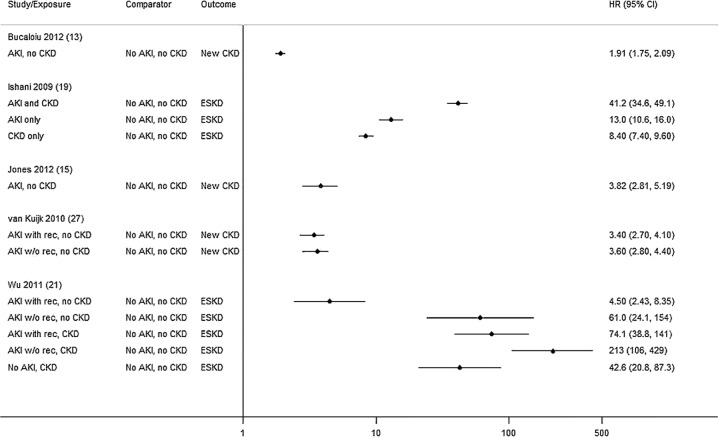
CKD outcomes
In five studies, there was marked variation in CKD outcomes following AKI (HR 1.91 to 213) depending on pre-AKI baseline and post-AKI recovery (table 3 and figure 5).12 14 18 20 26 In the two studies stratifying by pre-AKI function, AKI with baseline CKD was associated with a substantially greater (fourfold to fivefold) risk of ESKD than AKI in cases without prior CKD18 20 although notably one study defined CKD at eGFR<45 mL/min/1.73m2 20 and the other by a hospital code.18 The two studies stratifying by post-AKI function had different findings. In one postoperative study26 there was no association between non-recovery post-AKI (vs patients with recovery) and additional CKD progression, when recovery was measured at day three,26 but association was substantial (5–10-fold) in the other study where recovery was defined at hospital discharge.20 Again only one study stratified by both baseline and recovery, finding both associated with poorer prognosis.20
Table 3.
Studies of CKD outcomes in AKI by pre-AKI and post-AKI kidney function
| Progression | N | Clinical setting | Design | AKI exposure | Comparator | Follow-up | Pre-AKI baseline separation | Post-AKI recovery separation | Recovery definition (discharge unless otherwise stated) | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Bucaloiu et al12 | 1610 | Unselected | Cohort | Cr rise >50% | No AKI | 3.3 years* | CKD excluded | Recovered only | Within 10% baseline | Development of new CKD stage 3/1000 person-year 28.1 AKI vs 13.1 no AKI (HR 1.91†) |
| Ishani et al18 | 7197 | Unselected | Cohort | Code | No AKI or CKD code | 2 years | Y by code alone | N | – | ESKD/1000 person-year (HR vs no code) AKI and CKD 79.45 (HR 41.2†) AKI 24.52 (HR 13†) CKD only 19.88 (HR 8.43†) No Code 2.08 (reference) |
| Jones et al14 | 719 | Unselected | Cohort | Code | No AKI | 2.5 years* | CKD excluded | Recovered only | Within 10% Baseline | HR for new CKD stage 3 (15% of AKI vs 3% of no AKI patients) 3.82† |
| van Kuijk et al26 | 493 | Postoperative | Cohort | 10% Cr change by day 2 post-op | No AKI | 5 years* | CKD excluded | Y | Within 10% baseline at day 3 | Development of new CKD 11% if no AKI, 32% if AKI recovered (HR 3.4†), 36% if AKI without recovery (HR3.6†) |
| Wu et al20 | 4393 | Postoperative | Cohort | Cr rise (RIFLE, or 50% rise if previous CKD) | No AKI and no CKD | 4.76 years* | Y by GFR>/<45 | Y | Within 50% baseline | Development of ESKD (vs no code) AKI only 4.64†, CKD only 40.86†, AKI and CKD 91.69† Stratified by recovery AKI+CKD+without recovery 212.73† AKI+CKD+recovery 74.07† AKI only+without recovery 60.95† AKI only+recovery 4.5† |
*Mean/median.
†Statistically significant p<0.05.
–, Outcome not addressed by study; AKI, acute kidney injury; CKD, chronic kidney disease; Cr, serum creatinine; eGFR, estimated glomerular filtration rate; ESKD, end-stage renal disease (dialysis >90 days); ITU, intensive therapy unit; N, outcome addressed but not per quality criterion; RRT, renal replacement therapy.
Figure 5.
CKD outcomes (w/o rec, without recovery; with rec, with recovery; ESKD, end-stage kidney disease).
Timing of outcomes
Two studies considered whether HRs changed over time following the AKI episode.8 19 Lafrance et al,19 performed a sensitivity analysis of their study of AKI survivors and mortality, limiting analysis from 90-day to 6-month AKI survivors. The mortality HR (AKI vs non-AKI) fell from 1.41 (1.39 to 1.43) in 90-day survivors to 1.13 (1.11 to 1.14) in 6-month survivors.19 Ishani et al8 reported changes in HR of mortality and CKD outcomes from AKI (vs no AKI) when dividing follow-up into 6-month time discrete intervals. For both mortality and CKD outcomes, greatest relative risk (vs non-AKI) was in the first 6 months, with marked attenuation of risk (but still statistically significant) from 1 year.8 While recognising that risk may not be constant, the study also did not consider the importance of stratifying patients by pre-AKI baseline or post-AKI recovery.
Discussion
The introduction of a global definition for AKI has led to growing interest in the high incidence and poor outcomes. In this review of 7385 citations, there were only 30 studies that met the initial quality criteria for selecting patients with AKI with sufficient efforts to avoid misclassifying CKD. Of these studies only three mortality studies stratified by pre-AKI, six by post-AKI function, two CKD outcome studies by pre-AKI and two by post AKI function. Only one study considered both pre-AKI and post-AKI function. Only two studies considered the change in risk over time from AKI.
As with two previous reviews, we note that AKI is associated with overall poorer mortality and CKD outcomes,3 5 but with substantial variation in the magnitude of risk. We were transparent about the variation in definitions and clinical setting and identified heterogeneity as a problem with the design of AKI studies to date. The two previous reviews included studies with incomplete or estimated data on baseline kidney function,3 5 but in studies with available data we found that outcomes were modulated by pre-AKI baseline and post-AKI renal recovery. Unfortunately, these studies varied in definitions for AKI, recovery and follow-up preventing pooled meta-analysis. While proteinuria and oliguria may also be important factors, research on their association with long-term prognosis is sparse and we therefore focused on the course of serum creatinine prior and subsequent to the AKI episode.
Pre-AKI baseline CKD was associated with doubling of mortality outcomes and fourfold to fivefold increased risk of CKD outcomes compared to patients with non-AKI non-CKD. Unfortunately, only one study chose CKD comparators for patients with AKI at each level of pre-AKI function, finding that the additional risk from AKI diminished in those already at high risk due to advanced baseline CKD.19 It is likely the mortality and ESKD risk in advanced CKD is already high and less influenced by AKI.
Non-recovery of kidney function was associated with greater mortality and CKD outcomes in some studies, but the finding was inconsistent and dependent on study design. Studies with a less stringent threshold20 and later point of assessment15 showed a poorer prognosis with non-recovery, highlighting the significance of arbitrary thresholds in study design. We also note a further study outside the time period in this review reporting a cohort with poorer outcomes with non-recovery after AKI, in line with our overall findings.40
Increased risk of poor outcomes following AKI may be greatest early post-AKI, and therefore the timing of clinical assessment is important. Despite this, most studies followed a ‘proportional hazards’ assumption (ie, that the relative risk of AKI vs non-AKI comparator was constant over time), but this may not hold. Only two studies considered the timing of assessment post-AKI and found that additional risk of mortality as well as renal progression may diminish over time,8 19 but neither combined this with adjustment nor stratification by post-AKI recovery. Both factors are required to assess if the rate of CKD progression is more rapid in AKI than non-AKI (but baseline CKD) comparators with equal kidney function at the time of hospital discharge. Thus, understanding of how mortality and renal progression risks change over time remains inadequately addressed.
Overall while our findings broadly agree with previous reviews,3 5 we found that some of the variation in AKI prognosis may be explained by pre-AKI CKD and post-AKI non-recovery which both lead to a poorer prognosis. This is of great relevance given that these factors were infrequently addressed by stratification, and 28/61 studies had insufficient pre-AKI data to minimise misclassification between CKD and AKI. The relationships are complex with diminishing additional risk with advancing pre-AKI CKD. We also noted a non-linear clinical post-AKI course, with risk prediction dependent on the time point at which risk is assessed, necessitating fixed follow-up points to prevent overestimation of risk in a cohort with shorter follow-up. Importantly, few studies assessed these factors, using different definitions, and with only one study assessing both pre-AKI and post-AKI factors. This review included studies up to October 2012 and in the near future it is likely that AKI registries with additional routine outcome data will become available. Therefore there is now an opportunity to ensure that a concordance exists in definitions and reporting of future AKI studies before this review is revisited.
The NICE guidelines call for further studies of long-term AKI prognosis but without specific detail on which factors should be included. We suggest that future studies should adopt consistent definitions, incorporate baseline and recovery function and consider the timing of outcomes during follow-up. We therefore suggest that future long-term prognostic studies should adopt the KDIGO definition for identifying and stratifying AKI,41 the KDIGO CKD stages for stratifying AKI and non-AKI comparator groups by pre-AKI eGFR,42 the UK Renal Association definition of renal recovery for stratifying recovery (within 20% of baseline)2 and fixed interim points for establishing recovery (7, 30 and 90 days) and excluding early outcomes (up to 30, 90 and 180 days).
Conclusion
AKI has a poor, but variable prognosis influenced by clinical setting, underlying cause and comorbidity. There is a recognised need to understand which patients are at greater long-term risk and when AKI may carry additional risk beyond the underlying illness. In this review, pre-AKI CKD was associated with doubling of mortality outcomes and a fourfold to fivefold increase in renal outcomes. Non-recovery of kidney function may also affect prognosis, but the magnitude depended on both recovery definition and the timing of assessment. These factors are already considered by physicians in clinical practice, but unfortunately we found that few prognostic studies have addressed them. The heterogeneity of studies and accompanying lack of a clear consistent pattern of effect limit their clinical interpretation. As pre-AKI and post-AKI renal function may influence clinical practice, we suggest that consistent reporting of these factors is needed in future prognostic studies to establish how they modify AKI prognosis. This will inform the clinician as well as future research.
Supplementary Material
Footnotes
Contributors: SS, AM, NF and CB contributed to the design of the review, SS and MM acquired the data. SS, AM and CB contributed to analysis. SS wrote the draft and SS, AM, NF and CB critically revised the intellectual content of the work. All authors have given final approval for this paper to be published.
Funding: SS is supported by a research training fellowship from the Wellcome Trust (Reference Number 102729/Z/13/Z).
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Ftouh S, Lewington A; on behalf of the Acute Kidney Injury Guideline Development Group convened by the National Clinical Guidelines Centre and commissioned by the National Institute for Health and Care Excellence, in association with The Royal College of Physicians’ Clinical Effectiveness and Evaluation Unit. Prevention, detection and management of acute kidney injury: concise guideline . Clin Med 2014;14:61–5. 10.7861/clinmedicine.14-1-61 [DOI] [Google Scholar]
- 2.Lewington A, Kanagasundaram S. Clinical practice guidelines. Acute kidney injury. 5th edn UK Renal Association, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Yusuf B, Shlipak MG et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;53:961–73. 10.1053/j.ajkd.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bydash JR, Ishani A. Acute kidney injury and chronic kidney disease: a work in progress. Clin J Am Soc Nephrol 2011;6:2555–7. 10.2215/CJN.09560911 [DOI] [PubMed] [Google Scholar]
- 5.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 2012;81:442–8. 10.1038/ki.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012;82:516–24. 10.1038/ki.2012.208 [DOI] [PubMed] [Google Scholar]
- 7.James MT, Hemmelgarn BR, Wiebe N et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet 2010;376:2096–103. 10.1016/S0140-6736(10)61271-8 [DOI] [PubMed] [Google Scholar]
- 8.Ishani A, Nelson D, Clothier B et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 2011;171:226–33. 10.1001/archinternmed.2010.514 [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 10.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. 10.7326/0003-4819-144-6-200603210-00010 [DOI] [PubMed] [Google Scholar]
- 11.Schiffl H, Fischer R. Five-year outcomes of severe acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant 2008;23:2235–41. 10.1093/ndt/gfn182 [DOI] [PubMed] [Google Scholar]
- 12.Bucaloiu ID, Kirchner HL, Norfolk ER et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012;81:477–85. 10.1038/ki.2011.405 [DOI] [PubMed] [Google Scholar]
- 13.Hsu CY, Chertow GM, McCulloch CE et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol 2009;4:891–8. 10.2215/CJN.05571008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones J, Holmen J, De Graauw J et al. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 2012;60:402–8. 10.1053/j.ajkd.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maioli M, Toso A, Leoncini M et al. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation 2012;125:3099–107. 10.1161/CIRCULATIONAHA.111.085290 [DOI] [PubMed] [Google Scholar]
- 16.Kheterpal S, Tremper KK, Englesbe MJ et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007;107:892–902. 10.1097/01.anes.0000290588.29668.38 [DOI] [PubMed] [Google Scholar]
- 17.Triverio PA, Martin PY, Romand J et al. Long-term prognosis after acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant 2009;24:2186–9. 10.1093/ndt/gfp072 [DOI] [PubMed] [Google Scholar]
- 18.Ishani A, Xue JL, Himmelfarb J et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 2009;20:223–8. 10.1681/ASN.2007080837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafrance JP, Miller DR. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 2010;21:345–52. 10.1681/ASN.2009060636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu VC, Huang TM, Lai CF et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 2011;80:1222–30. 10.1038/ki.2011.259 [DOI] [PubMed] [Google Scholar]
- 21.Ponte B, Felipe C, Muriel A et al. Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transplant 2008;23:3859–66. 10.1093/ndt/gfn398 [DOI] [PubMed] [Google Scholar]
- 22.Brown JR, Kramer RS, Coca SG et al. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 2010;90:1142–8. 10.1016/j.athoracsur.2010.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coca SG, King JT Jr, Rosenthal RA et al. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int 2010;78:926–33. 10.1038/ki.2010.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loef BG, Epema AH, Smilde TD et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 2005;16:195–200. 10.1681/ASN.2003100875 [DOI] [PubMed] [Google Scholar]
- 25.Mehta RH, Honeycutt E, Patel UD et al. Impact of recovery of renal function on long-term mortality after coronary artery bypass grafting. Am J Cardiol 2010;106:1728–34. 10.1016/j.amjcard.2010.07.045 [DOI] [PubMed] [Google Scholar]
- 26.van Kuijk JP, Flu WJ, Chonchol M et al. Temporary perioperative decline of renal function is an independent predictor for chronic kidney disease. Clin J Am Soc Nephrol 2010;5:1198–204. 10.2215/CJN.00020110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoste EA, Doom S, De Waele J et al. Epidemiology of contrast-associated acute kidney injury in ICU patients: a retrospective cohort analysis. Intensive Care Med 2011;37:1921–31. 10.1007/s00134-011-2389-8 [DOI] [PubMed] [Google Scholar]
- 28.Lopes JA, Fernandes P, Jorge S et al. Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrol 2010;11:9 10.1186/1471-2369-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manns B, Doig CJ, Lee H et al. Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med 2003;31:449–55. 10.1097/01.CCM.0000045182.90302.B3 [DOI] [PubMed] [Google Scholar]
- 30.Lo LJ, Go AS, Chertow GM et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 2009;76:893–9. 10.1038/ki.2009.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng KP, Chanouzas D, Fallouh B et al. Short and long-term outcome of patients with severe acute kidney injury requiring renal replacement therapy. QJM 2012;105:33–9. 10.1093/qjmed/hcr133 [DOI] [PubMed] [Google Scholar]
- 32.Wald R, Quinn RR, Adhikari NK et al. Risk of chronic dialysis and death following acute kidney injury. Am J Med 2012;125:585–93. 10.1016/j.amjmed.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 33.Gupta R, Gurm HS, Bhatt DL et al. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv 2005;64:442–8. 10.1002/ccd.20316 [DOI] [PubMed] [Google Scholar]
- 34.Kimura T, Obi Y, Yasuda K et al. Effects of chronic kidney disease and post-angiographic acute kidney injury on long-term prognosis after coronary artery angiography. Nephrol Dial Transplant 2011;26:1838–46. 10.1093/ndt/gfq631 [DOI] [PubMed] [Google Scholar]
- 35.Lindsay J, Apple S, Pinnow EE et al. Percutaneous coronary intervention-associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv 2003;59:338–43. 10.1002/ccd.10534 [DOI] [PubMed] [Google Scholar]
- 36.Rihal CS, Textor SC, Grill DE et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105:2259–64. 10.1161/01.CIR.0000016043.87291.33 [DOI] [PubMed] [Google Scholar]
- 37.Roghi A, Savonitto S, Cavallini C et al. Impact of acute renal failure following percutaneous coronary intervention on long-term mortality. J Cardiovasc Med 2008;9:375–81. 10.2459/JCM.0b013e3282eee979 [DOI] [PubMed] [Google Scholar]
- 38.Luckraz H, Gravenor MB, George R et al. Long and short-term outcomes in patients requiring continuous renal replacement therapy post cardiopulmonary bypass. Eur J Cardiothorac Surg 2005;27:906–9. 10.1016/j.ejcts.2005.01.057 [DOI] [PubMed] [Google Scholar]
- 39.Swaminathan M, Hudson CC, Phillips-Bute BG et al. Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg 2010;89:1098–104. 10.1016/j.athoracsur.2009.12.018 [DOI] [PubMed] [Google Scholar]
- 40.Pannu N, James M, Hemmelgarn B et al. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 2013;8:194–202. 10.2215/CJN.06480612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012;2(Suppl 1):1–138. [Google Scholar]
- 42.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.