Abstract
Japanese encephalitis (JE) is a potentially serious form of viral encephalitis with varied clinicoradiological manifestations. We report the case of a 19-year-old girl admitted with headache, vomiting and altered sensorium in the absence of fever, whose cerebrospinal fluid analysis showed lymphocytic pleocytosis with significant protein content and positive serum IgM JE antibodies. MRI with venography revealed bilateral thalamic haemorrhage and cerebral venous sinus thrombosis. Although thalamic hypodensities are a well-described feature, thalamic haemorrhage and cerebral venous thrombosis are distinctly rare in JE. This report highlights the role of imaging in cases of encephalitis in general and JE in particular, in the early detection of uncommon manifestations that may complicate these diseases.
Background
Japanese encephalitis (JE) is a mosquito-borne viral encephalitis caused by members of the Flavivirus family. While humans are only incidental hosts, transmission of the virus between natural host such as pigs, birds and other domestic animals is effected by Culex mosquitoes.1 2 JE is reported to be the most common form of epidemic and endemic viral encephalitis in Asia, and predominantly affects children and young adults, with a seasonal peak between July and September.3–5 JE virus infection is subclinical in a majority of cases, with the ratio between symptomatic and asymptomatic infection ranging from 1:25 to 1:1000.6–8 Symptomatic JE virus infections manifest with acute onset of fever, headache, impaired consciousness and seizures.5 The immunoglobulin M (IgM) antibody captures ELISA (MAC ELISA) in serum and cerebrospinal fluid (CSF), and has good sensitivity and specificity for the diagnosis of JE.1 Cerebral venous sinus thrombosis (CVST) is an uncommon condition responsible for about 0.5% of strokes and may occur in a variety of settings including infections (central nervous system infections, vasculitis, ear and face infections) and non-infectious disorders (pregnancy and puerperium, haematological diseases, hypercoagulable states, drug use, head trauma, connective tissue disorders and dehydration).9 Although CVST complicating herpes virus encephalitis has been reported frequently,10 only one case of JE with CVST has been reported to date in the literature.11 We report the second case of JE with this unusual complication.
Case presentation
A 19-year-old unmarried woman with no comorbidities presented with headache, vomiting and gradual depression of sensorium, without fever, for 2 days. She also had a history of involuntary micturition for 1 day. There was no history of seizures, diplopia, blurring of vision or weakness of limbs. She denied any drug intake or substance abuse. There was no history of recent travel or vaccination. Her menstrual cycles were regular and last menstrual periods were 10 days prior to admission.
On examination, the patient was drowsy and disoriented. She was pale with a blood pressure of 110/70 mm Hg and pulse rate of 80/min. The rest of the general physical examination was unrevealing. Central nervous system examination demonstrated that she was not oriented to time, place or person. Her memory and other higher mental functions could not be assessed. Pupils were 3 mm bilaterally and reacting to light. There was no facial asymmetry or papilloedema. She was moving all four limbs to pain. Meningeal signs were conspicuously absent. Other systemic examination was unremarkable.
The patient's investigations showed haemoglobin of 7.5 g/dL and leucocytosis (total white cell count −17 700/mm3) with 92% neutrophils. Her renal, liver and thyroid function tests as well as serum levels of calcium, magnesium, sodium and potassium were normal. Her urine examination, chest radiograph and ECG were normal. Analysis of CSF showed lymphocytic pleocytosis with elevation of protein (308 mg/dL; reference: 20–40 mg/dL) and a normal glucose level (table 1). Gram stain, acid-fast stain and India ink staining were non-contributory. Adenosine deaminase and tubercle bacilli-PCR (TB-PCR) in the CSF were negative, ruling out tuberculous meningitis. PCR for herpes simplex virus DNA in the CSF was also negative.
Table 1.
Results of CSF analysis at admission
| Parameter | Patient's value | Normal range/value |
|---|---|---|
| Protein (mg/dL) | 308 | 20–40 |
| Glucose (mg/dL) | 67 | 40–60 |
| CSF glucose/blood glucose ratio | 0.65 | >0.6 |
| Total white cell count (cells/mm3) | 12 | 0–4 |
| Lymphocytes (%) | 90 | 100 |
| Polymorphs (%) | 10 | Usually absent |
| Red cells (cells/mm3) | 35 | Absent |
| CSF ADA (U/L) | 0.9 | <10 |
| India ink | Negative | – |
| Gram stain | Negative | – |
ADA, adenosine deaminase; CSF, cerebrospinal fluid.
Plain and contrast-enhanced CT of the brain showed meningeal enhancement with symmetrical hypodensities involving bilateral gangliocapsular regions and thalami (figure 1). In view of imaging abnormalities confined to bilateral thalami and the fact that the patient hailed from an area endemic for JE, we performed ELISA for IgM JE antibodies in serum and CSF, both of which were weakly positive. She was empirically managed with intravenous acyclovir, nasogastric feeds and supportive measures. On the second day in hospital, she developed extrapyramidal symptoms and generalised tonic–clonic seizures for which she was treated with intravenous promethazine and levitiracetam. Interictal electro-encephalogram (EEG) was abnormal with evidence of bilateral cerebral dysfunction without active epileptiform activity, consistent with diffuse encephalopathy.
Figure 1.
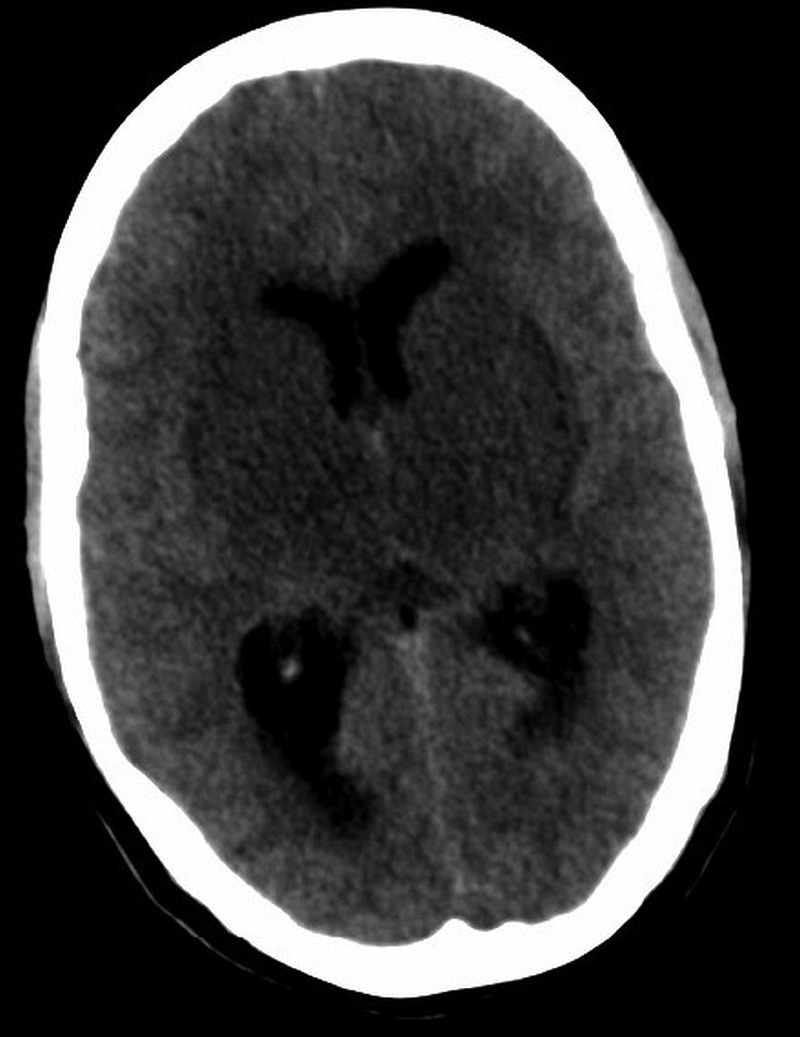
CT of the brain showing symmetric hypodensities involving bilateral thalami and gangliocapsular regions.
The clinical course was remarkable for the absence of fever throughout the illness. Antinuclear antibodies, antineutrophil cytoplasmic antibodies, antiphospholipid antibodies and serology for HIV (HIV I and II) were negative. Serum IgM ELISA for JE repeated on day 9 of the illness was strongly positive with significant rise in titres. By the fourth day of hospitalisation, the patient's sensorium improved and seizures were controlled. On day 5, she developed minimal weakness of right upper limb (power of grade 4/5) following which MRI with MR venography (MRV) of the brain showed bleeding in the bilateral thalami and heads of both caudate nuclei, and thrombosis of right transverse, right sigmoid, left transverse and straight sinuses (figures 2 and 3). MR arteriogram was normal. She was started on low-molecular-weight heparin, continued on oral anticoagulation with warfarin and discharged with an international normalisation ratio of 2.4. At the time of discharge her neurological status was normal with intact higher mental functions and normal power in all the limbs.
Figure 2.
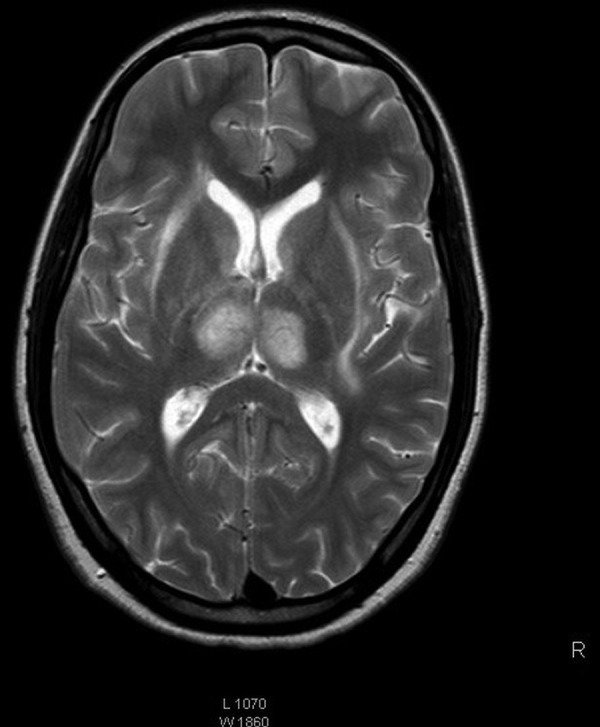
MRI of the brain showing haemorrhage in bilateral thalami and heads of both caudate nuclei.
Figure 3.
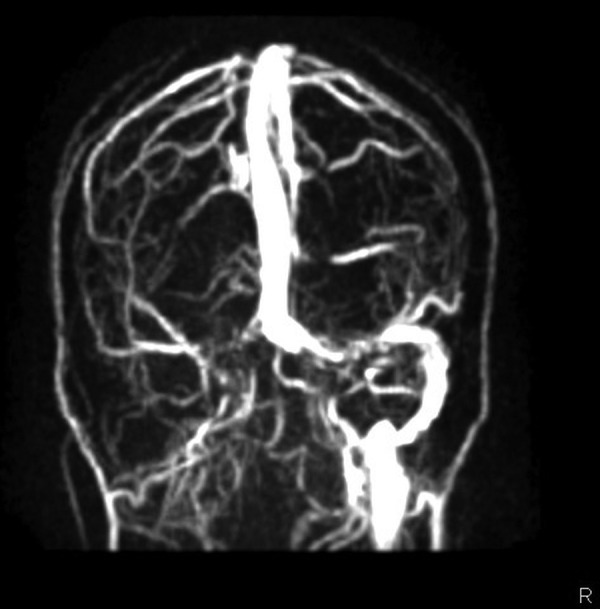
MR venogram showing absence of filling in the right transverse and right sigmoid sinuses.
Discussion
JE typically begins as a non-specific febrile illness with coryza, malaise or rigours. This is often followed by headache, vomiting and altered sensorium, usually heralded by a seizure. In older children and adults, altered mental status may be the initial presentation.2 Our patient presented with all these of typical features except fever. However, since she came from a northern district of Tamil Nadu, which is endemic for JE,12 and presented during the summer season, we considered JE among the differential diagnosis. This fact along with the neuroimaging findings prompted us to investigate further along the lines of JE by IgM JE antibodies in serum and CSF. Thus, a high index of suspicion and awareness of epidemiology regarding JE helped us clinch the primary diagnosis.
During the course of illness, our patient developed extrapyramidal symptoms. These symptoms are known to occur in JE and, as in our case, may include pill-rolling movements, head nodding, facial grimacing and choreoathetosis. Opisthotonus and rigidity, in particular, portend a poor prognosis.13 14 Seizures also occur quite commonly in JE and have been reported to be much more frequent in children than adults, generalised tonic–clonic convulsion being the most common.13 14 Hence the generalised seizures in our patient were not surprising.
Although most viral infections produce leucopenia, JE is known to result in anaemia and leucocytosis, which were present in our patient. A study from Thailand by Watt and Jongsakul15 found thrombocytopenia and altered liver enzymes in cases of JE. However, in our case, neither of these was evident. The prototype CSF findings in JE include mild to moderate pleocytosis (10–100 cells/mm3) with lymphocytic predominance, mild elevation of protein and normal glucose, although rarely a patient may not have any pleocytosis at all.13 Raised CSF pressure has been reported as a poor prognostic factor in JE, and fortunately our patient had normal pressure, which may have contributed to her remarkable recovery.
Neuroimaging has an important role in diagnosis of viral encephalitis, especially in differentiating between common aetiologies such as herpes simplex encephalitis and JE. Non-enhancing hypodensities in one or both thalami and basal ganglia, midbrain, pons and medulla are classic findings of JE, and can be seen in up to 50% of patients.16 17 Our patient had hypodensities involving bilateral thalami and caudate nuclei, which led us to suspect JE. Since she later developed mild weakness of her right upper limb, we performed MRI with MRV and arteriography, which revealed thalamic haemorrhage and CVST. CVST is very rare in JE, with only one case being reported to date. Bleeding in thalami and caudate nuclei is also rare in JE, occurring early in the course of illness, possibly from excessive vascularity secondary to inflammation.18 Our patient had thalamic hypodensities on CT early in the course of illness and developed bleeding on the seventh day. This pattern of neuroimaging has not been described previously in JE. One possible explanation may be the haemorrhagic transformation of venous infarcts secondary to cerebral venous thrombosis involving thalami. Improvement of limb weakness and neurological status following institution of anticoagulation further supports this.
Learning points.
Japanese encephalitis (JE) may have varied clinical manifestations, including behavioural abnormalities and extrapyramidal symptoms in the absence of fever. JE should be considered in the differential diagnosis of patients with such atypical features, especially if they hail from endemic areas.
Clinical suspicion must be strengthened if neuroimaging shows abnormalities involving the thalami or basal ganglia (including haemorrhage).
Cerebral venous sinus thrombosis must be considered in patients with JE who develop new neurodeficits during the course of illness. MRI with MR venography should be performed in such cases so that timely anticoagulant therapy may be started.
Acknowledgments
The author would like to acknowledge Department of Radiodiagnosis and Neurology.
Footnotes
Contributors: AB and SM were involved in conceptualisation, design and writing the manuscript. NI edited the manuscript and SC provided data on clinical details and photographs.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Innis BL. Japanese encephalitis. In: Porterfield JS. ed. Exotic viral infections. London, UK: Chapman & Hall, 1995:147–74. [Google Scholar]
- 2.Solomon T. Viral encephalitis in Southeast Asia. Neurol Infect Epidemiol 1997;2:191–9. [Google Scholar]
- 3.Burke DS, Leake CJ. Japanese encephalitis. In: Monath TP. ed. Arboviruses: epidemiology and ecology. Vol 3 Boca Raton, FL: CRC Press, 1988:63–92. [Google Scholar]
- 4.Knipe DM, Howley PM. Flaviviridae: the virus and their replication. In: Fields BN. ed Fields virology. 4th edn Philadelphia, PA: Lippincott-Raven, 2001;991–1029. [Google Scholar]
- 5.Wang H, Li Y, Liang X et al. Japanese encephalitis in Mainland China. Jpn J Infect Dis 2009;62:331–6. [PubMed] [Google Scholar]
- 6.Shope RE. Other flavivirus infections. Guerrant RL, Walker DH, Weller PF eds. Tropical infectious diseases: principles, pathogens, and practice. Philadelphia, PA: Churchill Livingstone, 1999:1275–9. [Google Scholar]
- 7.Solomon T, Dung NM, Kneen R et al. Japanese encephalitis. J Neurol Neurosurg Psychiatry 2000;68:405–15. 10.1136/jnnp.68.4.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benenson MW, Top FH Jr, Gresso W et al. The virulence to man of Japanese encephalitis virus in Thailand. Am J Trop Med Hyg 1975;24:974–80. [DOI] [PubMed] [Google Scholar]
- 9.Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol 2007;6:162–70. 10.1016/S1474-4422(07)70029-7 [DOI] [PubMed] [Google Scholar]
- 10.Kashyap AS, Anand KP, Kashyap S. Thrombosis of the cerebral veins and sinuses. N Engl J Med 2005;353:314–15. 10.1056/NEJM200507213530319 [DOI] [PubMed] [Google Scholar]
- 11.Jia M, Xiong N, Huang J et al. Japanese encephalitis accompanied by cerebral venous sinus thrombosis: a case report. BMC Neurol 2012;12:43 10.1186/1471-2377-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victor TJ, Malathi M, Ravi V et al. First outbreak of Japanese encephalitis in two villages of Dharmapuri district in Tamil Nadu. Indian J Med Res 2000;112:193–7. [PubMed] [Google Scholar]
- 13.Kumar R, Mathur A, Kumar A et al. Clinical features and prognostic indicators of Japanese encephalitis in children in Lucknow. Indian J Med Res 1990;91:321–7. [PubMed] [Google Scholar]
- 14.Poneprasert B. Japanese encephalitis in children in northern Thailand. Southeast Asian J Trop Med Public Health 1989;20:599–603. [PubMed] [Google Scholar]
- 15.Watt G, Jongsakul K. Acute undifferentiated fever caused by infection with Japanese encephalitis virus. Am J Trop Med Hyg 2003;68:704–6. [PubMed] [Google Scholar]
- 16.Shoji H, Murakamo T, Murai I et al. A follow-up study by CT and MRI in 3 cases of Japanese encephalitis. Neuroradiology 1990;32:215–9. 10.1007/BF00589115 [DOI] [PubMed] [Google Scholar]
- 17.Misra UK, Kalita J, Jain SK et al. Radiological and neurophysiological changes in Japanese encephalitis. J Neurol Neurosurg Psychiatry 1994;57:1484–7. 10.1136/jnnp.57.12.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalita J, Misra UK. Neurophysiological changes in Japanese encephalitis. Neurol India 2002;50:262–6. [PubMed] [Google Scholar]


