Abstract
A paraneoplastic leukemoid reaction is a rare condition of extreme leucocytosis in patients with solid malignancies. The differential diagnosis is often a true challenge. We present a case of a 56-year old woman with a history of stage IIIA malignant melanoma resected in 2004 that was diagnosed in May 2013 with BRAF V600E-mutated metastatic disease (left arm mass, lungs and adrenal glands). The laboratory findings revealed leucocytosis with granulocytosis that increased progressively to values up to 120.0×109/L. After a diagnostic work-up, a diagnosis of a paraneoplastic leukemoid reaction was established. We report the response of leucocytosis to radiation and BRAF inhibitor therapy, albeit short-lived. To the best of our knowledge, this is the first case report of a paraneoplastic leukemoid reaction in metastatic melanoma with characterisation of BRAF V600 mutation status. It remains unclear whether the aggressive tumour phenotype is related to the leukemoid reaction and whether this is related to the BRAF mutation.
Background
A paraneoplastic leukemoid reaction is a rare condition of extreme leucocytosis occurring in patients with solid malignancies. The major causes of a leukemoid reaction (usually defined as a persistent leucocytosis greater than 50×109/L) include malignancies, severe infections, intoxications, corticosteroid administration, severe haemorrhage and acute haemolysis.1 2 The differential diagnosis of a leukemoid reaction in patients with cancer is often a true challenge. Diagnostic work-up in these patients must rule out other underlying causes such as infection and acute and chronic leukaemia. A paraneoplastic leukemoid reaction has been reported in several solid tumour types and appears to be associated with a poor prognosis.3–9 Malignant melanoma has been associated with a variety of paraneoplastic manifestations including hormonal and dermatological syndromes.10 Some previous reports have described the extraordinarily rare occurrence of a paraneoplastic leukemoid reaction in patients with metastatic malignant melanoma.11–14 The underlying mechanism of paraneoplastic granulocytosis in melanoma and in other solid malignancies is the abnormal secretion of cytokines such as granulocyte colony-stimulating factor (G-CSF).13 15 16 Although previous reports have suggested that this is related to rapid disease progression, it is still unclear whether the paraneoplastic process is indicative of an aggressive tumour phenotype.13 16 It also remains unclear if this is related to different responses to therapy. As far as we know, this is the first report of a paraneoplastic leukemoid reaction related to BRAF-mutated metastatic melanoma and its response to radiotherapy and the BRAF inhibitor.
Case presentation
A 56-year-old woman presented in April 2013 with severe persistent pain and an abnormal swelling in her left arm. Nine years earlier (in 2004), she was subjected to excision of a right leg superficial spread malignant melanoma, Breslow 1.52 mm, with no ulceration. Sentinel lymph node biopsy was positive and she underwent right inguinal lymph node dissection, with seven negative nodes. The thoracic, abdominal and pelvic CT (TAP-CT) did not revealed distant metastasis (stage IIIA, American Joint Committee on Cancer (AJCC) 7th edition). Seven years later in 2011, the patient was found to have a local recurrence and underwent surgery. Until 2013, she had always had a normal white cell count (WCC). Her medical history was unremarkable apart from the melanoma, and there was no history of corticosteroid consumption. Owing to the pain in the left arm, a plain radiography was performed that revealed a suspicious humeral lesion (figure 1). A bone scan confirmed a single bone metastasis in the left humerus. She was referred to our medical oncology department and a CT-guided biopsy to the left arm lesion was performed, revealing BRAF V600E-mutated malignant melanoma metastasis (cobas® 4800 BRAF V600 Mutation Test). The staging TAP-CT showed a soft-tissue large mass (130×100 mm) involving the humerus with bone destruction and vessel incarceration (figure 2) and also bilateral lung and adrenal metastasis. Laboratory findings at this time included: haemoglobin (Hb) 12.4 g/dL, platelet count 315×109/L, leucocyte count 40.2×109/L, neutrophils 36.5×109/L (91%), lymphocytes 2.21×109/L (5.5%), increased lactate dehydrogenase 424 UI/L, increased alkaline phosphatase 183 UI/l) and C reactive protein (CRP) levels not elevated. There was neither fever nor signs of infection. The patient was given four cycles of chemotherapy with dacarbazine 1000 mg/m2 on day 1 every 21 days with dexamethasone as a prophylactic antiemetic for 3 days after each cycle of chemotherapy. After the fourth cycle of dacarbazine, there was an increase of the swelling (figure 3) and worsening pain in the left arm requiring increasing doses of opioid analgesics. The revaluation scan performed at this time revealed a mixed response, with an increase of the left arm lesion and reduction in the size of pulmonary and adrenal metastasis. The leucocytosis increased during chemotherapy from a leucocyte count of 65.4×109/L to 120.0×109/L (figure 4), with neutrophil predominance (93–97%) and macrocytic anaemia developed (Hb range 7.3–8 g/dL; mean corpuscular volume 105.2 fL). The patient remained afebrile and without any signs of infection, and a peripheral smear demonstrated marked leucocytosis primarily attributable to mature neutrophils. Bone marrow biopsy showed hypercellular bone marrow with all cell lines represented, with a marked right shift in granulopoiesis. There was no infiltration by melanoma tumour cells (figure 5). Testing for BCR/ABL1 translocation and the JAK2 V617F mutation were negative. A diagnosis of a paraneoplastic leukemoid reaction was then established.
Figure 1.
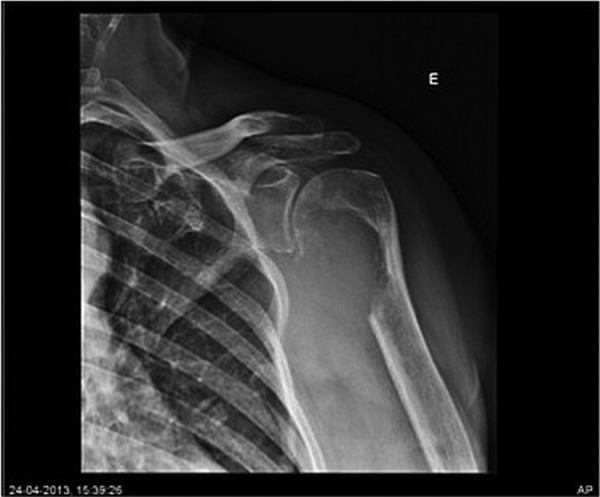
Plain radiography revealing a left humeral lesion with marked bone erosion.
Figure 2.
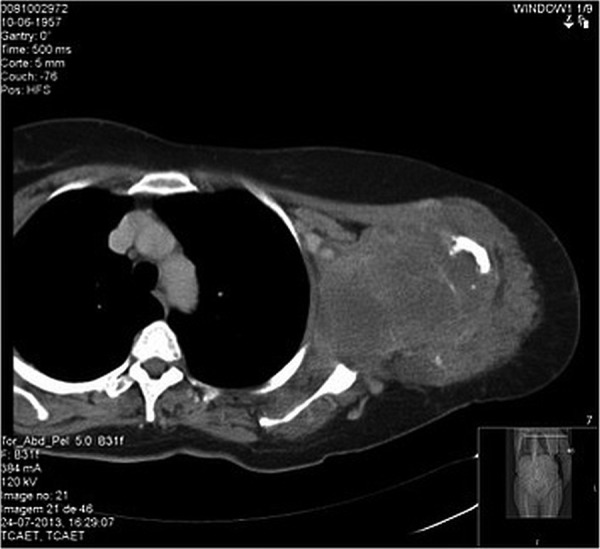
Soft-tissue mass involving the humerus with bone destruction.
Figure 3.
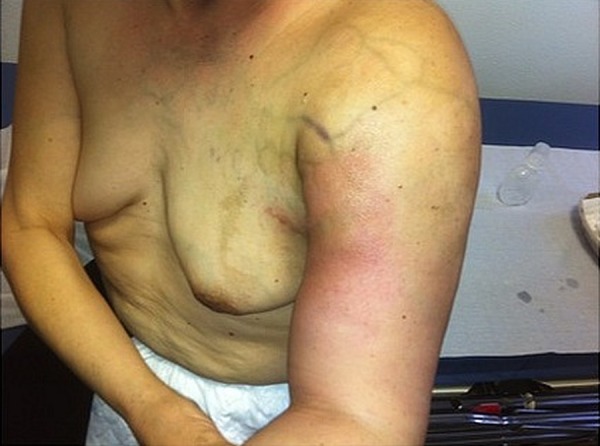
Left arm clinical appearance after four cycles of chemotherapy with dacarbazine.
Figure 4.
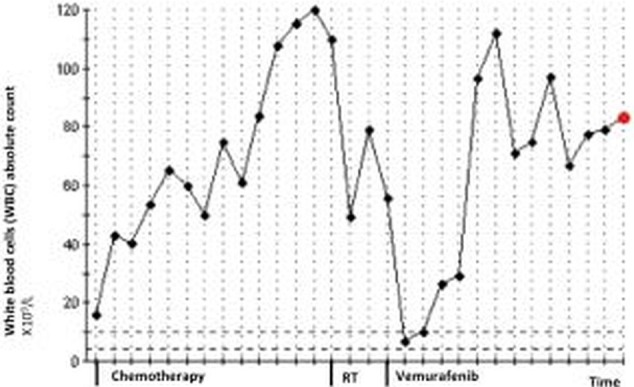
Evolution of white cell count since metastatic disease diagnosis until the patient's death.
Figure 5.
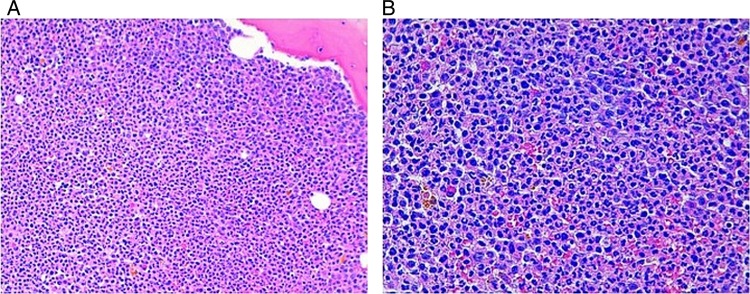
(A and B) Bone marrow biopsy with all cell lines represented, a marked right shift in granulopoiesis and no evidence of melanoma tumour cells.
Owing to of the increasing pain, the patient was subsequently treated with external-beam radiotherapy to the left arm lesion (30 Gy in 10 fractions). After the radiotherapy, there was a slight improvement in the swelling, and in a few weeks the patient reported that her pain intensity lessened for lower levels allowing a reduction of opioid dosages. Over the same period, there was a significant reduction in the WCC levels from 110.0×109/L (at the beginning of radiation therapy) to 49.0×109 in the next 4 weeks after treatment completion (figure 4). Unfortunately, the radiotherapy effect was of short duration and the pain worsened, as well as the swelling. After the national regulatory drugs authority approval, the patient started vemurafenib 960 mg twice daily initially with rapid improvement in the left arm swelling. After 15 days of treatment with vemurafenib, the laboratorial evaluation revealed a significant fall in the WCC to normal values (7×109/L) (figure 4). Moreover, as observed previously with radiotherapy, there was an improvement of anaemia. In the first month, there were no major toxicities with this drug. However, after 2 months of objective clinical response to vemurafenib, an impressive ulceration of the lesion on the left arm occurred and an exudate emerged (figure 6). She reported no fever and there were no other obvious infection sites. By this time, antibiotic therapy was started first with a second-generation cephalosporin and next with trimethoprim-sulfamethoxazole with slight improvement. Exudate and blood cultures were negative. Over the same time period, the patient presented with grade 3 mucositis, which justified the interruption of the BRAF inhibitor for 15 days. After mucositis resolution, vemurafenib was resumed at 720 mg twice daily dose. During the next 2 months, the leucocytosis increased to 83.3×109 (figure 4). The patient's condition worsened with progressive fatigue, increasing pain and a rapidly increasing haemorrhagic malignant wound. Owing to the rapid deterioration of performance status, vemurafenib was permanently discontinued. She was admitted in our oncology department ward and single dose radiotherapy to control bleeding was considered but not performed due to the patient's condition.
Figure 6.
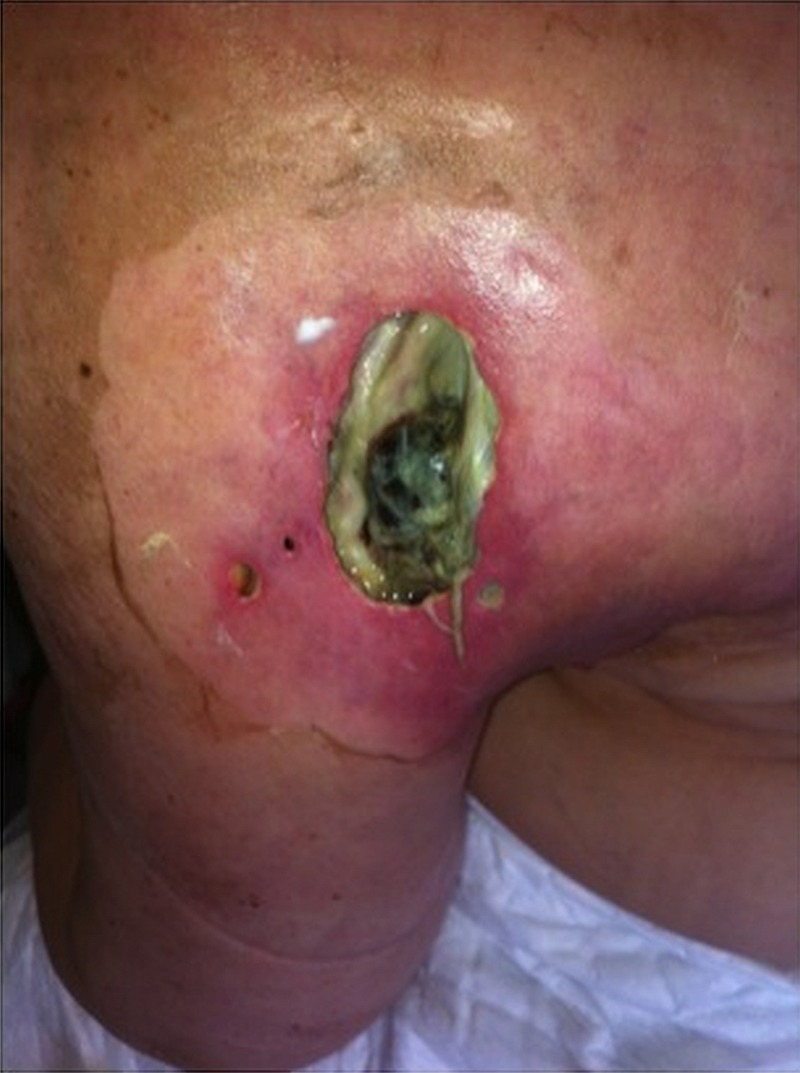
Left arm ulceration.
Outcome and follow-up
Unfortunately, her clinical condition continued to deteriorate and she died 5 days after admission.
Discussion
Paraneoplastic syndromes are rare events that result from the indirect effect of cancer through the production of humoral, neural or immune factors including cytokines, protein hormones or antigen-antibody interactions. Previous reports have described the occurrence of paraneoplastic manifestations in metastatic malignant melanoma such as dermatological and hormonal syndromes.10 Haematological manifestations are common in solid malignancies, most frequently cytopenias. The underlying causes for cytopenias in cancer include the tumour infiltration of bone marrow, the effects of chemotherapy and radiation therapy and the chronic disease condition by itself. The abnormal elevation of blood cell counts, including leucocytosis is less frequent. A leukemoid reaction is defined as a persistent leucocytosis greater than 50×109/L and can be neutrophilic or lymphocytic. This condition is observed in a variety of clinical settings. Causes of a neutrophilic leukemoid reaction include infections, intoxications or medications (eg, steroids, G-CSF therapy and mercury poisoning), serious burns, haemorrhage and acute haemolysis.1 2 Besides all these causes, the diagnostic work-up requires the exclusion of haematological diseases like chronic myelogenous leukaemia (CML) and myeloproliferative disorders. It is also crucial to rule out bone marrow invasion and replacement with metastatic solid tumour cells causing a leucoerythroblastic picture. In our patient, infectious causes were considered unlikely when leucocytosis first appeared. She remained afebrile, there were no evident signs of obvious infection and CRP was not increased. When the first signs of wound infection appeared in the left arm, leucocytosis was already established. Exudate and blood cultures also remained sterile. We also thought it was unlikely that the dosage of corticosteroids was responsible for causing leucocytosis. Moreover, although the patient was treated with antibiotics, the leucocytosis remained unchanged. She was not taking any medication possibly associated with a raised WCC. Given the size of the left arm mass and rapid growth, tumour necrosis could be a possible cause of leucocytosis. However, since CRP was not increased, this hypothesis was unlikely. We performed a peripheral smear that revealed marked leucocytosis that was primarily attributable to mature neutrophils. Additional investigation included bone marrow biopsy and testing for BCR/ABL1 translocation and the JAK2 V617F mutation. The bone marrow biopsy excluded infiltration by malignant melanoma cells and revealed characteristics consistent with hyperstimulation of granulopoiesis. The diagnosis of CML was very unlikely due to the absence of BCR/ABL fusion on cytogenetics and the normal basophil count. Similarly, negativity of the JAK2 mutation made the diagnosis of myeloproliferative disorders unlikely. Likewise, other less obvious causes of the leucocytosis listed were excluded. The presumptive diagnosis of a paraneoplastic leukemoid reaction was thus established.
A paraneoplastic leukemoid reaction has been described in a variety of malignant solid tumours including, among others, bladder carcinoma, sarcoma, lung cancer and cervical carcinoma.3–9 This event is extraordinarily rare in patients with melanoma ,with only a few cases reported in the literature. 11–14 In a study conducted in 626 patients with metastatic melanoma, Davis et al14 point out the rarity of paraneoplastic granulocytosis in this population, with only six patients presenting an elevated WCC and significantly elevated serum G-CSF levels. Most commonly, the secretion of G-CSF has been reported as the predominant mechanism of a leukemoid reaction in solid malignancies. G-CSF was indeed first purified from a bladder carcinoma cell line.17 Malignant melanoma has also been reported to secrete G-CSF. In 1987, Lilly et al15 reported that a human melanoma cell line (LD-1) was isolated from a patient with melanoma and unexplained leucocytosis. In this report, production of G-CSF in vitro was confirmed by molecular studies. Later in 2005, Schniewind et al13 reported the rapid progression of a patient with metastatic malignant melanoma with a paraneoplastic leukemoid reaction. This patient presented an elevated serum G-CSF level and the investigators isolated a G-CSF secreting melanoma cell line (KT293). They suggested that the paracrine effects of G-CSF secretion and a paraneoplastic leukemoid reaction might promote an aggressive disease phenotype.13 Safarians et al16 have suggested the same in an in vitro study that hypothesised G-CSF expression in human melanoma cell lines as a marker of a pathway linked to tumour progression and metastasis. In this study, Safarians et al ruled out an autocrine mechanism, noting the absence of G-CSF receptors in the G-CSF secreting melanoma cells. In other case reports, the paraneoplastic leukemoid reactions in distinct malignancy types have also been associated with worse prognosis, although the link between rapidly progressing cancer and leukemoid reactions has not been clearly established.6 7 13 In our case report, we pointed out the aggressiveness of the disease and rapid progression despite chemotherapy and BRAF inhibitor therapy. This is confirmed by those reports of a possibly more aggressive disease phenotype in patients with a leukemoid reaction. It remains unknown to what extent this phenotype of aggressive malignant melanoma is related to the presence of BRAF V600 mutation or other mutations (eg, NRAS, cKIT). As far as we know, this is the first case of a paraneoplastic leukemoid reaction in metastatic melanoma with characterisation of BRAF V600 mutation status, since this was not available at the time of the previous reports. Another interesting point in our case is the link between clinical response to radiotherapy and BRAF inhibitor therapy and lowering of WCCs. In the case of palliative radiotherapy, there was a positive tumour effect, albeit short-lived. It remains unclear what is the mechanism by which local control of the primary site of disease leads to an improvement of leukemoid reaction. After initiation of therapy with vemurafenib, the decline in WCCs was extremely rapid, reaching normal levels within 15 days. Unfortunately, the clinical response was also short-lived and there was a progressive increase in WCC that accompanied the patient's clinical deterioration. Some previous reports have noted that the monitoring of WCCs could be an indicator of tumour response to chemotherapy or radiotherapy or, on the contrary, a marker of recurrence.8 9 A previous report by Carey and Kunz11 in 1988 related the reversal of leucocytosis after bilateral adrenalectomy in a patient with malignant melanoma with adrenal metastases, and this was related with improved survival. Interestingly, our patient also presented with bilateral adrenal metastasis. However, we report for the first time the response of a paraneoplastic leukemoid reaction to radiation and BRAF inhibitor therapy in a patient with BRAF V600E-mutated metastatic melanoma, albeit short-lived. Unfortunately, a more detailed molecular analysis of our patient's tumour cell lines was not performed. It remains uncertain why some melanoma cell lines secrete G-CSF and whether this is related to already known mutations (eg, BRAF, NRAS and cKIT). It is also unclear whether there are different mechanisms of resistance to therapy in these particular cases, leading to a poor response or responses of shorter duration. These hypotheses need further investigation.
Learning points.
A paraneoplastic leukemoid reaction is extremely rare in patients with malignant melanoma, and the differential diagnosis is often a true challenge.
We report the first case described of a paraneoplastic leukemoid reaction in metastatic melanoma with characterisation of BRAF V600 mutation status and the response of leucocytosis to radiation and BRAF inhibitor therapy, albeit short-lived.
In line with previous reports, we describe an aggressive tumour phenotype related to a leukemoid reaction.
It remains unclear whether the aggressive tumour phenotype is related to a leukemoid reaction and whether this is related to the BRAF mutation.
Acknowledgments
The authors would like to thank Dr José Cabeçadas, Head of Pathology Department at our institution, for the supply of images of bone marrow biopsy and for the review of our manuscript. The authors also thank Dr Albertina Nunes and Dr Filipa Moita, Department of Hematology, for the clinical advice during the diagnostic work-up.
Footnotes
Contributors: EG, MS and MJP contributed to the conception, design and drafting of the manuscript. MJP and AM coordinated the drafting of the manuscript. All authors read and approved the final manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program 2012;2012:475–84. 10.1182/asheducation-2012.1.475 [DOI] [PubMed] [Google Scholar]
- 2.Sakka V, Tsiodras S, Giamarellos-Bourboulis EJ et al. An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur J Intern Med 2006;17:394–8. 10.1016/j.ejim.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 3.Ito N, Matsuda T, Kakehi Y et al. Bladder cancer producing granulocyte colony-stimulating factor. N Engl J Med 1990;323:1709–10. [DOI] [PubMed] [Google Scholar]
- 4.Kasuga I, Makino S, Kiyokawa H et al. Tumor-related leucocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer 2001;92:2399–405. [DOI] [PubMed] [Google Scholar]
- 5.Satoh H, Abe Y, Katoh Y et al. Bladder carcinoma producing granulocyte colony-stimulating factor: A case report. J Urol 1993;149:843–5. [DOI] [PubMed] [Google Scholar]
- 6.Miller JI, Sarver RG, Drach GW. Leukemoid reaction: a rare paraneoplastic syndrome associated with advanced bladder carcinoma. Urology 1994;44:444–6. 10.1016/S0090-4295(94)80114-2 [DOI] [PubMed] [Google Scholar]
- 7.Jardin F, Vasse M, Debled M et al. Intense paraneoplastic neutrophilic leukemoid reaction related to a G-CSF secreting lung sarcoma. Am J Hematol 2005;80:243–5. 10.1002/ajh.20454 [DOI] [PubMed] [Google Scholar]
- 8.Dukes JW, Tierney LM Jr. Paraneoplastic leukemoid reaction as marker for transitional cell carcinoma recurrence. Urology 2009;73:928.e 17–19. 10.1016/j.urology.2008.05.023 [DOI] [PubMed] [Google Scholar]
- 9.Nimieri HS, Makoni SN, Madziwa FH et al. Leukemoid reaction response to chemotherapy and radiotherapy in a patient with cervical carcinoma. Ann Hematol 2003;82:316–17. [DOI] [PubMed] [Google Scholar]
- 10.Wagner RF Jr, Nathanson L. Paraneoplastic syndromes, tumor markers, and other unusual features of malignant melanoma. J Am Acad Dermatol 1986;14(2 Pt 1):249–56. 10.1016/S0190-9622(86)70029-7 [DOI] [PubMed] [Google Scholar]
- 11.Carey RW, Kunz VS. Leukocytosis due to adrenal metastases from malignant melanoma: reversal after bilateral adrenalectomy with long-term survival. Am J Hematol 1988;27:228–9. 10.1002/ajh.2830270317 [DOI] [PubMed] [Google Scholar]
- 12.de Wolff JF, Planken EV, den Ottolander GJ. Extreme leucocytosis and splenomegaly in metastasised melanoma. Neth J Med 2004;62:164–7. [PubMed] [Google Scholar]
- 13.Schniewind B, Christgen M, Hauschild A et al. Paraneoplastic leukemoid reaction and rapid progression in a patient with malignant melanoma: establishment of KT293, a novel G-CSF-secreting cell line. Cancer Biol Ther 2005;4:23–7. 10.4161/cbt.4.1.1447 [DOI] [PubMed] [Google Scholar]
- 14.Davis JL, Ripley RT, Frankel TL et al. Paraneoplastic granulocytosis in metastatic melanoma. Melanoma Res 2010;20:326–9. 10.1097/CMR.0b013e328339da1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lilly MB, Devlin PE, Devlin JJ et al. Production of granulocyte colony stimulating factor by a human melanoma cell line. Exp Hematol 1987;15:966–71. [PubMed] [Google Scholar]
- 16.Safarians S, Rivera SP, Sternlicht MD et al. Ectopic G-CSF expression in human melanoma lines marks a trans-dominant pathway of tumor progression. Am J Pathol 1997;150:949–62. [PMC free article] [PubMed] [Google Scholar]
- 17.Welte K, Platzer E, Lu L et al. Purification and biochemical characterization of a human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci USA 1985;82:1526–30. 10.1073/pnas.82.5.1526 [DOI] [PMC free article] [PubMed] [Google Scholar]


