Abstract
Purpose
To report a long-term follow-up of a young woman affected by cystic fibrosis (CF) with a residual retroperitoneal mass of neuroblastoma (NBL) after treatment.
Case report
We reviewed the patient's database and analysed a 20-year follow-up by considering pulmonary exacerbation, nutritional condition, pulmonary function (forced expiratory volume in 1 s), microbiological data and residual retroperitoneal mass volume. We observed stable pulmonary and nutritional conditions. No variation was found in the residual retroperitoneal mass volume.
Discussion
We report this case of a patient with CF with previous NBL because such a long time of follow-up of a NBL with a stable retroperitoneal remaining tumour is uncommon and needs to be reported. Multidisciplinary management has been crucial in this case because of the presence of concomitant diseases and consequently, differential diagnosis challenges.
Background
Neuroblastoma (NBL) is the most common extracranial solid tumour in children.
NBL originates from cells of the primitive neural crest and accounts for 8–10% of all paediatric malignancies. It most typically involves adrenal glands followed by abdominal, thoracic, pelvic, head and neck sympathetic ganglions, plexuses and nerves. Most common metastatic sites are the bone marrow, liver and lymph nodes.1
Clinical manifestations of NBL range from spontaneous regression up to eminently malignant development. The prognosis for most patients, especially the older patients with metastatic disease, remains poor.2
NBL is a biologically active tumour secreting vanillylmandelic acid (VMA) and homovanillic acid (HVA) or other metabolites, such as catecholamines, neuron-specific enolase (NSE) and vasoactive intestinal peptides.3 4 NBL can be radiologically diagnosed by ultrasonography (US), CT and MRI with different sensitivities and specificities.5 Approximately, 50% of the patients have a localised tumour with high chances of cure, while the others have disseminated disease which could have dismal outcomes.6
We report a case of multidisciplinary management of chronic disease (cystic fibrosis, CF) complicated by NBL and the long-term follow-up of the remaining mass.
Case presentation
We report the case of a 22-year-old woman affected by CF diagnosed by evocative symptoms and confirmed by positive sweat test (Cl− levels 106 mEq/L) at the age of 2 months; genotype M1V/del 17a, 17b, 18. M1V is a rare CFTR mutation and its clinical impact is not known. It has reported a frequency of pancreatic insufficiency of 56% in M1V carriers.
Our patient developed pancreatic insufficiency during the first year of infancy.
At 22 months of age, in 1993, as part of a routine monitoring planned for the underlying disease, abdominal ultrasound was performed which showed a retroperitoneal mass. MRI of the abdomen showed a space-occupying process in the right adrenal region, with infiltration of median retroperitoneum and compression of the abdominal aorta and inferior vena cava. Metaiodobenzylguanidine (MIBG) scintigraphy showed an intense uptake in the retroperitoneum, suggesting a localised stage of the tumour. Urinary levels of VMA and HVA were high, while serum NSE and ferritin were normal.
The biopsy of the mass gave the diagnosis of differentiated NBL with low mitotic index. Bone marrow aspirates and biopsies were negative. Staging concluded with definition of stage 3 NBL.
Treatment
She received antiblastic therapy, following AIEOP NB92 protocol (deferoxamine 7.5 g/m2, days 1–5; cyclophosphamide 600 mg/m2, days 5–6; thiotepa 30 mg/m2, days 5–7; vepeside 450 mg/m2, days 5–7; carboplatin 1 g/m2, days 6–7 for 4 cycles), without a significant reduction of the mass. AIEOP NB92 protocol was followed by surgical therapy with the removal of almost 70% of the neoplastic mass, resulting in a remaining retroperitoneal mass (84×67×72 mm). The patient underwent four metabolic radiotherapeutic cycles with MIBG and was then taken off therapy.
Outcome and follow-up
The patient has been in regular follow-up at our CF centre for diagnosis: during this long-term follow-up, we observed stable pulmonary and nutritional conditions, with rare pulmonary exacerbations. The patient presents chronic colonisation by methicillin-sensible Staphylococcus aureus, no sputum positive for Pseudomonas aeruginosa; last forced expiratory volume in 1 s 69% predicted; actual body mass index 23.1. She had pancreatic insufficiency from the first year of infancy and at the age of 10 years, she developed CF-related diabetes mellitus.
We also carried out regular checks of serum tumour markers level (NSE, carbohydrate antigen 19-9 (CA19.9), carcinoembryonic antigen (CEA), vanillylmandelic acid (VMA), homovanillic acid (HVA)); these have been always negative. No variations were found in the residual retroperitoneal mass volume (actually 55×40×115 mm; figure 1).
Figure 1.
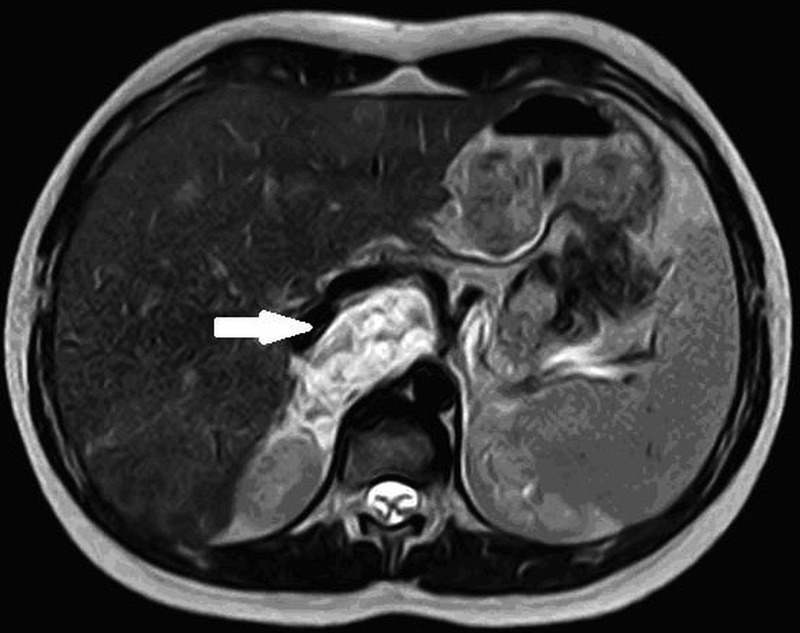
Last MRI abdomen performed (2010) shows the residual neuroblastoma mass localised in paramedian right retroperitoneum; measures 55×40×115 mm, in contact with splenic vein, celiac trunk and hepatic artery, with incorporation of right renal vascular pedicle.
During the clinical follow-up, the patient developed some complications from the adopted chemo-radiotherapy treatment:
At age 4 years, diagnosis of iatrogenic hypothyroidism treated with substitutive therapy (levothyroxine).
At age 12 years, onset of icterus, hypertransaminasaemia, hyperbilirubinaemia along with a diagnosis of hepatic nodular hyperplasia demonstrated by abdominal US tomography and confirmed by high resolution CT. MIBG scintigraphy showed accumulated MIBG in midline epigastric region. Positron emission tomography scintigraphy ruled out an active disease. These results confirmed the diagnosis of focal nodular hepatic hyperplasia. A multidisciplinary approach was necessary in the differential diagnosis of these hepatic lesions because the patient was not affected by CF-liver disease.
At age 21 years, the patient underwent total thyroidectomy after diagnosis of papillary thyroid carcinoma.
Discussion
NBL is a tumour, primarily seen in infants and younger children, from sympathetic neuroblasts. A familial history of NBL is observed in ∼1% of patients. Two main neural crest-derived developmental disorders are associated with an increased risk to develop NBL: Hirschsprung's disease and Ondine's curse, a disorder characterised by a failure of the autonomic control of ventilation during sleep. Both diseases are frequently associated with each other and most cases are linked to mutation of the PHOX2B gene. Associations between neuroblastic tumours and various ‘neuro-cardio-facial-cutaneous’ syndromes, including Noonan and Costello syndromes and neurofibromatosis type 1, have been reported. The anaplastic lymphoma kinase (ALK) gene was recently identified as a second NBL predisposition gene. However, the vast majority of cases of NB occur sporadically and in these cases, the adverse prognostic effect of MYCN amplification (NMA) and of other chromosomal segmental alterations has been confirmed in many studies.1
Treatment plans vary from close clinical observation to multimodal interventions, including surgery, chemotherapy and radiotherapy. Complete surgical removal of the primary tumour is an essential component of treatment for the majority of patients with NBL.7
Complete surgical excision alone can provide useful treatment for stages I and II tumours (International Neuroblastoma staging System, INSS).8 9 In patients with INSS stages III or IV tumours, complete excision is not always feasible and could trigger of severe complications.9
Most NBL treatment results have been reported after complete excision; few studies evaluate incomplete resection outcome. In the literature, no significant differences have been found between patients who had complete and incomplete resection.10 However, a recent study shows that gross total resection should be a part of the management of high-risk NB, considering the positive outcomes reported.9 Gross total resection is a serious and often life-threatening intervention and in some cases, complete resection cannot be performed.
No association with CF has been reported. Only two previous cases of coexistent CF and NBL are reported. In one patient, NBL was congenital; in the other NBL was diagnosed at 11 months of age. In both infants, complete surgical resection of the tumours have been successful.11
The overall incidence of malignancies in CF children is low; however, these patients have an increased risk in adult age of digestive tract cancer, particularly after transplantation. In the literature is also reported an increased risk of lymphoid leukaemia and testicular cancer.12
We report this case because such a long time of follow-up (20 years) in a patient with CF with a NBL stable remaining mass is uncommon and needs to be reported. Unfortunately, we cannot define the real impact of the antiblastic treatments performed on lung function because of the young age of the patient at the time of diagnosis of NBL.
It is important to underline the multidisciplinary management of this patient affected by two serious diseases. On several occasions, differential diagnosis has been challenging and a co-ordinated follow-up between the CF centre and oncologic unit was crucial.
Learning points.
Long-term (20 years) follow-up of stage 3 neuroblastoma with stable residual mass.
Long-term complication of radiotherapy and chemotherapy.
Multidisciplinary management in a patient with complex concomitant diseases.
Acknowledgments
The authors thank Dr Alberto Garaventa (IRCCS Istituto G. Gaslini, Oncology Department, Genoa, Italy) for his collaboration.
Footnotes
Contributors: AN, FC and FF analysed the case history and wrote the manuscript. RC reviewed the paper.
Competing interests: None.
Patient consent: Obtained.
References
- 1.Mueller S, Matthay KK. Neuroblastoma: biology and staging. Curr Oncol Rep 2009;11:431–8. 10.1007/s11912-009-0059-6 [DOI] [PubMed] [Google Scholar]
- 2.Humpl T. Neuroblastoma. World J Urol 1995;13:233–9. 10.1007/BF00182969 [DOI] [PubMed] [Google Scholar]
- 3.Brodeur GM, Marris JM. Neuroblastoma. In: Pizzo PA, Poplack BG eds. Principles and practive of pediatric oncology. 5th edn Philadelphia: Lippincott Williams & Wilkins, 2006:933–70. [Google Scholar]
- 4.Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am 2010;24:65–86. 10.1016/j.hoc.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 5.Pfluger T, Schmied C, Porn U et al. Integrated imaging using MRI and 123I metaiodobenzylguanidine scintigraphy to improve sensitivity and specificity in the diagnosis of pediatric neuroblastoma. AJR Am J Roentgenol 2003;181:1115–24. 10.2214/ajr.181.4.1811115 [DOI] [PubMed] [Google Scholar]
- 6.De Bernardi B, Pistoia V, Gambini C et al. Peripheral neuroblastic tumors. Clinical endocrine oncology. 2nd edn Oxford: Blackwell Science, 2008:360–9. [Google Scholar]
- 7.Grosfeld JL. Neuroblastoma. In: Grosfeld JL, O'Neill JA, Fonkalsrud EW, Coran AG eds. Pediatric surgery. 6th edn St Louis: Mosby, 2006:467–94. [Google Scholar]
- 8.Michon J, De Bernardi B, Rubie H et al. Surgery as the only treatment for INSS stage 2 neuroblastoma: risk factors predicting relapse. Med Pediatr Oncol 2000;35:758. [Google Scholar]
- 9.La Quaglia MP, Kurshner BH, Su W et al. The impact of gross resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg 2004;39:412–17. 10.1016/j.jpedsurg.2003.11.028 [DOI] [PubMed] [Google Scholar]
- 10.Kiely EM. Radical surgery for abdominal neuroblastoma. Semin Surg Oncol 1993;9:489–92. 10.1002/ssu.2980090606 [DOI] [PubMed] [Google Scholar]
- 11.Moss RB, Blessing-Moore J, Bender SW et al. Cystic fibrosis and neuroblastoma. Pediatrics 1985;76:814–17. [PubMed] [Google Scholar]
- 12.Maisonneuve P, Marshall BC, Knapp EA et al. Cancer risk in cystic fibrosis: a 20-year nationwide study from the United States. J Natl Cancer Inst 2013;105:122–9. 10.1093/jnci/djs481 [DOI] [PubMed] [Google Scholar]


