Abstract
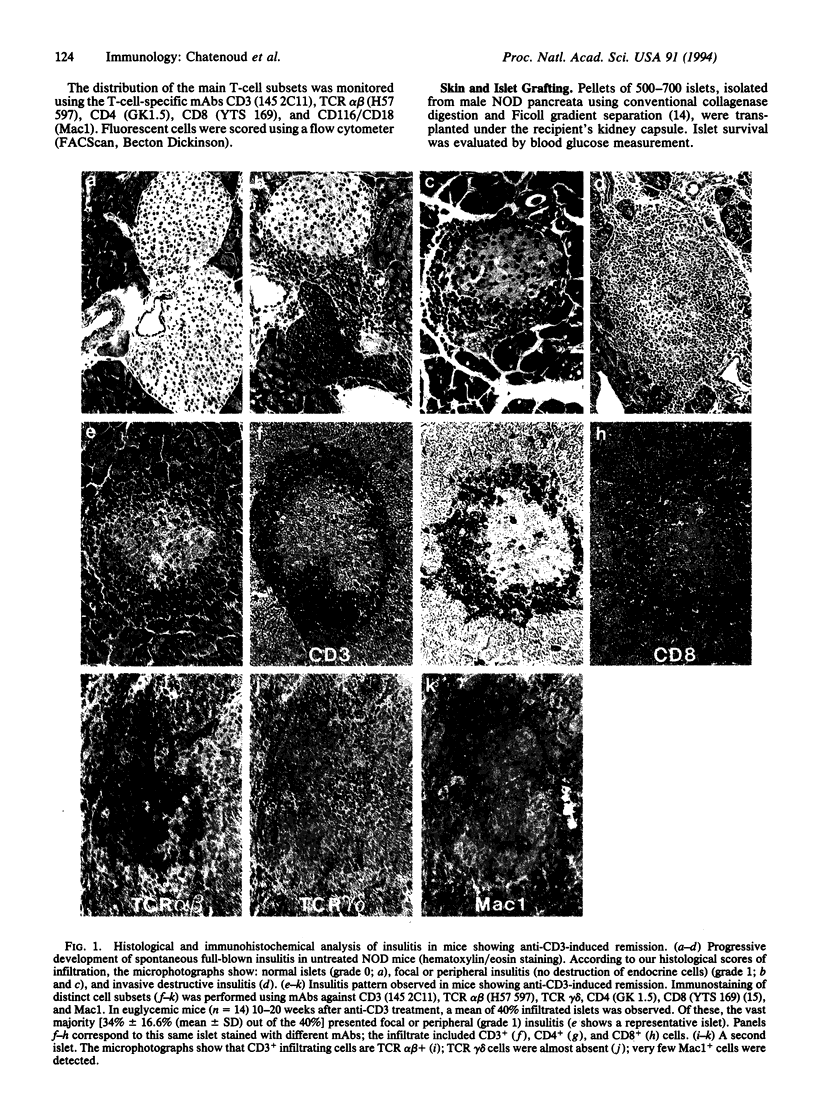
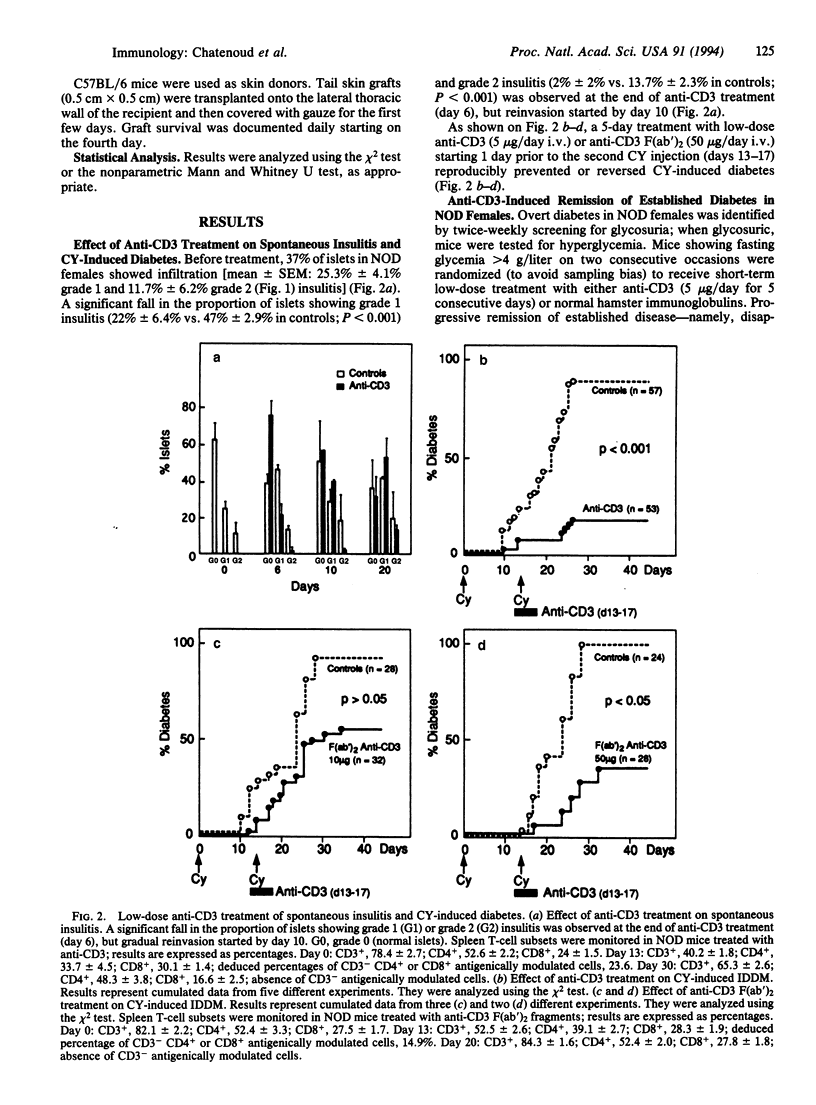
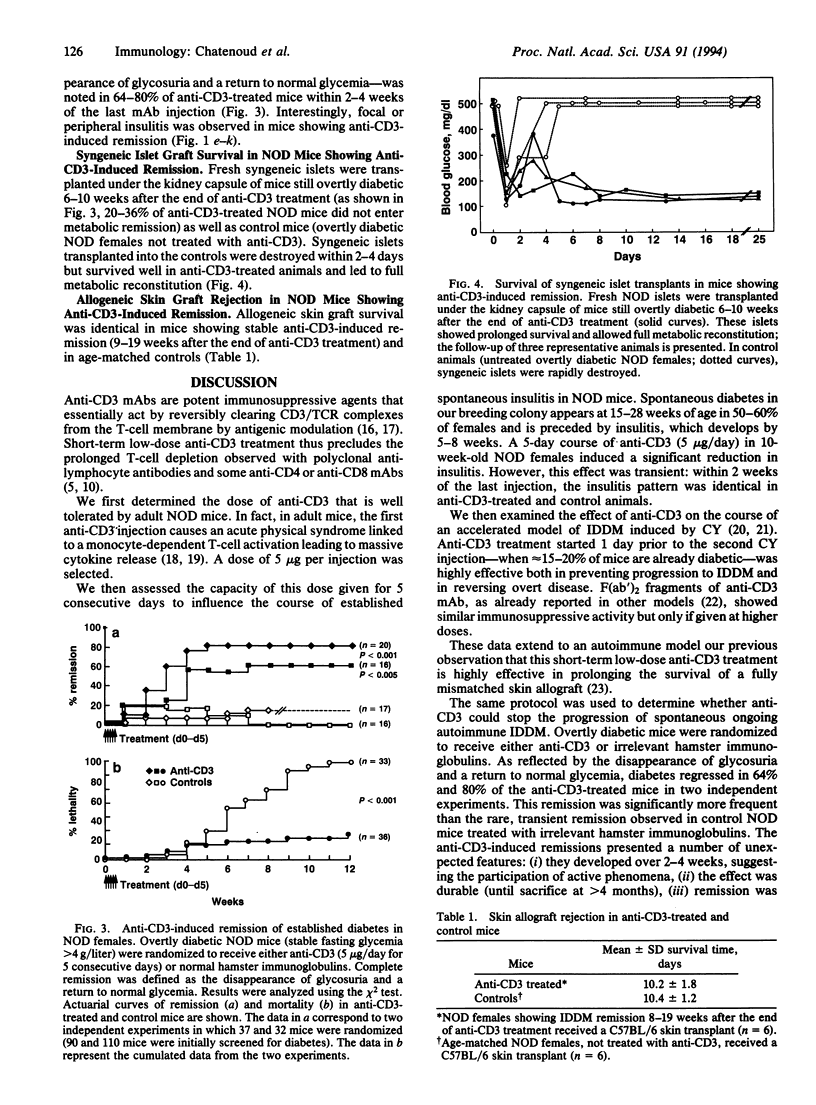
Anti-CD3 monoclonal antibodies suppress immune responses by transient T-cell depletion and antigenic modulation of the CD3/T-cell receptor complex. Anti-CD3 treatment of adult nonobese diabetic (NOD) mice, a spontaneous model of T-cell-mediated autoimmune insulin-dependent diabetes mellitus, significantly inhibits the autoimmune process. Short-term low-dose anti-CD3 treatment (5 micrograms/day i.v. for 5 consecutive days) prevented the occurrence of an accelerated form of the disease induced by cyclophosphamide. More unexpectedly, when applied to adult NOD females within 7 days of the onset of full-blown diabetes, the same anti-CD3 regimen induced a complete remission of overt disease (i.e., a return to permanent normoglycemia) in 64-80% of mice. This remission was durable (> 4 months) and was not associated with the disappearance of insulitis (mononuclear cell infiltration of the islets). The immunosuppression was apparently specific for beta-cell-associated antigens, since mice showing anti-CD3-induced remission rejected histoincompatible skin grafts normally, whereas they did not destroy syngeneic islet grafts, unlike control untreated overtly diabetic NOD females. These results open major therapeutic perspectives. They strongly suggest that self-tolerance can be restored in adult mice once autoimmunity is fully established and confirm that this effect can be obtained by transient targeting of the CD3/T-cell receptor without massive T-cell debulking.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C., Yasunami R., Dardenne M., Bach J. F. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989 May 1;169(5):1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos H. H., Bach J. F., Chatenoud L. Devising murine models to better adapt clinical protocols: sequential low-dose treatment with anti-CD3 and anti-CD4 monoclonal antibodies to prevent fully mismatched allograft rejection. Transplant Proc. 1993 Feb;25(1 Pt 1):798–799. [PubMed] [Google Scholar]
- Castaño L., Eisenbarth G. S. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Slattery R. M., Mandel T. E. Cyclophosphamide-induced diabetes in NOD/WEHI mice. Evidence for suppression in spontaneous autoimmune diabetes mellitus. Diabetes. 1989 Apr;38(4):441–447. doi: 10.2337/diab.38.4.441. [DOI] [PubMed] [Google Scholar]
- Chatenoud L., Baudrihaye M. F., Kreis H., Goldstein G., Schindler J., Bach J. F. Human in vivo antigenic modulation induced by the anti-T cell OKT3 monoclonal antibody. Eur J Immunol. 1982 Nov;12(11):979–982. doi: 10.1002/eji.1830121116. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Ferran C., Dy M., Sheehan K., Merite S., Schreiber R., Landais P., Grau G., Bluestone J., Bach J. F., Chatenoud L. Inter-mouse strain differences in the in vivo anti-CD3 induced cytokine release. Clin Exp Immunol. 1991 Dec;86(3):537–543. doi: 10.1111/j.1365-2249.1991.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran C., Sheehan K., Dy M., Schreiber R., Merite S., Landais P., Noel L. H., Grau G., Bluestone J., Bach J. F. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990 Mar;20(3):509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- Gotoh M., Maki T., Satomi S., Porter J., Bonner-Weir S., O'Hara C. J., Monaco A. P. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987 May;43(5):725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Shreiber M. Neonatal injection of CD3 antibody into nonobese diabetic mice reduces the incidence of insulitis and diabetes. J Immunol. 1989 Sep 1;143(5):1555–1559. [PubMed] [Google Scholar]
- Herold K. C., Bluestone J. A., Montag A. G., Parihar A., Wiegner A., Gress R. E., Hirsch R. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes. 1992 Mar;41(3):385–391. doi: 10.2337/diab.41.3.385. [DOI] [PubMed] [Google Scholar]
- Hutchings P., Rosen H., O'Reilly L., Simpson E., Gordon S., Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990 Dec 13;348(6302):639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T., Ichikawa T., Blanco R., Porter J. Long-term abrogation of autoimmune diabetes in nonobese diabetic mice by immunotherapy with anti-lymphocyte serum. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3434–3438. doi: 10.1073/pnas.89.8.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T. C., Madsen J. C., Larsen C. P., Morris P. J., Wood K. J. Induction of transplantation tolerance in adults using donor antigen and anti-CD4 monoclonal antibody. Transplantation. 1992 Sep;54(3):475–483. doi: 10.1097/00007890-199209000-00018. [DOI] [PubMed] [Google Scholar]
- Qin S. X., Cobbold S., Benjamin R., Waldmann H. Induction of classical transplantation tolerance in the adult. J Exp Med. 1989 Mar 1;169(3):779–794. doi: 10.1084/jem.169.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Cobbold S. P., Pope H., Elliott J., Kioussis D., Davies J., Waldmann H. "Infectious" transplantation tolerance. Science. 1993 Feb 12;259(5097):974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- Sempé P., Bédossa P., Richard M. F., Villà M. C., Bach J. F., Boitard C. Anti-alpha/beta T cell receptor monoclonal antibody provides an efficient therapy for autoimmune diabetes in nonobese diabetic (NOD) mice. Eur J Immunol. 1991 May;21(5):1163–1169. doi: 10.1002/eji.1830210511. [DOI] [PubMed] [Google Scholar]
- Shah S. C., Malone J. I., Simpson N. E. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989 Mar 2;320(9):550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Taylor-Edwards C., Banks B. A., Gregory A. K., Fathman C. G. Immunotherapy of the nonobese diabetic mouse: treatment with an antibody to T-helper lymphocytes. Science. 1988 Apr 29;240(4852):659–662. doi: 10.1126/science.2966437. [DOI] [PubMed] [Google Scholar]
- Yasunami R., Bach J. F. Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol. 1988 Mar;18(3):481–484. doi: 10.1002/eji.1830180325. [DOI] [PubMed] [Google Scholar]




