Abstract
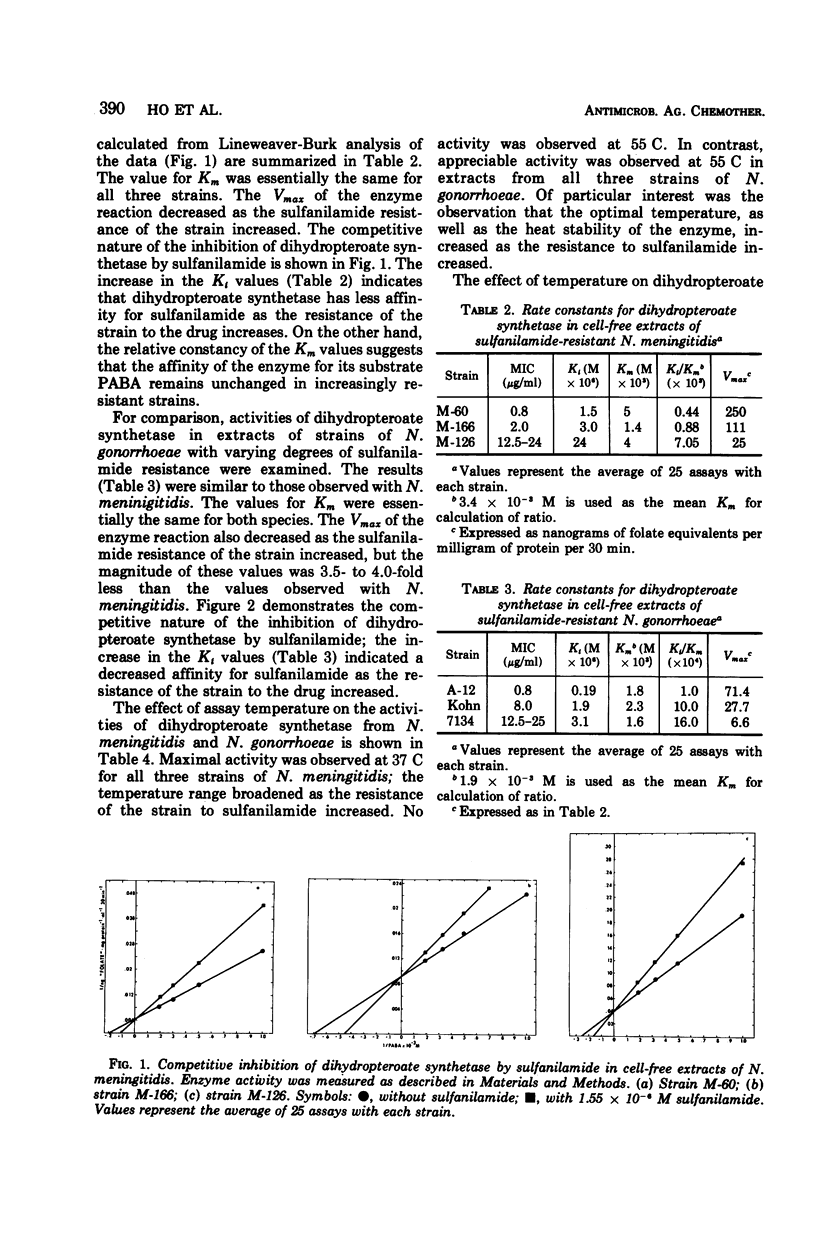
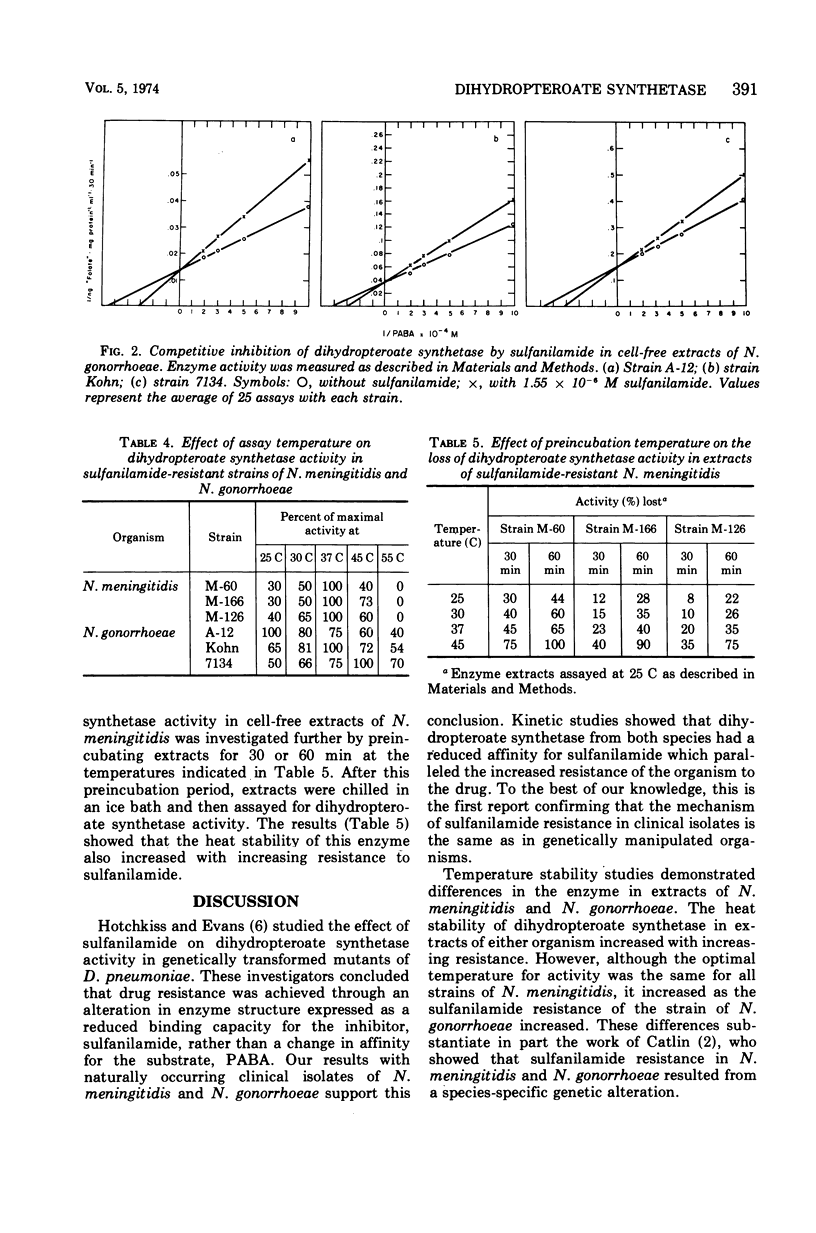
Extracts from Neisseria meningitidis and N. gonorrhoeae with varying susceptibility to sulfanilamide have been investigated for dihydropteroate synthetase activity. Sulfanilamide was a competitive inhibitor of dihydropteroate synthetase with respect to p-aminobenzoate in extracts from both species. Though the Km for p-aminobenzoate was unaffected, the Ki for sulfanilamide increased and the Vmax decreased as the strains' resistance to sulfanilamide increased. Temperature studies have revealed differences in the dihydropteroate synthetase from N. meningitidis and N. gonorrhoeae. A direct relationship was observed between the minimal inhibitory concentration of sulfanilamide determined in vitro and the ratio of Ki/Km. This ratio may be a molecular explanation of sulfanilamide resistance for both N. meningitidis and N. gonorrhoeae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catlin B. W. Genetic studies of sulfadiazine-resistant and methionine-requiring Neisseria isolated from clinical material. J Bacteriol. 1967 Sep;94(3):719–733. doi: 10.1128/jb.94.3.719-733.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic reduction of folic acid and dihydrofolic acid to tetrahydrofolic acid. J Biol Chem. 1957 Oct;228(2):1031–1038. [PubMed] [Google Scholar]
- HOTCHKISS R. D., EVANS A. H. Analysis of the complex sulfonamide resistance locus of pneumococcus. Cold Spring Harb Symp Quant Biol. 1958;23:85–97. doi: 10.1101/sqb.1958.023.01.012. [DOI] [PubMed] [Google Scholar]
- LASCELLES J., WOODS D. D. The synthesis of folic acid by Bacterium coll and Staphylococcus aureus and its inhibition by sulphonamides. Br J Exp Pathol. 1952 Jun;33(3):288–303. [PMC free article] [PubMed] [Google Scholar]
- MITSUDA H., SUZUKI Y., TADERA K., KAWAI F. BIOCHEMICAL STUDIES ON PTERIDINES IN PLANTS. I. BIOGENESIS OF FOLIC ACID IN GREEN LEAVES: CONFIRMATION OF ENZYMATIC SYNTHESIS OF FOLATE COMPOUNDS BY THE ENZYME SYSTEM FROM THE SPINACH. J Vitaminol (Kyoto) 1965 Jun 10;11:122–138. [PubMed] [Google Scholar]
- McCullough J. L., Maren T. H. Inhibition of dihydropteroate synthetase from Escherichia coli by sulfones and sulfonamides. Antimicrob Agents Chemother. 1973 Jun;3(6):665–669. doi: 10.1128/aac.3.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Goto M. Syntheses of 2-amino-4-hydroxy-6-hydroxymethylpteridine-10-C14 and 2-amino-4-hydroxypteridine-10-C14 and their metabolism in Drosophila melanogaster. J Biochem. 1965 Nov;58(5):458–462. doi: 10.1093/oxfordjournals.jbchem.a128225. [DOI] [PubMed] [Google Scholar]
- Ortiz P. J. Dihydrofolate and dihydropteroate synthesis by partially purified enzynes from wild-type and sulfonamide-resistant pneumonococcus. Biochemistry. 1970 Jan 20;9(2):355–361. doi: 10.1021/bi00804a024. [DOI] [PubMed] [Google Scholar]
- SHIOTA T., DISRAELY M. N., MCCANN M. P. THE ENZYMATIC SYNTHESIS OF FOLATE-LIKE COMPOUNDS FROM HYDROXYMETHYLDIHYDROPTERIDINE PYROPHOSPHATE. J Biol Chem. 1964 Jul;239:2259–2266. [PubMed] [Google Scholar]
- Thijssen H. H. A simplified radioassay method of dihydropteroate synthetase activity in Escherichia coli and its application for an inhibition study of p-aminobenzoi acid derivatives. Anal Biochem. 1973 Jun;53(2):579–585. doi: 10.1016/0003-2697(73)90109-7. [DOI] [PubMed] [Google Scholar]
- WOLF B., HOTCHKISS R. D. Genetically modified folic acid synthesizing enzymes of pneumococcus. Biochemistry. 1963 Jan-Feb;2:145–150. doi: 10.1021/bi00901a026. [DOI] [PubMed] [Google Scholar]


