Abstract
Epidermolysis bullosa (EB) is comprised of a group of hereditary mechanobullous disorders that are characterised by extremely fragile skin and mucous membranes. This results in blister formation and non-healing wounds. This case report describes the results of an innovative treatment of two large skin lesions in a newborn with dystrophic recessive EB (DEB) who experienced bacterial superinfections and progressive anaemisation. The lesions were treated with platelet gels derived from allogeneic cord blood (cord blood platelet gel, CBPGs). The skin lesions were clinically evaluated and treated with CBPG weekly until they completely healed. The first and second lesion required CBPG applications for 2 and 4 weeks, respectively. Both lesions were monitored weekly for 6 weeks after the last CBPG application, and no significant relapses were observed during the follow-up period. This case indicates that CBPG is an effective and safe therapeutic option for managing newborns with DEB, particularly as treatment and prevention of fluid loss and superinfection.
Background
Epidermolysis bullosa (EB) is comprised of a heterogeneous group of hereditary mechanobullous disorders that are characterised by extremely fragile skin and mucous membranes. Consequently, patients with EB often develop blisters and non-healing wounds following minor trauma.1 In infants, the most important aspects of EB management are preventing blisters and caring for wounds. These decrease the risk of sepsis and the loss of fluids and electrolytes from lesions, which can lead to dehydration, anaemia and electrolyte imbalance.2 Autologous or allogeneic platelet preparations have been shown to be useful for treating non-healing wounds in adults,3 but their efficacy in infants and children is unknown. We describe a case report where a newborn with dystrophic recessive EB (DEB) was treated with a platelet gel derived from allogeneic cord blood (cord blood platelet gel, CBPG).
Case presentation
A newborn female was referred to the Neonatal Intensive Care Unit at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, University of Milan, Milan, Italy, because of skin lesions. She was born at 39 weeks gestation from healthy, non-consanguineous parents after an unremarkable pregnancy. On physical examination, her weight (3200 g), length (50 cm) and head circumference (35 cm) were normal. However, she had multiple diffuse skin erosions, especially on the lower limbs, which included oral and rectal lesions. EB was suspected, and a skin biopsy was taken from the edge of a fresh blister for ultrastructural assessment. Direct immunofluorescence and electron microscopy showed subepidermal blistering with reduced collagen VII. Molecular analysis showed mutations leading to the formation of premature termination codons (PTCs) of translation in the gene COL7 encoding type VII collagen (COL7) with a marked reduction or complete absence of COL7 mRNA and the patient was diagnosed with recessive DEB.
Initially, the diffuse skin lesions were managed conservatively by applying antiseptic Eosin staining solution (2% w/v) and dressings of wet gauze immersed in petroleum to prevent infection and dryness. During the first days of life, the patient experienced progressive anaemisation due to bleeding from the skin lesions and was treated with concentrated red blood cells and erythropoietin infusions. In addition, she received antibiotic therapy because of a skin bacterial superinfection.
Treatment
On day 8 of the patient's life, the severity of the anaemisation and the continuous presence of skin superinfection led us to seek and obtain approval for treating the patient with CBPGs from the Institutional Ethics Committee of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico. Written informed consent was obtained from the patient's parents. We decided to treat two of the larger lesions on the patient's legs with CBPGs. The first lesion (Lesion 1) was a deep ulceration that covered the left leg from her knee to the back of her foot (figure 1); this lesion bled continuously and forced the foot into a bent position. The second lesion (lesion 2) was a smaller ulceration at a friction and bending zone at her right ankle at the back of the foot and big toe (figure 2); this lesion had delayed healing.
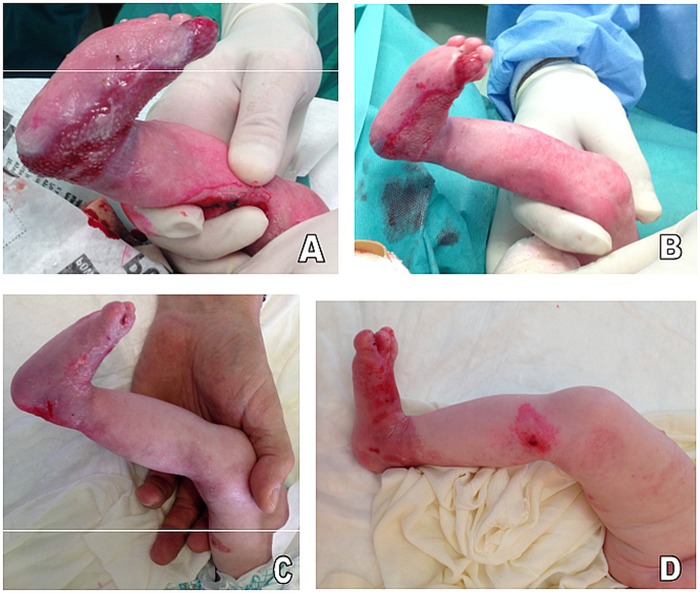
Figure 1.
(A) Lesion 1 at birth. Note the large and deep skin erosion on the shin that extends from the knee to the back of the foot and the consequent bent foot. (B) Partial re-epithelialisation of the wound after one cord blood platelet gel (CBPG) application (7 days from treatment initiation). (C) Healthy skin with some milia 4 weeks after the second CBPG application (see Results section). The red–pink marks on the knee and foot are residue from previously applied Eosin solution. (D) Slight erosion on the distal shin and lasting healing in the other skin areas 6 weeks after the last CBPG application.
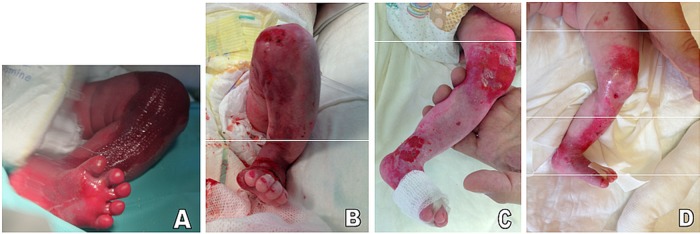
Figure 2.
(A) Lesion 2 at birth was a smaller exudating erosion in a friction and bending zone. (B) Partial re-epithelialisation after 1 week of treatment. (C) Healthy skin 4 weeks after the last cord blood platelet gel (CBPG) application. (D) Slight erosion on the medial side of the ankle. The red–pink mark on the back of the foot is residue from previously applied Eosin solution.
The CBPGs were prepared according to standard procedures as previously described.4 Briefly, a platelet concentrate of about 10 mL containing 1.5–2.0×106 platelets/µL was obtained by differential centrifugation of allogeneic cord blood units donated to the Milano Cord Blood Bank. The platelet concentrate was frozen and gelification was obtained after thawing by the addition of batroxobin (Plateltex, Praha, Czech Republic). Each CBPG was applied to the wound surface under sterile conditions, fixed with fine mesh paraffin gauze, and then bandaged to retain the CBPG in situ. The skin lesions were clinically evaluated and treated weekly with CBPG until completely healed. The CBPG was removed after 3 days, and the lesion site was fixed with clean paraffin gauze and a bandage by nurses. The lesions were cleaned with an antiseptic (Eosin staining solution) until the next CBPG application. All CBPG applications were preceded by paracetamol or morphine administration to prevent pain. Each lesion was photographed over the course of the study period, and any adverse events were noted.
Outcome and follow-up
Lesion 1 partially healed with a single CBPG application (figure 1). After the second week of treatment, the lesion completely resolved. Unfortunately, the same area was accidentally traumatised at a later time, resulting in the partial re-opening of the lesion at the knee. This lesion was then re-treated with two additional CBPG applications with good results. Lesion 2 was treated for 4 weeks, and evidence of re-epithelialisation was observed after the first two applications (figure 2). The lesion was completely resolved at the end of treatment (figure 2).
Both lesions were monitored weekly for 6 weeks after the last CBPG application. No significant relapses were observed during this period with the exception of two small bullous lesions on the left and right ankle, which arose roughly 6 weeks after treatment. Overall, the skin appeared healthy and had a well-structured corneal layer with only some milia appearing on the left leg. No further anaemisation, skin bacterial superinfection or other clinical problems were observed.
Discussion
This case report highlights new potentials in managing newborns affected by DEB. Specifically, the CBPG treatment was able to treat and prevent fluid loss and superinfections, and was also aesthetically and functionally beneficial for serious skin lesions. CBPG treatment appeared to be an effective and safe therapeutic option that positively contributed to our patient's clinical outcome.
Newborns with EB may present with localised or widespread blistering at birth or within the first few days of life.1 Currently, there is no cure for EB. Managing EB in newborns centres around preventing new blister formation and careful wound medication to ensure fast and lasting healing.2 Previous studies have found that autologous and allogeneic platelet preparations are safe and effective for treating skin lesions.3 4 Therefore, we decided to treat two large skin lesions on this newborn with EB using CBPGs to prevent or at least contain some of the possible wound complications. To the best of our knowledge the grafting of donor cells has never been reported from patients receiving topical treatment with allogeneic platelet gel. The lack of evidence in this regard is supported by the very small number of red blood cells and lymphocytes present in CBPG, and their total loss of viability following freezing at −80°C of the platelet suspension before thawing and activation of the platelet gel.
Before CBPG application, our patient experienced excessive bleeding and general fluid loss from the wounds, which caused an electrolyte imbalance and anaemia. The prompt resolution of her large lesions by the CBPG treatment helped control the bleeding and improved nourishment. This led to a slow but constant improvement in the newborn's health. Moreover, our patient also experienced skin superinfection, which is typical of DEB.1 Skin superinfection results in slower wound healing and, consequently, fluid loss. We used CBPG because of its antimicrobial activity.4 We hypothesised that, when combined with faster healing, applying CBPG would re-establish a complete skin barrier and prevent new infections. In our patient, no further infections were observed during and after the CBPG treatment.
Finally, we note that lesion 1 was positioned at a site where lesions are frequently observed in patients with EB since it is a position prone to friction in uterus.5 These deep skin ulcers, which are localised on the shin, often force the newborn's foot into a bent pose. Treatment is usually conservative early and may result in a retracting scar, which could require additional reconstructive surgery for aesthetic and/or functional reasons.2 Therefore, a fast and lasting resolution of this lesion type should be considered a priority when managing DEB in newborns. In our patient, the foot quickly returned to normal, and the skin appeared healthy after 6 weeks.
In conclusion, this case report indicates that CBPG is a promising and safe option for treating skin lesions in patients with EB. Further controlled studies are needed to confirm these preliminary findings and expand the use of CBPG in chronic wound management in newborns and infants.
Patient's perspective.
As parents, we were very happy on the clinical outcome of our daughter after CBPG treatment and we would like to thank the teams of Susanna Esposito, Fabio Mosca and Maurizio Marconi as well as Debra Italia Onlus (ie, the NGO dedicated to EB) for all their efforts.
Learning points.
Epidermolysis bullosa (EB) is comprised of a heterogeneous group of hereditary mechanobullous disorders that are characterised by extremely fragile skin and mucous membranes.
Patients with EB often develop blisters and non-healing wounds following minor trauma.
In infants, EB is associated with an increased risk of sepsis, and the loss of fluids and electrolytes from lesions.
Autologous or allogeneic platelet preparations have been shown to be useful for treating non-healing wounds in adults, but their efficacy in infants and children is unknown.
Platelet gel derived from allogeneic cord blood (cord blood platelet gel, CBPG) appears as a promising and safe option for the treating of skin lesions in patients with EB.
Acknowledgments
The authors would like to thank Debra Italy, the staff of the Milano Cord Blood Bank and Centro Nazionale Sangue, Istituto Superiore di Sanità, Rome, Italy, for their support in the project. Moreover, they would like to thank all those who gave their support to the cure of this patient: Sophie Guez, Pediatric Highly Intensive Care Unit, Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Maurizio Marconi and Noemi Greppi, Blood Transfusion Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy.
Footnotes
Contributors: All authors contributed to the planning, drafting, revising and final approval of the manuscript. SE is the guarantor.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.El Hachem M, Zambruno G, Bourdon-Lanoy E et al. Multicentre consensus recommendations for skin care in inherited epidermolysis bullosa. Orphanet J Rare Dis 2014;9:76 10.1186/1750-1172-9-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol 2013;37:32–9. 10.1053/j.semperi.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 3.Greppi N, Mazzucco L, Galetti G et al. Treatment of recalcitrant ulcers with allogeneic platelet gel from pooled platelets in aged hypomobile patients. Biologicals 2011;39:73–80. 10.1016/j.biologicals.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 4.Crovetti G, Martinelli G, Issi M et al. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 2004;30:145–51. 10.1016/j.transci.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Chiaverini C, Charlesworth A, Fernandez A et al. Aplasia cutis congenita with dystrophic epidermolysis bullosa: clinical and mutational study. Br J Dermatol 2014;170:901–6. 10.1111/bjd.12741 [DOI] [PubMed] [Google Scholar]