Abstract
Virchow-Robin spaces (VRS) are extensions of the subarachnoid space surrounding perforating blood vessels entering the brain parenchyma. VRS are fluid filled, but almost virtual and only visible on MRI of the brain when dilated. Such dilations are commonly asymptomatic. In rare cases, extreme dilations can be observed; the clinical repercussions of which remain unclear. We report the case of a patient presenting symptoms of normal pressure hydrocephalus due to extreme VRS mesencephalon dilations.
Background
Virchow-Robin spaces (VRS) are extensions of the subarachnoid space surrounding perforating blood vessels entering the brain parenchyma.1 VRS are fluid filled, but usually almost virtual and only visible on brain MRI when dilated. Dilations of VRS are commonly asymptomatic and of low significance. Extreme dilations of VRS are rare. Diverse symptoms have been described, depending on their localisation.2 3 Most frequently, radiological signs of hydrocephalus (with various clinical impacts) have been associated with cystic widening of VRS. We report the case of a patient presenting gait disorders and occasioning multiple falls, most probably linked with upstream hydrocephalus brought about by extreme VRS dilations.
Case presentation
A 74-year-old man was hospitalised for repeated falls, recently complicated by rib fractures and rhabdomyolysis due to prolonged periods of post-fall immobility on the ground. The patient had a history abdominal surgery, significant especially for appendectomy and partial colectomy in the treatment of diverticulosis. Intermittent urinary incontinence had begun to appear a few months earlier, and the patient described weakness in his right leg. In addition, the patient's daughter reported that progressive cognitive decline accompanied by memory loss had been developing for about 5 months.
The patient's body mass index was 28.4 kg/m2 (88 kg for 1 m76). Basic and instrumental activities of daily living were moderately impaired (B-ADL 3/6; IADL 5/8).
Physical neurological examination showed no significant sensory or motor deficit. Gait examination revealed that the patient was afflicted by an enlarged support polygon (with negative Romberg test) and discrete non-lateralised imbalance. There were no signs of Parkinsonism. Evaluation of oculomotricity was normal. Although gait capacities were relatively intact, fall risk was considered high as assessed by the Tinetti scale (21/28), as well as the timed get up and go test (22 s) and a recorded comfortable walking speed of 0.71 m/s. Neuropsychological evaluation highlighted discrete alterations in executive functions such as planning or inhibition control, but no significant impairment of memory storage. Minimental state examination was 28/30.
Investigations
The results of standard laboratory tests as well as echocardiography and holter-ECG monitoring all proved normal. Functional balance exploration through platform posturography and video nystagmography showed signs of vestibular omission and high visual dependency requiring re-education. Brain MRI revealed multiple fluid-filled cavities corresponding to extreme dilations of VRS, mostly located in the mesencephalon. These were causing compression on the third ventricle responsible for upstream hydrocephalus (figures 1–4).
Figure 1.
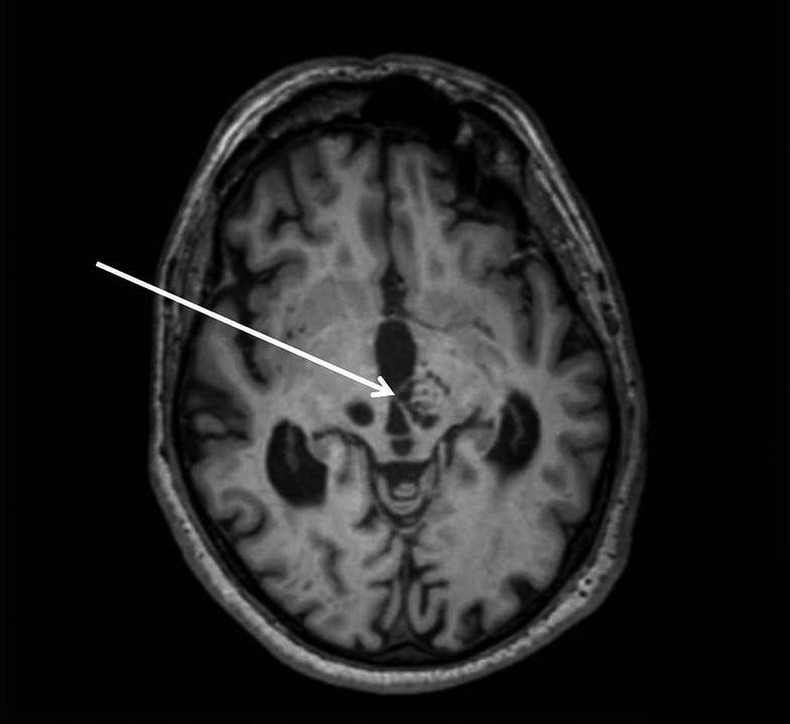
Axial T1-weighted MRI showing important kystic dilations of Virchow-Robin spaces in the mesencephalon. The arrow points to the compression on the third ventricle.
Figure 2.
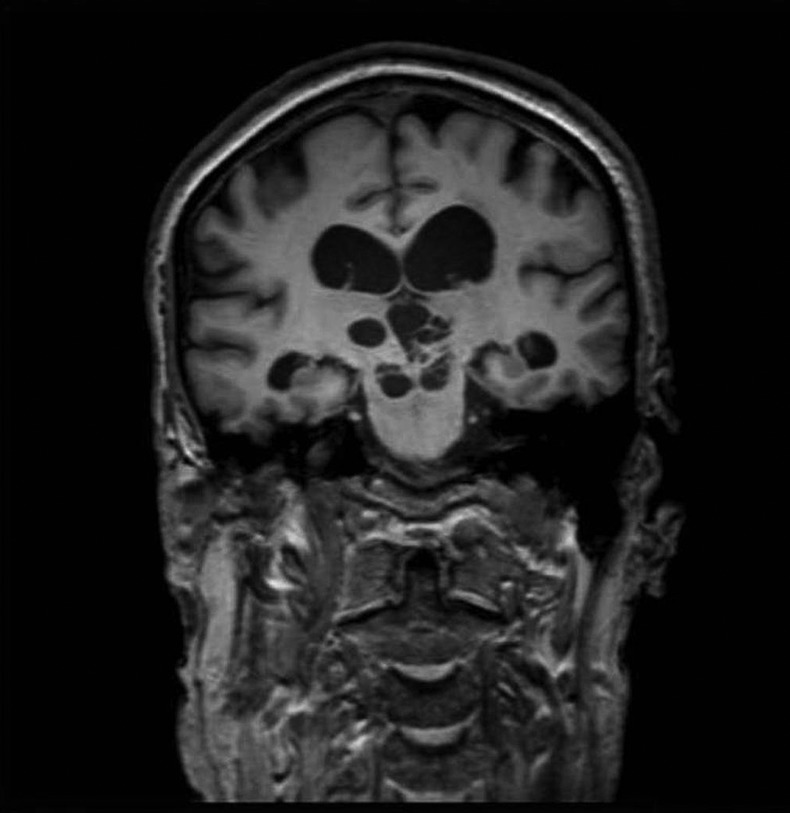
Coronal T1 MRI showing kystic dilations of Virchow-Robin spaces in the mesencephalic and thalamic regions of the brain, with signs of upstream hydrocephalus.
Figure 3.
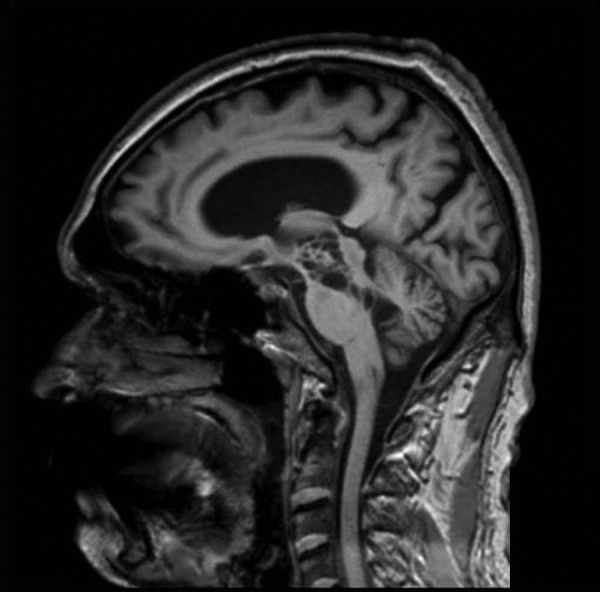
Sagittal T1 MRI showing the important dilations of Virchow-Robin spaces in the mesencephalon.
Figure 4.
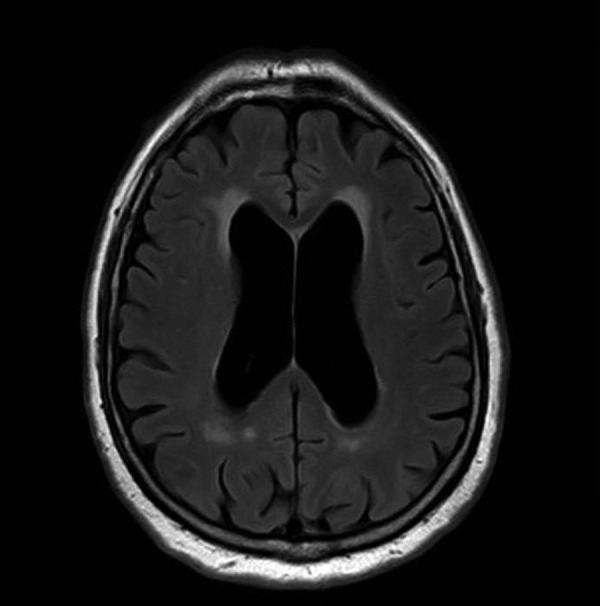
Axial fluidattenuated inversion recovery MRI showing the hydrocephalus with signs of cerebrospinal fluid resorption.
Differential diagnosis
On brain MRI, the fluid nature was confirmed as isointense to cerebrospinal fluid (CSF) on all sequences (hypointense fluidattenuated inversion recovery, T1 hypointense, T2 hyperintense, hypointense broadcast). Subarachnoid or squamous disease (hyperintense diffusion, not fluid), a neuroglial cyst, cystic tumours (no solid component) or an abscess (hyperintense diffusion, viscosity) could all be excluded. The typical anatomic location of VRS dilations (perivascular midbrain) helps to make the distinction with arachnoid cysts (middle cranial fossa, perisellar cisterns) or chronic lacunar infarctions (basal ganglia, thalamus, external or internal capsule, ventral pons, periventricular white matter).3 Clinically, there was no sign of infection or headache, no context of immunodeficiency pointing towards cryptococcosis. These factors all finally pointed towards the single diagnosis of VRS dilations.
With regard to the hydrocephalus, the patient presented all the symptoms of ‘Hakim's triad’—the association of gait disturbances, urinary tract incontinence and cognitive disorders involving alterations of executive functions that are typical of normal pressure—or idiopathic—hydrocephalus. These symptoms strongly suggested that the hydrocephalus observed on the MRI was responsible for the clinical presentation of our patient. Furthermore, the compression of the aqueduct pictured on figure 1 clearly excludes idiopathic hydrocephalus.
Treatment
As the patient was initially reluctant to go through investigations, we decided to introduce a diuretic treatment by acetazolamide and to limit ourselves to clinical and radiological observation. After a year characterised by failing sight with repeated falls, however, further support was proposed and accepted by the patient. An evacuation of 30 mL of CSF via lumbar puncture was performed, leading to swift amelioration of gait and balance. An improvement of cognitive functions was also obtained. Given these encouraging results, an indication of ventriculoatrial shunt was revealed, preferred to ventriculoperitoneal derivation given the history of abdominal surgery. The patient agreed to undergo an operation.
Outcome and follow-up
Immediate outcome after surgery was positive, despite a tendency towards hyperdrainage needing pressure adjustments on the valve. One month later, the patient presented with intense headaches, myoclonia of the head and neck, and dysarthria. CT-scan revealed signs of hyperdrainage with ventricular collapse and a subacute subdural haematoma of the left hemisphere. Surgeons proceeded urgent drainage of the subdural haematoma with the twist-drill technique and valve replacement.
The patient is currently in the rehabilitation ward with a good clinical evolution on gait and cognitive functions.
Discussion
VRS are virtual spaces surrounding short sections of perforating blood vessels as they enter the brain parenchyma.1 They are extensions of the subpial space, which is distinct from the subarachnoid space.4 5 Depending on their localisation, dilated VRS may be categorised according to three types: type I, which can be observed along lenticulostriate arteries towards the basal ganglia; type II, which are located in the cortex following the path of the medullary arteries; and type III, which are situated in the midbrain, surrounding the arteries from the diencephalon-mesencephalon junction.1 3 6 This latter type corresponds to the diagnosis of our patient. An alternative classification was established by Poirier et al7 based on neuropathological criteria (lacunas). This approach also classifies our case as type III.
Certainty of diagnosis can be confirmed by MRI.8 VRS dilations appear as fluid-filled cavities, isointense to CSF (ie, T1-hyposignal and T2-hypersignal). The commonest localisations are in the inferior third of the basal ganglia, in the mesencephalon, or in the hemispheric white matter in the subcortical regions of the convexity.8–10 Although dilations of VRS are often noticed on brain MRI, dilations as extreme as those observed in our case have rarely been reported.2 11 12 Poirier et al7 were the first to describe cognitive decline with similar cerebral lesions, presented in that instance by a 54-year-old patient. We can note that, for our patient, Alzheimer disease's biomarkers were found to be normal.
VRS dilations are most usually small and asymptomatic. As such, they are viewed as non-pathological anatomic variations. Dilations exceeding 2 mm are more common among the elderly, and generally associated with microcirculation alterations and subcortical atrophy.2 13 They may also be linked with cognitive decline,7 14 or strokes.15 Hydrocephalus appears to be the most frequent indicator of extreme VRS dilations, sometimes associated with local signs of cranial hypertension.2 6 9
The causative factors for VRS dilations are unclear.16 Several hypotheses have been put forward, including structural anomalies in blood vessel layers, focal arteritis,7 pulsating increase in CSF pressure, and accumulation of cerebral interstitial fluids.4 5 17 Impairment of the ventricular system's drainage capacities has been suggested as a possible mechanism for progressive increase in VRS dilations.18 Conversely, other authors consider these VRS dilations as a cause of hydrocephalus, due to local compressions altering CSF circulation and absorption. By reference to previously reported cases, the effects of surgical treatment (notably ventricular shunting) have been variable. Some cases record a diminution, while others a persistence of the VRS dilations.6 19
Treatment for symptomatic VRS dilations is not codified, and depends on the type as well as the localisation of the lesions. No specific management or monitoring is needed for minor dilations, even if they are numerous.6 Important dilations often require surgical treatment, especially in cases of badly-tolerated symptoms of cranial hypertension.20 Third ventriculocisternostomy is more commonly proposed than ventriculoperitoneal shunt. According to a recent meta-analysis, both methods are acceptable, but third ventriculocisternostomy is probably preferable.21
Learning points.
Virchow-Robin spaces (VRS) are virtual spaces surrounding the vessels in the cortex.
Giant VRS dilations are rare entities.
Hydrocephalus appears to be the most frequent indicator of extreme VRS dilations, but stroke or dementia can be observed.
Neuroimaging enables these anomalies to be identified, with confirmation of diagnosis performed by MRI.
The therapeutic management is not codified. It depends on the type and location of the expansion of VRS.
Acknowledgments
The authors thank Professor Marc Bonnefoy for his help in the preparation and reviewing of this manuscript.
Footnotes
Contributors: FR involved in the bibliography and elaboration of initial manuscript in French. TG involved in the translation of original French manuscript to English, contribution to elaboration of original manuscript and revision. MH involved in the reviewing. FC involved in the picture selection and description, contribution to the differential diagnosis section, reviewing.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ogawa T, Okudera T, Fukasawa H et al. Unusual widening of Virchow-Robin spaces: MR appearance. AJNR Am J Neuroradiol 1995;16:1238–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Caner B, Bekar A, Hakyemez B et al. Dilatation of Virchow-Robin perivascular spaces: report of 3 cases with different localizations. Minim Invasive Neurosurg 2008;51:11–14. 10.1055/s-2007-1022538 [DOI] [PubMed] [Google Scholar]
- 3.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics 2007;27:1071–86. 10.1148/rg.274065722 [DOI] [PubMed] [Google Scholar]
- 4.Adachi M, Hosoya T, Haku T et al. Dilated Virchow-Robin spaces: MRI pathological study. Neuroradiology 1998;40:27–31. 10.1007/s002340050533 [DOI] [PubMed] [Google Scholar]
- 5.Oztürk MH, Aydingöz U. Comparison of MR signal intensities of cerebral perivascular (Virchow-Robin) and subarachnoid spaces. J Comput Assist Tomogr 2002;26:902–4. 10.1097/00004728-200211000-00008 [DOI] [PubMed] [Google Scholar]
- 6.Marnet D, Noudel R, Peruzzi P et al. Dilatation des espaces périvasculaires de Virchow-Robin (Lacunes de type III): corrélations radio-cliniques. Rev Neurol (Paris) 2007;163:561–71. 10.1016/S0035-3787(07)90462-2 [DOI] [PubMed] [Google Scholar]
- 7.Poirier J, Barbizet J, Gaston A, et al. . [Thalamic dementia. Expansive lacunae of the thalamo-paramedian mesencephalic area. Hydrocephalus caused by stenosis of the aqueduct of Sylvius]. Rev Neurol (Paris) 1983;139:349–58. [PubMed] [Google Scholar]
- 8.Salzman KL, Osborn AG, House P et al. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol 2005;26:298–305. [PMC free article] [PubMed] [Google Scholar]
- 9.Kanamalla US, Calabrò F, Jinkins JR et al. Cavernous dilatation of mesencephalic Virchow-Robin spaces with obstructive hydrocephalus. Neuroradiology 2000;42:881–4. 10.1007/s002340000440 [DOI] [PubMed] [Google Scholar]
- 10.Rohlfs J, Riegel T, Khalil M et al. Enlarged perivascular spaces mimicking multicystic brain tumors. Report of two cases and review of the literature. J Neurosurg 2005;102:1142–6. 10.3171/jns.2005.102.6.1142 [DOI] [PubMed] [Google Scholar]
- 11.Lefranc M, Peltier J, Bugnicourt J-M, et al. . [Giant cystic widening of Virchow-Robin spaces, case report]. Morphol Bull Assoc Anat 2008;92:82–6. [DOI] [PubMed] [Google Scholar]
- 12.Stephens T, Parmar H, Cornblath W et al. Giant tumefactive perivascular spaces. J Neurol Sci 2008;266:171–3. 10.1016/j.jns.2007.08.032 [DOI] [PubMed] [Google Scholar]
- 13.Patankar TF, Mitra D, Varma A et al. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol 2005;26:1512–20. [PMC free article] [PubMed] [Google Scholar]
- 14.Maclullich AMJ, Wardlaw JM, Ferguson KJ et al. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 2004;75:1519–23. 10.1136/jnnp.2003.030858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pialat JB, Hermier M, Lion-François L et al. Acute mesencephalic stroke associated with dilated cystic perivascular spaces. Neurology 2005;65:648 10.1212/01.wnl.0000178167.96418.ff [DOI] [PubMed] [Google Scholar]
- 16.Derouesné C, Poirier J. [Cerebral lacunae: still under debate]. Rev Neurol (Paris) 1999;155:823–31. [PubMed] [Google Scholar]
- 17.Nishi K, Tanegashima A, Yamamoto Y et al. Histochemical characteristic of perivascular space in the brain with an advanced edema. Leg Med Tokyo Jpn 2003;5(Suppl 1):S280–4. 10.1016/S1344-6223(02)00150-5 [DOI] [PubMed] [Google Scholar]
- 18.Derouesné C, Gray F, Escourolle R et al. Expanding cerebral lacunae in a hypertensive patient with normal pressure hydrocephalus. Neuropathol Appl Neurobiol 1987;13:309–20. 10.1111/j.1365-2990.1987.tb00070.x [DOI] [PubMed] [Google Scholar]
- 19.Mascalchi M, Salvi F, Godano U et al. Expanding lacunae causing triventricular hydrocephalus. Report of two cases. J Neurosurg 1999;91:669–74. 10.3171/jns.1999.91.4.0669 [DOI] [PubMed] [Google Scholar]
- 20.Longatti PL, Fiorindi A, Carteri A et al. Expanding cerebral cysts (lacunae): a treatable cause of progressive midbrain syndrome. J Neurol Neurosurg Psychiatry 2003;74:393–4. 10.1136/jnnp.74.3.393-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottenhausen M, Meier U, Tittel A et al. Acute Decompensation of noncommunicating hydrocephalus caused by dilated Virchow-Robin spaces type III in a woman treated by endoscopic third ventriculostomy: a case report and review of the literature. J Neurol Surg Part Cent Eur Neurosurg 2013;74(Suppl 1):e242–7. 10.1055/s-0033-1349339 [DOI] [PubMed] [Google Scholar]


