Abstract
Native fluorescence, or autofluorescence (AF), consists in the emission of light in the UV-visible, near-IR spectral range when biological substrates are excited with light at suitable wavelength. This is a well-known phenomenon, and the strict relationship of many endogenous fluorophores with morphofunctional properties of the living systems, influencing their AF emission features, offers an extremely powerful resource for directly monitoring the biological substrate condition. Starting from the last century, the technological progresses in microscopy and spectrofluorometry were convoying attention of the scientific community to this phenomenon. In the future, the interest in the autofluorescence will certainly continue. Current instrumentation and analytical procedures will likely be overcome by the unceasing progress in new devices for AF detection and data interpretation, while a progress is expected in the search and characterization of endogenous fluorophores and their roles as intrinsic biomarkers.
Key words: Endogenous-fluorophores, energeticmetabolism, functionality-monitoring
Introduction
The native fluorescence, or autofluorescence (AF) is a widespread phenomenon, because of the common presence of intrinsic biomolecules acting as endogenous fluorophores in the organisms of the whole life kingdom. The strict relationship of many endogenous fluorophores with morpho-functional properties of the living systems, which are influencing their AF emission features, affords an outstandingly powerful resource for the direct monitoring of the biological substrate condition.
The attention of scientists to autofluorescence was accompanying the technological progresses in microscopy and spectrofluorometry starting from the 1900’s, and during the years, several articles and books reviewed the advancements in the investigation and use of AF in biological research and biomedical diagnosis.1-8 These AF applications are part of a wider range of analytical procedures based on the interaction between electromagnetic radiations and biological material, defined as Optical biopsy. This term is actually used to describe the possibility to obtain diagnostic information in situ, in a non-invasive or minimally invasive manner, without removal of tissue specimens and in the absence of exogenous markes.8-16
Given the huge number of AF based studies that makes a complete report of the overall studies almost impossible, in this review the history and technological progresses related to AF are briefly summarized, followed by the description of some selected topics of interest in biomedical research and diagnosis, implicating some experience of the authors.
Autofluorescence: a brief history
The emission of light from calcium fluoride and from the organic compounds quinine and chlorophyll in solution was reported early in 1838 by David Brewster in Scotland, and named fluorescence by George Stokes in Cambridge. As reviewed by Frederick H. Kasten,2 the scientific community offered increasing interest in fluorescence observations in biological compounds in parallel with the development of suitable instrumentation, leading to the set-up of the first fluorescence microscopes. Autofluorescence is reported, in 1911, as the first form of fluorescence observed at the microscope by Stübel, a physiologist at Jena University who investigated AF of single cells such as bacteria and protozoa, of animal tissues as in the teeth, and of various biological substances. In the immediate next years, several researchers paid attention to ashes, plant tissues and products, and the eye lens, and attempts were made to use AF to discriminate bacterial pathogens. In comparison with animal substrates, the plant endogenous fluorophores were found to give rise to much more appreciable emission signals, because of their more favorable spectral properties and quantum efficiency. As a consequence, AF was considered a powerful tool to study plant morphology and physiology,17-19 and many fluorochromes naturally present in plants such as quinones, coumarins, cyanines, tetrapyrroles and alkaloids were commercially extracted to be used as exogenous markers.
The availability of these fluorochromes, in addition to those provided by chemical synthesis or modification of natural substances to make them fluorescent (e.g., the biogenic amines treated with formaldehyde),20 led to an increasing development of highly selective and sensitive procedures for labeling subcellular components in cytology and histology.4 In this concern, an increasing number of new products is made available by a continuous search and synthesis activity, as from the numerous commercial catalogues of fluorochromes/fluorescent dyes.
In the application of exogenous and induced fluorescence-based assays, AF was often perceived as a nuisance, its signal resulting in a background which may hinder the specific detection of the exogenous marker emission. Different strategies were proposed to address this problem, for instance avoiding AF by proper optical filtering or its removal. The latter chance is important, in particular, when AF results in a wide wavelength range and/or high amplitude emission. AF background can be reduced by bleaching it through pre-irradiation, as successfully demonstrated to improve the DNA measurements after Feulgen staining in single cells, or the immunofluorescence labelling of the arterial vessels.21,22 The high photolability of vitamin A was also exploited to selectively eliminate its unwanted AF signal from Ito stellate cells during the in vivo analysis of NADH fluorescence in the liver.23 The signal from highly emitting endogenous fluorophores such as lipofuscins and elastin was also demonstrated to be reducible by chemical treatments before the staining procedures.24,25
The use of fixatives also deserved attention. Aldehyde derivatives, for example, are well known to undergo condensation reactions with amines and proteins generating fluorescent products.20,26 The consequent increase in the overall AF emission can thus affect the assays requiring fixation, in particular when specific fluorochromized biological probes (i.e., antibodies, lectins, receptor probes) are used to demonstrate the occurrence of targets which are present in very low amounts. In these cases, in the attempt to maximize the signal/background ratio, it can be suggested to use fluorophores with high quantum yield or with excitation/emission ranges longer than the blue region (which is the predominant one for most endogenous fluorophores). At present, the major chances to circumvent the AF adverse effects are actually offered by the improvement in the emission properties of exogenous fluorophores, in terms of quantum yield and of spectral position and narrowing to favor a selective optical filtering.
While representing a complication when cells and tissue are labeled with exogenous fluorochromes, AF by itself encompasses a great potential in the analysis of animal organs and fluids for both research and diagnostic purposes. Actually, the morphological and metabolic conditions of cells and tissues are strictly influencing the nature, amount, physico-chemical state, intra-tissue distribution and microenvironment of the endogenous fluorophores: therefore, the consequent changes in the emission properties of these biomolecules make them to act as intrinsic biomarkers. The whole AF emission signal is thus carrying comprehensive information suitable for the direct and real-time characterization and monitoring of physiological or altered morpho-functional properties of cells and tissues, in the absence of exogenous marker perturbations.
The first observations on the dependence of AF variations on tissue functional changes concerned porphyrins.27-29As reviewed by Kasten,2 porphyrins were observed to be present in different sites, from the sebaceous plugs of human hair follicles to the Harderian gland of rodents, and their red fluorescence was considered as diagnostically meaningful, since at that time porphyrins were not otherwise detectable at the microscope. It is worth recalling that a noticeable increase of porphyrin fluorescence was remarked in relation with both metabolic disorders and the presence of neoplasia,27,30 these observations being particularly predictive of the later development leading to the currently applied Photo-Dynamic Diagnosis and Photo-Dynamic Therapy (PDD PDT).31 In the same early times, a variety of vitamins (e.g., vitamin A, riboflavin, thiamin) and other substrates (such as lipofuscins, structural proteins, ceroid pigments) were identified as natural fluorophores by Herwig Hampler at the University of Vienna (Austria), who began the fluorescence microscopy applications in human pathology.2,4,32,33
At the end of the mid-20th century, particular attention was given to the coenzymes NAD(P)H and flavins, and to the monitoring of their redox equilibria in isolated mitochondria, single cultured cells and intact tissues under different metabolic conditions. These pioneering investigations by Britton Chance and his coworkers divided the period of sporadic AF observations on various kinds of biological substrates from the new way of systematic AF investigation in biomedicine, focused on specific targets and functions. The development of instruments such as the first microspectrofluorometers and flow cytofluorometers made these first studies possible, and the progresses in AF investigations were accompanied by the continuous advancement in the instrumental equipment and analytical procedures, allowing to transfer the results of experimental studies to the clinical application.34-42
In general, NAD(P)H and flavins are the almost unique responsible for the AF signal rising from the cell cytoplasm:43 this allows to perform investigations on cell redox metabolism without the need of supplementary analyses to distinguish them from other fluorophores. Figure 1 shows examples of AF distribution patterns in living cultured cells under different biological conditions.44
Figure 1.
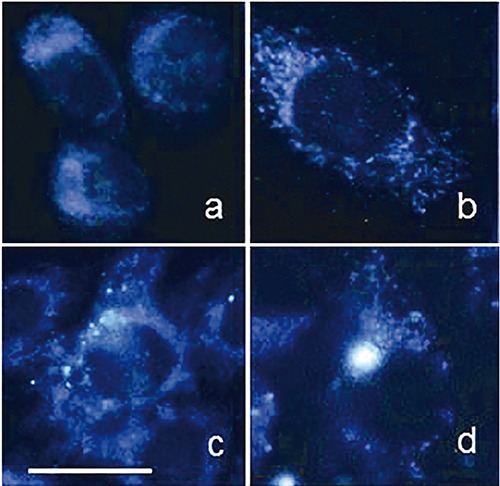
Examples of autofluorescence distribution patterns recorded from cultured, living pig cells. The emission signal rising from the cytoplasm around darker nuclei is mainly ascribable to NAD(P)H, the contribution of globular proteins being almost negligible under the conditions used for detection (exc 366 nm; em 420-600 nm). The signal is more diffusely distributed in stem cells (a) than in a mature one (b), where structures ascribable to the typical morphology of mitochondria can be easily recognized. These findings are consistent with a prevalent engagement in anaerobic energetic metabolism in stem cells, and in aerobic – mitochondrial – one in the mature one. Intracytoplasmic brightly fluorescing granules (c,d) are ascribable to lipofuscins, accumulated as undigested material from autophagic processes contributing to the maintenance of the stemness homeostasis or in cell eldering. Scale bar. 20 μm.
The continuously increasing interest in investigating tissues rather than single cells was accompanied by a progressive attention to additional fluorophores which may be found depending on the morpho-functional properties of the target substrates. The endogenous fluorophores mainly recurrent in biomedical AF-based studies are summarized in Table 1, and their representative spectral profiles are shown in Figure 2. The AF studies were thereafter focused on two main topics: i) monitoring of metabolic functions of cells and tissues under normal and experimental conditions, ii) in situ, real-time diagnosis of oncological and non-oncological diseases. Actually, the boundary between these two subjects of investigation cannot be clearly drawn, since a different extent of early and subtle metabolic changes as well as structural alterations of cells and tissues are likely to occur, as the first signs of disorders, degenerating diseases or neoplasia.45-47 Therefore, the ability of AF analysis to detect primary changes in cell and tissue morphofunctional properties is a promising potential for diagnostic applications. In this concern, AF analysis of cells and tissue properties fully belongs to Optical biopsy,15,48 its applications being developed to address and support different diagnostic procedures in biomedicine.
Table 1.
Endogenous fluorophores recurrently exploited as intrinsic biomarkers in autofluorescence studies.
| Endogenous fluorophores | Biological constituents | Autofluorescence (exc) / (em) ranges | Autofluorescence photophysical fingerprints and possible correlated alterations |
|---|---|---|---|
| Aromatic amino acids: Phe, Tyr, Trp | Functional proteins | (240-280 nm) / (280-350 nm) | Spectral shape and amplitude (near UV, blue region tail) |
| Cytokeratins | Intracellular fibrous proteins | (280-325 nm) / (495-525 nm) | Spectral shape and emission amplitude |
| Collagen/Elastin | Extracellular fibrous proteins | (330-340 nm) / (400-410 nm) (350-420 nm) / (420-510 nm) | Excitation light birifrangence effects spectral shape and emission amplitude, depending on maturation degree in eldering and fibrosis |
| NAD(P)H | Coenzymes of key enzymes in redox reactions | (330-380 nm) / (440, 462 nm, bound, free) | Spectral shape, emission amplitude |
| Flavins | Coenzymes of key enzymes in redox reactions | (350-370;440-450 nm) / (480/540 nm) | (NAD(P)Hbound/free, NAD(P)Htotal/oxidized flavins ratios, depending on aerobic/anaerobic energetic metabolism, antioxidant defense, inflammation, carcinogenesis |
| Fatty acids | Accumulated lipids | (330-350 nm) / (470-480 nm) | Spectral shape, emission amplitude and photosensitivity, depending on altered lipid metabolism |
| Vitamin A | Retinols and carotenoids | (370-380 nm) / (490-510 nm) | Spectral shape, emission amplitude and photosensitivity, depending on multiple functions including antioxidant and vision roles, and altered retinol metabolism |
| Protoporpyrin IX and porphyrin derivatives | Protein prostetic group | (405 nm) / (630-700 nm) | Spectral shape, emission amplitude and photosensitivity, depending on heme and iron altered metabolism |
| Lipofuscins/Lipofuscin like-lipopigments/ceroids | Miscellaneous (proteins, lipids, retinoids) | (UV, 400-500 nm) / (480-700 nm) | Spectral shape, emission amplitude depending on eldering, oxidation degree, cell stemness degree |
Figure 2.
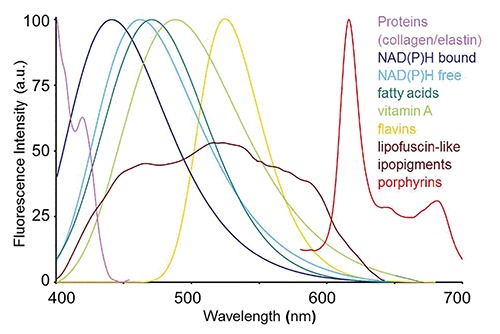
Typical spectral profiles of autofluorescence emission from single endogenous fluorophores. Spectra were recorded by microspectrofluorometry from pure compounds in solution, except for the fibrous proteins collagen and elastin and lipopigments, recorded respectively from connective tissue of hepatic portal selected areas of a liver cryostatic tissue section, or from remnant material collected after organic extraction of liver tissue homogenates. Spectra were normalized to the maximum emission peak for presentation, except for the broad emission of lipopigments. Excitation light: 366 nm.
Technologies and data processing
The advances in excitation sources, detection devices (including fiber optic probes for both excitation and AF light guide),49 and data analysis procedures promoted great progresses in the applications of Optical biopsy on tissue sites which can be either directly or endoscopically accessed, as recently reviewed by Alfano and Pu.15 The improvement in techniques and devices used for AF signal collection and analysis (such as spectroscopy, microspectroscopy and imaging) is continuously growing to improve the sensitivity and specificity in the detection of different fluorophores in single cells and tissues. As to the in situ imaging of endogenous fluorophores, the first applications of multiphoton microscopy allowing sub-micron resolution were followed by continuous progresses in the optical techniques to investigate the cell metabolism through the microscope.50,51 A careful choice of the Near InfraRed (NIR) excitation wavelength and power was however recommended, to preserve the reliability of the results obtained for living cells minimizing photobleaching and damages, and the undesired occurrence of photoinduced fluorescent granules containing lipofuscins.52,53 For example, suitable NIR measuring conditions resulted in an efficient, non-invasive detection of NAD(P)H and flavins in ratiometric redox fluorometry to assess in situ mitochondrial metabolic states.54 The two coenzymes NAD(P)H and flavins, along with lipofuscins and the second armonic generation from collagen fibers were also exploited as intracellular and extracellular exclusive sources of imaging contrast to monitor the differentiation of human mesenchymal stem cells in culture.55
In addition to the steady-state techniques, time-resolved AF contributed to improve the study of the respiratory chain functions as well as of different metabolic activities in cells and tissues under normal and altered conditions. These applications took advantage of the different fluorescence lifetimes characterizing the signal decay of NAD(P)H in its free and bound state, and of flavins, being respectively of about 0.4-0.5 ns, 2.0-2.5 ns and 6 ns.56-58 In this relationship, technical set-ups for direct lifetime detection and monitoring of tissue AF were proposed, aiming to improve routine biomedical and bio-analytical online analyses.59 Recently, a phasor approach has also been developed allowing an easier fluorescence lifetime data processing and interpretation, through a non-invasive, label-free, fit-free lifetime imaging microscopy technique, escaping the problems of exponential analysis to assess the presence of multiple fluorescing species. A graphical global view is given as an image, each pixel contributing as a point to the plot. The position of each point identifies a specific fluorophore depending on its typical decay properties, and a picture is provided allowing an overall and direct interpretation of data in terms of the fluorophores presented.60
As to the processing and diagnostic interpretation of AF data in single cells, the mere measurement of the overall emission signals allowed to detect and isolate granulocytes and in particular eosinophils by means of flow cytometric or more generally of microfluidic systems, the analysis of the spectral shape detected under different excitation conditions even leading to a complete discrimination of the leukocyte families.61-64 A multispectral imaging AF microscopy technique, based on the simple collection of monochrome images in the blue, green and red regions under 365 nm excitation, was also demonstrated to distinguish between non-neoplastic and malignant lymphoid tissues.65 In bulk tissues, complex multivariate analysis procedures were proposed to process overall emission data, and assess their potential for a specific and sensitive discrimination of normal from diseased areas.66-69
Nearly in the meantime, a device was developed, based on a high sensitivity camera to be applied at endoscopy to collect AF signals in the green and red regions, and to manage them in a ratio imaging by a processing unit to display then a color image.70 The validity of this apparatus as a support for the detection of lung and colon neoplasia resulted in the development of commercial devices, currently used in the clinics for pre-screening and diagnostic purposes through endoscopy. The first-generation Xillix® Laser-Induced Fluorescence Endoscopy (LIFE) (Xillix Technologies Corporation, Richmond, BC, Canada) received an FDA-PMA approval in USA in 1996, and the European CE Mark in 1997. A Xillix’s next device, Onco-LIFE, incorporating fluorescence and white-light endoscopy was then developed for an improved guide in the localization of lung and gastrointestinal cancers. The differences observed in AF emission between neoplastic and non-neoplastic regions were correctly foreseen to be ascribable to multiple alterations, in tissue metabolism, presence of porphyrins, blood circulation and histological architecture. Actually, progresses in the knowledge on the nature of single endogenous fluorophores present, their AF properties and their changes depending on both tissue structure and biological alteration, confirmed the suggestions of Takehana,70 helping the progression of optical techniques for diagnostic applications.11
The role of tissue optical properties, in terms of absorption, reflection and scatter were also considered, as factors influencing the propagation of both excitation light and the resulting fluorescence within bulk tissues, thus affecting the overall signal collected at the tissue surface. Monte Carlo simulation models were therefore developed, with particular reference to the neoplastic lesions in multilayered epithelia, to investigate and validate the dependence of the overall AF signal collected at the tissue surface on the contribution of the typical spectra of their biological components, their histological arrangement, localization depth and optical properties.71-76 In the colon, for example, the structural alteration induced by the rising of the tumor mass affecting the presence and localization depth of sub-mucosa was demonstrated as the main responsible for the marked decrease of the whole AF signal in neoplastic with respect to non-neoplastic tissue (Figure 3).77-79 These findings are explained considering the essential role played by submucosa, the tissue layer with a strong fluorescence emission because of the presence of collagen, and its involvement in AF excitation/emission depending on its localization depth: submucosa AF signal accounts for a great part of the whole AF emission detected at the tissue surface in normal mucosa, while it disappears following the submucosa derangement and loss induced by the rising of neoplasia. Monte Carlo simulation modeling procedures were also applied in non-epithelial tissues, as in the case of AF imaging of NADH and flavoproteins to map brain activity in cortical areas.80
Figure 3.
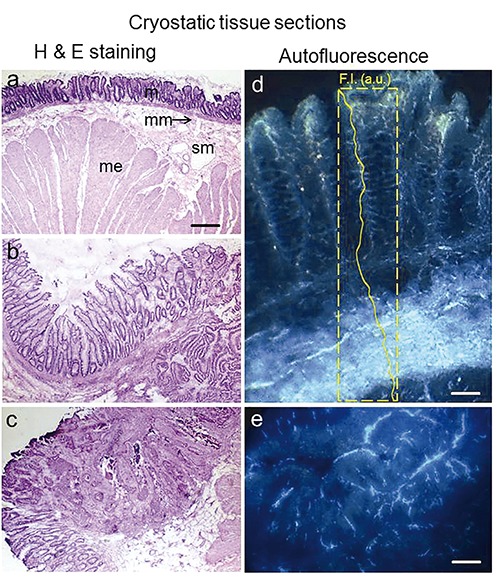
Unfixed cryostatic tissue sections from human colon, non-neoplastic mucosa (a) and adenocarcinoma at different staging (b,c), after Hematoxylin and Eosin staining. Non-neoplastic tissue (a) shows the layer organization: mucosa (m) at the inner surface, muscolaris mucosa (mm), submucosa (sm) with blood vessels and muscolaris esterna (me). The histological organization of non-neoplastic mucosa is subversed by the rising of the neoplasia. The highly fluorescing connective tissue in submucosa (d, and AF signal plot profile inside) is strongly affected, only some remnants being appreciable in the neoplastic mass (e). Excitation: 366 nm, emission: 420-640 nm. Scale bars: a-c), 650 µm; d), 100 µm; e), 170 µm.
Considering the differences in the spectral profiles of the endogenous fluorophores relevant for each kind of tissue, spectroscopy, spectrofluorometry and microspectrofluorometry provided a powerful support to imaging investigations.81 In this view, following the assessment of typical excitation-emission spectra of single endogenous fluorophores, excitation-emission matrices were defined, in the attempt to better select the measurement conditions and improve data interpretation in tissue spectroscopy.82 Analytical procedures such as spectral curve fitting or differential imaging were developed to detect the nature and estimate the relative amount of the fluorophores contributing to the whole AF emission of a cell or a tissue. An essential condition for the application of these procedures is obviously the definition of the spectral parameters for each single fluorophore considered in the biological substrate under investigation. Spectral fitting analysis is thus based on the use of functions representing typical emission profiles, as represented in Figure 4.83,84 It is worthwhile reminding that in the steady-state analysis of AF, the ability of the near-UV excitation to excite simultaneously the great part of endogenous fluorophores can be an acceptable compromise between a relatively simple and cost effective instrumentation for both spectroscopy and imaging studies, and the advantage to collect comprehensive data on the different fluorophores in the substrate under investigation. The data collection from a single measuring set can minimize or avoid the artifacts which may likely derive from photobleacing in sequential measurements of the same area when changing excitation/emission conditions, or from the sampling variability among different measured sites when investigating metabolic equilibria. This could be the case of NAD(P)H and flavins when studying energetic metabolism,34-44,53-56,80,84 or metabolism of biomolecules involved in altered lipid turnover and oxidative stress in fatty livers.85-87
Figure 4.
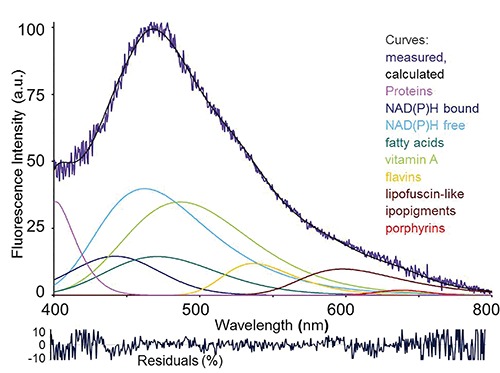
Example of fitting analysis of an autofluorescence spectrum collected via fiber optic probe from a rat liver under living conditions. Real measured spectrum and calculated curve as the sum of the endogenous fluorophore contributions are shown, along with spectral functions representing each endogenous fluorophore. Before analysis, spectra are normalized to 100% at the peak maximum. Curve-fitting procedure is then performed to evaluate the relative contributions of each fluorophore to the overall emission. The procedure consists in an iterative non-linear analysis, based on the Marquardt–Levenberg algorithm through the finding of the true absolute minimum value of the sum of squared deviations of a combination of GMG (half-Gaussian Modified Gaussian) spectral functions, each of them representing the emission profile of a pure fluorophore. The goodness of fitting is verified trough the residual analysis and r2 coefficient of determination, ≥0.898 in this case. Fitting parameters are reported as peak center wavelength (λ) / full width at half intensity maximum (FWHM): NAD(P)H free, 463 nm / 115 nm; NAD(P)H bound, 444 nm / 105 nm; vitamin A, 488 nm /102 nm; fatty acids, 470 nm / 90 nm; flavins, 526 nm / 81 nm. Due to parameter variability because of heterogeneity in composition and fluorescing properties, the functions of lipopigments and proteins are left free to adjust, respectively in the 530-600 nm range, and at λ <440 nm, to reach the goodness of fitting analysis results. Excitation: 366 nm.
NAD(P)H, flavins and energetic metabolism
Starting from the already reminded pioneering works by Britton Chance,34-39 NAD(P)H and flavins are up to now the most extensively investigated endogenous fluorophores, finding unceasing applications in biomedical research and clinics as dynamic biomarkers of energetic metabolism in single cells and in the monitoring of organ vitality and function. The properties of NAD(P)H and flavins are briefly reminded, considering their great role in AF based Optical biopsy. The NAD(P)H is fluorescent in the reduced state and flavins in the oxidized state, the AF emission properties being strictly dependent on the bound/free condition.88-90 These coenzymes are the main responsible for the AF rising from cell cytoplasm, their amount, and redox, bound-free state being in close relationship with their engagement in energetic metabolism, cell oxidative defense, reductive biosynthesis, and signal transduction.37,38,89,91-94
The flavin free derivatives, flavin mononucleotide (FMN) and flavin-adenin dinucleotide (FAD), exhibit very similar excitation/emission properties in aqueous solution (pH 7), their absorption/excitation and emission maxima being respectively at about 440-450 nm and 525 nm.95,96 The fluorescence emission from FAD, in turn, was reported to be strongly affected by the nature of the protein to which the prosthetic group is bound. Studies on isolated mitochondria indicated that, as to the flavoproteins engaged in energetic metabolism, α-lipoamide dehydrogenase (FP5) and electron transfer flavoprotein were the major responsible for the flavoprotein fluorescence signal in the cell, contributing respectively for about 50% and 25% of the total flavin signal. The remaining part was ascribed to the not better defined, dithionite reducible flavoproteins. The emission of FP5 was found to occur in the 510-550 nm region, while that of FP3 showed a maximum at about 480 nm.90,91
The fluorescence properties of NAD(P)H depend on the nicotinamide group, absorbing at about 365 nm and fluorescing in the 420-490 nm region. The AF spectral shape does not show appreciable differences between the two derivatives NADH and NADPH, in general the binding to enzyme molecules resulting in a blue shift, an increase in the quantum yield efficiency (the ratio between free and bound forms being of about 0.33), and a lengthening of the fluorescence decay time.40,56,88,89 While NADPH is engaged as an electron donor for reductive biosynthesis and antioxidant defense, NADH is mainly involved in reactions leading to energy production. Glycolysis and the citric acid Kreb’s cycle, taking place respectively in the cytoplasm or in the mitochondrial matrix, produce the reduced form of NADH, while NADH reoxidation occurs mainly through oxidative phosphorylation, resulting in ATP and heat production. The NADH inside mitochondria cannot diffuse across their membrane, the transport of the reducing equivalents from the cytosol to mitochondria being carried out by shuttle systems, ensuring an equilibrium between cytoplasm and mitochondria. Transhydrogenases, in turn, are enzymes able to transfer electrons from NAD(P)H to NAD(P)+ in a reversible reaction, contributing to the cell resources to maintain redox homeostasis. A simplified representation of the several interrelated metabolic pathways involving changes in the redox state of NAD(P)H and flavins is shown in Figure 5. Such complex situation can explain apparently conflicting results on the ability to induced changes in the redox state of NAD(P)H and flavins by drugs acting on mitochondria. For instance, blocking the respiratory chain by means of potassium cyanide is expected to produce an increase in the NAD(P)H fluorescence intensity and, conversely, a decrease in flavin emission. Yet, evidences inconsistent with this assumption were reported,41 leading to define bistable the reciprocal changes in the NAD(P)H and flavin reciprocal redox state, in dependence of the metabolic steady state of the cells undergoing metabolic alterations.89,97 Despite this complexity, these coenzymes were successfully used as intrinsic biomarkers of cell energetic metabolism, consistently with the proposal of Huang,54 to represent the redox – fluorescent state of the cells by means of a very simplified equation:
where FP5 belongs to the dehydrogenase multienzyme complex catalyzing the electron transfer from pyruvate to NAD+, as a point of electron entry in the respiratory chain. In addition to the huge amount of studies in single cells, NAD(P)H and flavins were investigated as dynamic biomarker of energetic metabolism in tissues and organs.
Figure 5.
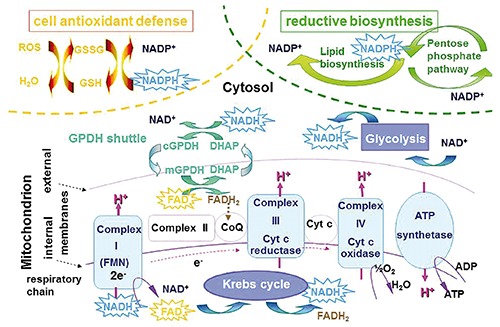
Simplified scheme of the main metabolic pathways affecting the redox state of NAD(P)H and flavins, the coenzymes participating to the reactions as reductive equivalent donor /acceptors. The star frames indicate the fluorescing reduced form of NAD(P)H and oxidized form of flavins.
Studies on pancreas, demonstrated the AF suitability to monitor the response to anoxia in the assessment of progressive damage after pancreatitis or transplantation.98 The particular metabolic engagement of pancreatic islet cells and their implications in the development of diabetes inspired NAD(P)H and flavin based AF studies aimed both at improving knowledge on functional mechanisms and at facilitating the flow cytometric sorting and enrichment of pure beta cell populations for transplantation.99-101 More generally, several experimental studies on different organs such as heart, kidney and liver were stimulated in order both to attain an effective real-time functional monitoring and to support investigations on strategies aimed at assessing and facing possible injuries from ischemic conditions during surgery or transplantation.102-107
An in vivo multiparametric assessment of tissue and organ dysfunctions under pathological states was also proposed, based on the measurement of whole NAD(P)H AF emission, together with oxyhemoglobin (Hb-O2) and blood hemodynamics.42 In addition, it is to remind that the spectrofluorometric, real-time monitoring of the tissue redox state allowed to detect and explain transient events, such as a temporary NAD(P)H oxidation accompanied by an increase in the NAD(P)Hbound/free ratio observed soon after the induction of liver ischemia. This phenomenon, opposite to an expected increase in the NAD(P)H reduced state after the block of its reoxidation through the respiratory chain in the absence of oxygen, was explained with a physiological compensatory vasodilatation attempt of liver microvasculature to respond to ischemia.108 Recently, the in vivo analysis of AF in a rat liver model of lipid accumulation made it possible to assess the maintenance of energetic metabolism homeostasis under oxidative stress. This effort was not detectable ex vivo, while the combined AF and biochemical analysis of tissue samples confirmed the expected alteration in mitochondrial activity.87 These findings further confirmed that changes in biological conditions can affect the cell engagement in aerobic and anaerobic energetic metabolism. In this respect, an example is given by recent AF-based studies on the metabolic plasticity of stem cells. This phenomenon entails the metabolic signatures of the stemness status, consisting in the switch from anaerobic to aerobic respiration during differentiation, the opposite occurring during the reprogramming of somatic cells to pluripotency.109 The consequent changes in AF emission at to both signal amplitude and spectral shape are mainly ascribable to alterations in the relative presence of bound and free NAD(P)H, as demonstrated by numerous works performed using different optical systems on single cultured cell and living tissues.44,55,110-113 The usefulness of AF for a comprehensive analysis in the assessment of the actual stem cell differentiation degree has been thus proved, the exclusive advantage to avoid undesired stimuli from exogenous-markers being of particular value when the very delicate metabolic equilibria of stem cells are investigated for regenerative medicine and zootechnology applications.
Apart from these recent studies, a prevalence of anaerobic on the aerobic energetic metabolism is known since a long time to characterize tumor cells, as early remarked by Warburg in 1956.114 This condition, likely favoring survival and invasiveness of tumor cells, results in general in low values in the NAD(P)Hbound/free ratio in neoplastic cells with respect to the non-neoplastic ones. These AF results have been justified by a favored pentose-phosphate pathway, reflecting a possible impairment of mitochondrial respiratory activity and involving fewer steps of interaction between the pyridinic coenzymes and enzymes as compared to the aerobic metabolism.89,115-118 This suggestion confirmed the strict relationship between the AF properties of cells and their actual metabolic engagement, consistently with the finding that the NAD(P) Hbound/free ratio may vary among cancerous cell lines, while remaining constant in a cell line under standard metabolic conditions.119
In solid tumors, the cell switch from the aerobic to the anaerobic energetic metabolism can contribute at a different extent to the whole AF signal alteration, depending on the tissue considered. In general, the effects of energetic metabolism are of minor diagnostic importance than tissue architecture in multilayered epithelia. In fact, as already described above, the growing of the neoplastic mass affects the integrity and localization depth of the highly fluorescing submucosa, resulting in a marked decrease of its contribution to the whole AF signal collected at the tissue surface.66,70,71,74,75,77-79 On the other hand, in the central nervous system the subversion of the energetic metabolism was demonstrated to contribute to the discrimination between brain tumor mass (in glioblastoma) and the non-neoplastic surrounding tissue.120,121 A role of the metabolic engagement was demonstrated by data from tissue sections, showing an alteration in the NAD(P)Hbound/free ratio accounting for both the decrease in amplitude emission and the red shift of AF spectra in the tumor with respect to the normal tissue. In vivo measurements performed via fiber optic probe during surgery, in turn, showed much higher differences between the neoplastic and non-neoplastic tissue (Figure 6). These findings indicated also a role of the tissue optical properties, since changes in blood supply and in architectural organization, leading to a markedly increased cellularity in the tumor mass, can alter absorption, reflectance and the scatter phenomena, influencing the migration of light, and the yield of excitation and fluorescence collection from different tissue depths.9, 122 The dependence of AF signals on both structural and physiological properties of brain areas, and their sensitivity to hemodynamic changes induced by sensory activation were further demonstrated by imaging studies of NADH and flavoproteins.80
Figure 6.
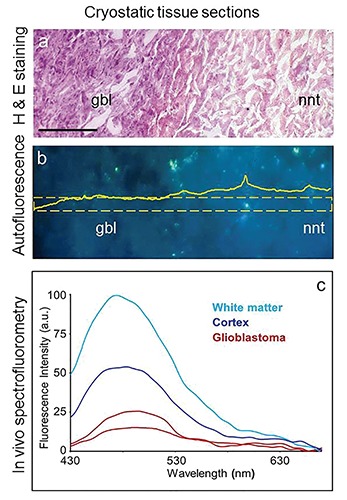
Unfixed cryostatic brain tissue sections from a glioblastoma bearing patient. a) Hematoxylin and Eosin staining evidences an altered stainability and cell density in neoplastic (glioblastoma, gbl) tissue as compared to the surrounding non-neoplastic tissue (nnt). b) Autofluorescence imaging of a serial, unstained tissue section, shows a lower emission signal in neoplastic as compared to the non-neoplastic area; a quantitative representation of this difference is given by the amplitude distribution profile of the selected area indicated by the frame overimposed to the picture. Excitation: 366 nm; emission: 420-640 nm; scale bars: 200 µm. c) Spectrofluorometry performed in vivo, via fiber optic probe during surgical operation showed an even more marked lower autofluorescence amplitude, and spectral profile changes in neoplastic tissue in comparison with the non-neoplastic tissues (white matter and cortex); spectra are shown as real values.
More generally, the importance of the cell metabolic engagement combined with tissue organization and cellularity was supported by different cases, concerning for example leukocytes becoming resident and accumulating in tissues with inflammation, or lymph node alterations in lymphoproliferative disorders affecting AF signals;65 similarly, it was possible to discriminate neoplastic degeneration or to detect in situ heart allograft rejection.123,124
Lipofuscins and lipofuscin-like fluorophores in aging, oxidative damage, and more
In addition to the diffuse fluorescence of NAD(P)H and flavins, brightly emitting particles ascribable to lipofuscin or lipofuscin-like lipopigments can be occasionally observed in the cell cytoplasm.
Lipofuscin fluorescence covers the yellow-reddish region, the spectral shape and emission amplitude being dependent on the variability in composition (proteins, lipids, carotenoids), crosslinks and oxidation degree of these heterogeneous compounds, and ageing.125 In general, lipofuscins consist in undigested material remaining from phagocytosis and autophagy processes, accumulating as intracytoplasmic granules depending on physiological cell metabolic engagement, and the occurrence of disorders and pathologies.115,126-130 The physiological intracellular accumulation of lipofuscins in ageing is commonly reported in the liver (see below), or in central nervous system. In the latter case, an anomalous increase in the presence of lipofuscins or lipofuscin-like lipopigments or ceroids has been detected as a consequence of oxidative stress, as a response to pathological conditions (i.e., the neuronal ceroid lipofuscinosis or Batten disease), or to toxic compounds.131-133 In this concern, the alteration of the postnatal development of the multilayered rat cerebellar cortex induced by cisplatin administration resulted in an increase in the lipofuscin emission signal and alterations in NAD(P)H and flavin contributions.132 Actually, cerebellum during development is known to be particularly vulnerable to toxic compounds and radiation, and these findings in response to the cisplatin oxidative damage confirmed that AF-based studies are especially appropriate to investigate the oxidative effects of chemical and physical agent.134
The presence of lipofuscins is inversely correlated with the differentiation degree in stem cells.44,55,113 This phenomenon has been ascribed to autophagic processes actively engaged in the removal and oxidation of undesired subcellular structures, with particular reference to mitochondria. Regulating the number of mitochondria would preserve the prevailing anaerobic energetic metabolism of stem cells, thus contributing to the stemness maintenance.135 The accumulation of lipofuscin in stem cells should therefore be ascribable to the action of reactive oxygen species on autophagocytosed mitochondria, rather than to the physiological ageing processes.
A particular case of brightly fluorescing intracellular granules in eosinophils was reported by Grossi and Zaccheo136 already in 1962. This AF emission was later ascribed at least in part to FAD, and found to undergo a further increase in amplitude and shift from the blue to the yellow region when the circulating cells become resident in the tissues.61,137,138 The relatively high AF emission of eosinophils was more recently exploited to isolate the much less fluorescent neutrophils by flow cytometric cell sorting, to generate a nearly pure quiescent cell population in an antibody-free environment, an essential condition for subsequent activation studies.139 Another particular case of remarkable intracellular AF emitting granules concerned the pigment cell components in the liver of some amphibians, likely involving melanin in addition to lipofuscin-like products. Alterations in both emission amplitude and spectral profile reflected variations in the composition of these pigment constituents in relation with summer activity and winter hibernation cycles, suggesting an engagement to compensate changes in oxidative conditions depending on the seasonal liver plasticity.140,141 A strong yellow fluorescence rising after UV irradiation was also demonstrated to be induced by peroxidation of melanins naturally present in different tissues, improving the possibility to distinguish them from lipopigments,142 while studies on synthetic melanins demonstrated that the emission signal depends on modifications in the composition and polymerization degree of indolic components.143 These findings opened wide-ranging perspectives for a further development of optic-based diagnostic clinical applications, for example in clinical dermatology as well as in the environment monitoring through sentinel wild animals.
Finally, the importance of lipofuscins and retinoid derivatives accumulating in retinalpigment-epithelial (RPE) cells as residues of undigested outer segments of photoreceptors is here only reminded, as compared with the huge amount of work carried on about their AF characterization as to etiology, diagnosis and monitoring of retinal diseases.144-146
Porphyrins and heme metabolism alteration
In general, the emission spectra from the above described fluorophores cover the whole visible range, the contributions in the red region consisting in the low, longer wavelength tails of the emission spectra. However, distinct and well defined emission bands beyond 600 nm can be detected, that can be ascribed to porphyrins and their derivatives.147
The porphyrin chemical structure is based on a tetrapyrrolic ring, the most frequently naturally recurrent protoporphyrin IX (PpIX) being the ultimate precursor before iron insertion along the biosynthesis pathways of the heme group. This latter is an ubiquitous molecule, involved as a prosthetic group in proteins and enzymes providing to functions ranging from transport of oxygen to catalysis and pigmentation. Heme group is produced following a series of reactions, and can induce a feedback-inhibition of the key enzymes involved: ᵟ-aminolevulinate synthase, ᵟ-aminolevulinate dehydrase and ferrochelatase.
The red emission from iron-free, heme-based compounds was among the early reported observations of natural fluorescence.2 In 1924, the occurrence of red fluorescence under ultraviolet light illumination was described in an experimental rat sarcoma and correctly attributed to the presence of endogenous porphyrins.27 In 1929 the detection of porphyrin fluorescence spectra in the tissues from a patient who died for congenital porphyria was one of the first examples of AF microspectrofluorometric assay.30 It cannot be neglected that the current PDD and PDT procedures were initiated by these observations, followed by the early experiences on the first hematoporphyrin derivatives, obtained from the blood as iron free products to be administered as exogenous compounds.148 These derivatives showed a preferential accumulation in tumor tissues, where they were able to activate photosensitizing phenomena under suitable light irradiation.149-151 Following the first indications by Samuel Schwartz on the heterogeneous composition of porphyrin derivatives, mixtures enriched in their aggregated species which are mostly responsible for the selective localization in target tissues (e.g., Photofrin) or specific chlorine derivatives (e.g., Foscan and Verteroporphyrin) were produced to improve PDD and PDT efficacy, resulting in preparations approved for the clinical use.31,147,152-156
The complex chemical composition of porphyrin preparations make their use in PDD and PDT difficult; in addition, porphyrins proved to be less selective than expected for tumor cells, while exhibiting low tissue depth photoactivation and inducing prolonged skin photosensitivity. As a consequence, several other photosensitizing molecules were proposed. Few of them, however, received market approval, while successful parallel research demonstrated the usefulness of ᵟ-aminolevulinic acid (ALA) or its chemically modified derivatives, as pro-drug precursors of PpIX.156-159
Although a contribution from bacteria could not be completely excluded, an increase in porphobilinogen deaminase and a decrease of ferrochelatase activities participating to heme synthesis in tumor cells explained the selective accumulation of PpIX in the neoplastic mass following the administration of ALA precursors.160 The same enzymes are likely responsible for the systemic accumulation of endogenous porphyrins in the absence of prodrug administration in subjects bearing tumors or metabolic disorders. While the increased presence of porphyrins in tumor tissue contributes to the above mentioned imaging ratio analysis for in situ detection of tumors,70 the increase of PpIX in the blood has been proposed to support the differential diagnosis of hidden tumors or altered metabolism.161-165 In this respect, AF imaging and microspectrofluorometric studies on a MMTV-neu (erbB-2) female C1 mouse, model of spontaneously growing mammary tumor,166 showed a systemic marked increase of PpIX and porphyrin derivatives along with their rising in the neoplasia, in particular in the necrotic mass, (Figure 7).167-169 The rising in PpIX fluorescence in the serum, however, could not be completely explained by a reversed activity of ferrochelatase removing iron from heme in death tissue,168 and by the blood porphyrin drainage from the tumor. In fact, the iron scarcity and porphyrin increase observed in particular in the spleen (the organ normally engaged in heme catabolism and iron recycling) supported the role of a systemic subversion of metabolism in tumor bearing subjects induced by the presence of tumor cells requiring this element to face their metabolic requirements.169,170
Figure 7.
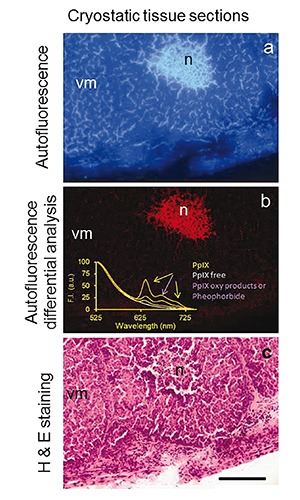
Unfixed cryostatic tissue section from a mouse mammary tumor. Autofluorescence image collected at t = 0 s of excitation-light irradiation from (a), and the distribution pattern of the signal lost (b), detected through the differential analysis of images recorded from the same tissue area at t = 0 s and t= 10 s of continuous irradiation; excitation: 330-385 nm; emission <420 nm. The lost signal representing the most photolabile fluorescing species occurring in this tissue is ascribable to porphyrins. The presence of PpIX, in particular, is confirmed by the spectra recorded under 405 nm excitation from the same tissue area, showing its typical emission bands centered at ≈ 630 nm and 700 nm. The band at about 670 nm is ascribable to pheophorbide or porphyrin oxidative products. The PpIX band amplitude is consistent with the low diffused signal in the vital mass and the much higher emission in necrosis, identified when the measured area has been retrieved after conventional Hematoxylin and Eosin staining (c), for a direct comparison with autofluorescence distribution in tumor living cell mass (vm) and necrosis (n). Scale bar: 100 µm.
Miscellanea of endogenous fluorophores in liver disorders, and more
In mammalian livers, lipofuscins resulting from the ageing processes are of no real pathological importance, while already at the beginning of the 1940’s a ceroid material with fluorescence properties was observed to accumulate in experimental cirrhotic rat livers, and ascribed to degradation products of unsaturated fatty acids.2,171,172
The nature of the lipofuscin-like lipopigments as peroxidized products of lipids and other intracellular macromolecules was supported by later investigations, even leading to their proposal as AF biomarkers of oxidative damages induced in livers with lipid accumulation and oxidative stress.87,127,173,174 Actually, an increasing number of endogenous fluorophores has been considered for comprehensive AF based diagnostic applications in hepatology, depending on the complex biochemical composition characterizing the liver tissue due to its engagement in several metabolic and detoxificating functions.84-87,175 In addition to NAD(P)H, flavins and lipofuscins, the overall liver tissue AF emission can originate from proteins, vitamin A and fatty acids likely playing a role as interconnected autofluorescent biomarkers of liver disorders. Under normal conditions, vitamin A accumulates mainly in stellate cells, the protein emission rising mainly from cytoskeletal and fibrous components, namely cytokeratins and collagen. This latter, in particular, is present in connective septa, i.e. the portal space, and in the delicate connective matrix of perisinusoidal reticular fibers.23 The collagen AF contribution increases when stellate cells are activated by inflammation messages, becoming fibrogenic and losing their role as vitamin A deposits during development of liver fibrosis,46 the emission amplitude and spectral shape being strongly dependent on the type, network density and ageing of this extracellular protein.176,177 The ability of AF analysis to detect such alterations in tissue organization was demonstrated by means of different approaches, such as multiphoton analysis and intravital microscopy in rodent models of developing cirrhosis or toxininduced fibrosis,178,179 and the role of collagen as early autofluorescent biomarker of developing fibrosis was further supported by ex vivo AF characterization of human liver specimens.180
Vitamin A was also proposed as an autofluorescent biomarker directly related to the content of lipids within hepatocytes; this was consistent with previous findings of a direct relationship between the changes in the amount and distribution of the vitamin A emission signal and lipids, as demonstrated by in situ Nile red fluorochromization performed on the same tissue section after AF analysis.175,180
The indications on the presence of vitamin A and fatty acids were obtained exploiting the different sensitivity to the light irradiation exhibited by the various endogenous fluorophores. Actually, photolability can be a further parameter improving the reliability of the information provided by both imaging and spectrofluorometry. In the normal liver parenchyma, continuous irradiation results in the decrease of the blue emission from the cytoplasm of hepatocytes, mainly due to the photobleacing of NAD(P)H, their anastomozing plates being bordered by a more photoresistant bright network ascribable to reticular fibers. In fatty livers the strong photolability of vitamin A and fluorescing fatty acids helped to demonstrate their localization in lipid vesicles, highlighted by means of differential imaging and microspectrofluorometric analysis, similarly to the procedure already described for porphyrins (Figures 8 and 9).
Figure 8.
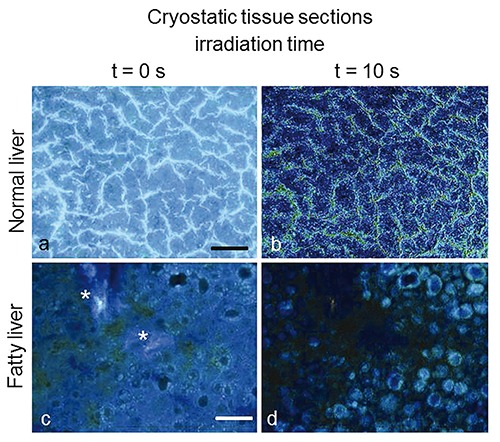
Normal (a,b) and fatty (c,d) liver from rat models. Autofluorescence images collected at t = 0 s of excitation light irradiation from unfixed, unstained cryostatic tissue sections (a,c), and topographical distribution of the whole signal lost (b,d), obtained through the differential analysis of images recorded from the same tissue area at t = 0 s and t= 10 s of continuous irradiation. In the normal liver a signal loss occurs mainly along sinusoids, likely involving vitamin A accumulated in Ito cells. Fatty liver shows marked signal decrease within vesicular structures likely corresponding to lipid droplets, while the bright light blue fluorescing connective component (*) observed at t = 0 s of irradiation undergoes a much lesser decrease. Image levels are adjusted to optimize image observation. Excitation: 366 nm. Scale bars: a), 80 µm; c), 100 µm.
Figure 9.
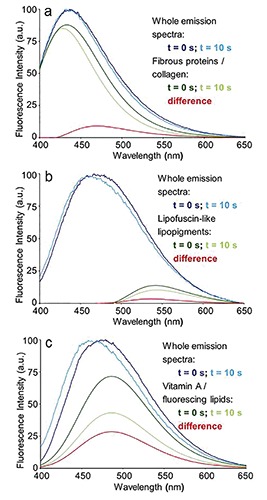
Autofluorescence emission spectra recorded at t = 0 and at t = 10 s of irradiation from unfixed, unstained cryostatic sections of rat livers: connective tissue of a portal area (a) and lipofuscin granules (b) from a normal liver parenchima, and lipid droplet (c) from a fatty liver. The changes induced in the whole emission profile by light irradiation are evidenced by the measured spectra normalized to the peak maximum. The response to irradiation in terms of spectral shape and amplitude changes in the AF emission from collagen, lipofuscin-like lipopigments and vitamin A/fluorescing lipids is represented by the curves of each single fluorophore spectral function. Excitation: 366 nm.
Autofluorescence properties can be exhibited also by some lipid compounds, depending on their molecular properties. Up to now, few indications have been provided on AF emission properties and high photolability of some fatty acids such as arachidonic, linoleic and stearic acid, while other derivatives such as butirric and palmitic acid, triglycerids and phospholipids are in general poorly fluorescent.84,86,87,175 This apparent limitation in the AF detection of lipids, however, may give indications on induced alterations in the fluorescent fatty acid pool. In fact, despite the complex liver lipidic composition and its dependence on several metabolic pathways, a rising in fluorescing fatty acids was indicated by spectral fitting analysis of AF signals recorded from livers submitted to experimental warm ischemia. The phenomenon was suggested to reflect an altered balance of acylation and deacylation rates, rather than of fatty acid β-oxidation, the ultimate steps of this energy production pathway requiring oxygen.86,181,182
As to diagnostic potential of AF in hepatology, promising advancements concern also bile composition and its dependence on liver functionality. Bilirubin is excreted in the bile as the breakdown product of systemic and intrahepatic heme degradation. Bile optical properties were first mainly investigated to set-up assays for its evaluation in the blood, with the aim to improve diagnostic and therapeutic procedures to face hyperbilirubinemia.183-185 More recently the bichromophore nature of bilirubin has been found to influence its AF amplitude and spectral profile, characterized by two main emission bands centered at about 517-523 nm and 570 nm. The phenomenon is due to an intramolecular energy transfer consequent to an exciton splitting phenomenon between the two chromophore groups composing the bilirubin molecule, consisting in two non-completely symmetric moieties each of them containing two pyrrole rings. The alterations in the molecule conformation depending on microenvironment will thus affect the yield of bilirubin intramolecular energy transfer, reflected by different extents of changes in its emission properties. The phenomenon, particularly favored by the near-UV excitation, is thus making bilirubin along with other bile components (proteins and biliary acids, fluorescing in the blue region) to act as sensitive intrinsic biomarkers of bile composition, and consequently of liver function.85,86
Being aware of the extensive applications based on several endogenous fluorophores as intrinsic diagnostic biomarkers in biomedicine, only few more examples are briefly reported. As to the extracellular structural proteins, apart the numerous studies on the diagnostic importance of the collagen-rich submucosa affecting the whole AF signal recorded at the surface of multilayered epithelial tissues,70,74,75,77-79,186 early spectroscopic characterization of laser induced emission signals from atherosclerotic aorta were followed by the development of multiphoton analytical procedures for elastin and collagen in situ analysis.187-190 In the case of collagen, as well as of other supramolecular periodically organized fibrous proteins with a non-centrosymmetric molecular structure, it is also to underline that multiphoton analysis offers the possibility to detect the Second Harmonic Generation (SHG), that is the light emission at half the incident laser wavelength.190,191 These studies paved the way to renewed histological analysis for diagnostic applications through in situ three-dimensional visualization in intact tissues.192-194 It is worth mentioning also the possibility to detect the accumulation of advanced glycation end-products in the skin, given by simple and non-invasive spectroscopy as a supportive method in the diagnosis and control of aging and of the glycemic complications in diabetes.195,196
At last, the UV absorption properties of monoamine-aromatic neuromediators stimulated the development of different and sophisticate strategies exploiting their AF properties for their detection and monitoring in living cells and in the central nervous system.197,198 Three-photon excitation microscopy, for example, allowed to measure and monitor the distribution and content of neurotransmitter granules within viable cells, providing a support to more conventional extracellular secretion assays, e.g. carbon-fiber amperometry.199,200 In particular, the possibility provided by AF analysis to differentiated sequestered from unsequestred serotonin indicated an otherwise hardly detectable non-linear relationship between the two fractions, a supplementary factor of particular value in differentiation and drug response studies of serotoninergic cells.201 In this respect, the AF spectroscopy potential for a direct monitoring of neurotransmitter was supported also by data obtained in vivo, through a optic fiber probe positioned via stereotaxis, from the hippocampus region of rats submitted to behavioral or pharmacological treatments affecting serotonin levels.202,203
Concluding remarks and future perspectives
Only some selected topics deserving interest in the biomedicine field have been considered in this review. This limited description, however, may sufficiently illustrate the long-time enduring and wide-ranging attention of the scientific community to AF of biological substrates. Endogenous fluorophores and their role as intrinsic biomarkers offer an exceptionally powerful tool to characterize in real time even subtle changes of interconnected morphological and metabolic properties of cells and tissues under physiological or altered conditions. In this concern, a very recent example of a powerful AF application has shown the possibility provided by two-photon excitation for noninvasive, automated and quantitative assessment of mitochondrial organization in intact, engineered epithelial tissues. Differences in the three-dimensional mitochondrial organization reflect alterations in metabolic activities and allow to discriminate normal and precancerous conditions, playing a role as hopeful early cancer diagnostic tool.204
In the future, the interest in the AF phenomenon will certainly continue. Current instrumentation and analytical procedures will likely be overcome by the unceasing progress in new devices for AF detection and data interpretation; nevertheless, a progress is expected in the search, identification, photophysical characterization and biological meaning of additional biomolecules present in cells and tissues and exploitable as AF intrinsic biomarkers.
Acknowledgments
The Authors are particularly grateful to all Partners contributing to their original studies mentioned in this review article, and wish to thank Fondazione Cariplo, grant n. 2011-0439, for supporting their work.
References
- 1.Udenfriend S. Fluorescence Assay in Biology and Medicine. Vol II London: Academic Press; 1969. [Google Scholar]
- 2.Kasten FH. The origins of modern fluorescence microscopy and fluorescence probes, p. 4-47 In: Kohen E., Hirschberg J.G. JG (eds.), Cell structure and function by microspectrofluorometry. Academic Press Inc., 1989. [Google Scholar]
- 3.Balaban RS, Mandel LJ. Optical methods for the study of metabolism in intact cells, p. 213-36 In: Forskett J.K., Grinstein S. (eds.), Non-invasive techniques in cell biology. Wiley Liss, 1990. [Google Scholar]
- 4.Rost FWD. Fluorescence microscopy, vol 2 Cambridge University Press, 1995. [Google Scholar]
- 5.Wagnières GA, Star WM, Wilson BC. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem Photobiol 1998;68:603-32. [PubMed] [Google Scholar]
- 6.Bottiroli G, Croce AC. Autofluorescence spectroscopy of cells and tissues as a tool for biomedical diagnosis, p. 189-210 In: Palumbo G., Pratesi R. (eds.), Photosciences, lasers and current optical techniques in biology. RSC Books and Databases, 2004. [PubMed] [Google Scholar]
- 7.Louie JS, Richards-Kortum R, Anandasabapathy S. Applications and advancements in the use of high-resolution microendoscopy for detection of gastrointestinal neoplasia. Clin Gastroenterol Hepatol 2014;12:1789-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annu Rev Physical Chem 1996;47:555-606. [DOI] [PubMed] [Google Scholar]
- 9.Ramanujam N. Fluorescence spectroscopy in vivo, p. 1-37 In: Meyers R.A. (ed.), Encyclopedia of analytical chemistry. John Wiley & Sons, 2000. [Google Scholar]
- 10.Ramanujam N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia 2000;2:89-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokolov K, Follen M, Richards-Kortum R. Optical spectroscopy for detection of neoplasia. Curr Opin Chem Biol 2002;6:651-8. [DOI] [PubMed] [Google Scholar]
- 12.Bigio IJ, Mourant JR. Optical biopsy, p. 1577-93 In: Driggers R.G. (ed.) Encyclopedia of optical engineering. Marcel Dekker, 2003. [Google Scholar]
- 13.Wang TD, Van Dam J. Optical biopsy: a new frontier in endoscopic detection and diagnosis. Clin Gastroenterol Hepatol 2004;2:744-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy HK, Backman V. Spectroscopic applications in gastrointestinal endoscopy. Clin Gastroenterol Hepatol 2012;10:1335-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfano R, Pu Y. Optical biopsy for cancer detection, p. 325-67 In: Jelinkova H. (ed.), Lasers for medical applications: diagnostics, therapy and surgery. Woudhead Publishing Ltd., 2013. [Google Scholar]
- 16.Liu J, Dlugosz A, Neumann H. Beyond white light endoscopy: the role of optical biopsy in inflammatory bowel disease. World J Gastroenterol 2013;19:7544-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haitinger M. Fluoreszenzmikroskopie. Ihre Anwendung in der Histologie und Chemie. Akademische Verlagsgellschaft MBH, 1938. [Google Scholar]
- 18.Goodwin RH. Fluorescent substances in plants. Ann Rev Plant Physiol 1953;4:283-304. [Google Scholar]
- 19.O’Brien TP, McCully ME. The study of plant structure. Principles and selected methods. Termacarphi Pty Ltd., 1981. [Google Scholar]
- 20.Baroni B. Contributo allo studio dei melanomi cutanei al lume di un moderno mezzo d’indagine: del microscopio a fluorescenza. Archivio Italiano di Dermatologia, Sifilogia e Venereologia 1933;9:543-86. [Google Scholar]
- 21.Fukuda M, Nakanishi K, Sawamura I, Fujita S. Standardization of the post-irradiation method to eliminate primary fluorescence in cytofluorometry. Histochemistry 1977;52:119-27. [DOI] [PubMed] [Google Scholar]
- 22.Kingsley K, Carroll K, Huff JL, Plopper GE. Photobleaching of arterial autofluorescence for immunofluorescence applications. Biotechniques 2001;30:794-7. [DOI] [PubMed] [Google Scholar]
- 23.Burkhardt M, Vollmar B, Menger MD. In vivo analysis of hepatic NADH fluorescence. Methodological approach to exclude Ito cell vitamin A-derived autofluorescence. Adv Exp Biol 1998;454:83-9. [PubMed] [Google Scholar]
- 24.Johnston NW, Bienenstock J. Abolition of non-specific fluorescent staining of eosinophils. J Immunol Methods 1974;4:189-94. [DOI] [PubMed] [Google Scholar]
- 25.University Health Network Research. Wright Cell Imaging Facility. Available from: http://www.uhnres.utoronto.ca/facilities/wcif/ [Google Scholar]
- 26.Eränko O. The practical histochemical demonstration of catecholamines by formaldehyde-induced fluorescence. J R Microsc Soc 1967;87:259-76. [PubMed] [Google Scholar]
- 27.Policard A. Etudes sur les aspects offerts par des tumeurs expérimentales examinées a la lumière de Wood. C R Soc Biol 1924;91:1423-8. [Google Scholar]
- 28.Bommer S. Über sichtbare Fluoreszenz beim Menschen. Acta Derm Venereol 1929;10:253-315. [Google Scholar]
- 29.Dhéré C. La fluorescence en biochimie. Paris, France, La Presse Universitaire de France, 1937. [Google Scholar]
- 30.Borst M, Königsdörffer H. Untersuchungen über Porphyrie, mit besonderer Berücksichtigung der Porphyria Congenita. Leipizig, Hirzel, 1929. [Google Scholar]
- 31.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011;61:250-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamperl H. Die Fluoreszenzmikroskopie menschlicher Gewebe. Virchows Arch Pathol Anat Physiol 1934;292:1-51. [Google Scholar]
- 33.Popper H. Distribution of vitamin A in tissue as visualized by fluorescence microscopy. Physiol Rev 1944;24:205-24. [Google Scholar]
- 34.Chance B, Legallias V. Rapid and sensitive spectrophotometry. II. A stopped-flow attachment for a stabilized quartz spectrophotometer. Rev Sci Instrum 1951;22:627-38. [Google Scholar]
- 35.Chance B, Baltscheffsky H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem 1958;233:736-9. [PubMed] [Google Scholar]
- 36.Chance B, Legallias V. Differential microfluorimeter for the localization of reduced pyridine nucleotide in living cells. Rev Sci Instrum 1959;30:732-5. [Google Scholar]
- 37.Chance B, Thorell B. Localization and kinetics of reduced pyridine nucleotide in living cells by microfluorometry. J Biol Chem 1959;234:3044-50. [PubMed] [Google Scholar]
- 38.Chance B, Thorell B. Fluorescence measurements of mitochondrial pyridine nucleotide in aerobiosis and anaerobiosis. Nature 1959;184:931-4. [DOI] [PubMed] [Google Scholar]
- 39.Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science 1962;137:499-508. [DOI] [PubMed] [Google Scholar]
- 40.Duysens LN, Amesz J. Fluorescence spectrophotometry of reduced phosphopyridine nucleotide in intact cells in the nearultraviolet and visible region. Biochim Biophys Acta 1957;24:19-26. [DOI] [PubMed] [Google Scholar]
- 41.Thorell B. Flow-cytometric monitoring of intracellular flavins simultaneously with NAD(P) levels. Cytometry 1983;4:61-5. [DOI] [PubMed] [Google Scholar]
- 42.Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion 2007;7:330-9. [DOI] [PubMed] [Google Scholar]
- 43.Aubin JE. Autofluorescence of viable cultured mammalian cells. J Histochem Cyto chem 1979;27:36-43. [DOI] [PubMed] [Google Scholar]
- 44.Croce AC, Bottiroli G, Santin G, Pacchiana G, Vezzoni P, Di Pasquale E. Autofluorescence and metabolic signatures in a pig model of differentiation based on induced pluripotent cells and embryonic bodies. Microscopie 2014:52-9. [Google Scholar]
- 45.Pedraza-Fariña LG. Mechanisms of oncogenic cooperation in cancer initiation and metastasis. Yale J Biol Med 2006;79:95-103. [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008;134: 1655-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masoudi-Nejad A, Asgari Y. Metabolic cancer biology: structural-based analysis of cancer as a metabolic disease, new sights and opportunities for disease treatment. Semin Cancer Biol 2014doi: 10.1016/j.semcancer.2014.01.007 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48.Alfano RR, Tata DB, Cordero J, Tomashefsky P, Longo FW, Alfano MA. Laser induced fluorescence spectroscopy from native cancerous and normal tissue. IEEE J. Quantum Elect 1984;20:1507-11. [Google Scholar]
- 49.Utzinger U, Richards-Kortum RR. Fiber optic probes for biomedical optical spectroscopy. J Biomed Opt 2003;8:121-47. [DOI] [PubMed] [Google Scholar]
- 50.Piston DW, Knobel SM. Real-time analysis of glucose metabolism by microscopy. Trends Endocrinol Metab 1999;10:413-7. [DOI] [PubMed] [Google Scholar]
- 51.Georgakoudi I, Quinn KP. Optical imaging using endogenous contrast to assess metabolic state. Annu Rev Biomed Eng 2012;14:351-67. [DOI] [PubMed] [Google Scholar]
- 52.Patterson GH, Piston DW. Photobleaching in two-photon excitation microscopy. Biophys J 2000;78:2159-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tiede LM, Nichols MG. Photobleaching of reduced nicotinamide adenine dinucleotide and the development of highly fluorescent lesions in rat basophilic leukemia cells during multiphoton microscopy. Photochem Photobiol 2006;82:656-64. [DOI] [PubMed] [Google Scholar]
- 54.Huang S, Heikal AA, Webb WW. Two-photon fluorescence spectroscopy of NAD(P)H and flavoprotein. Biophys J 2002;82:2811-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rice WL, Kaplan DL, Georgakoudi I. Two-photon microscopy for non-invasive, quantitative monitoring of stem cell differentiation. PLoS One 2010;5:e10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lakowicz JR, Szmacinski H, Nowaczyk K, Johnson ML. Fluorescence lifetime imaging of free and protein-bound NADH. Proc Natl Acad Sci USA 1992;89:1271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.König K, Schneckenburger H. Laser-induced autofluorescence for medical diagnosis. J Fluoresc 1994;4:17-40. [DOI] [PubMed] [Google Scholar]
- 58.Schneckenburger H, Wagner M, Weber P, Strauss WS, Sailer R. Autofluorescence lifetime imaging of cultivated cells using a UV picosecond laser diode. J Fluoresc 2004;14:649-54. [DOI] [PubMed] [Google Scholar]
- 59.Pfeifer L, Stein K, Fink U, Welker A, Wetzl B, Bastian P, et al. Improved routine biomedical and bio-analytical online fluorescence measurements using fluorescence lifetime resolution. J Fluoresc 2005;15: 423-32. [DOI] [PubMed] [Google Scholar]
- 60.Digman MA, Caiolfa VR, Zamai M, Gratton E. The phasor approach to fluorescence lifetime imaging analysis. Biophys J 2008; 94:L14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weil GJ, Chused TM. Eosinophil autofluorescence and its use in isolation and analysis of human eosinophils using flow microfluorometry. Blood 1981;57:1099-104. [PubMed] [Google Scholar]
- 62.Monici M, Pratesi R, Bernabei PA, Caporale R, Ferrini PR, Croce AC, et al. Natural fluorescence of white blood cells: spectroscopic and imaging study. J Photochem Photobiol B 1995;30:29-37. [DOI] [PubMed] [Google Scholar]
- 63.Carulli G, Sbrana S, Azzarà A, Minnucci S, Angiolini C, Marini A, et al. Detection of eosinophils in whole blood samples by flow cytometry. Cytometry 1998;34:272-9. [DOI] [PubMed] [Google Scholar]
- 64.Emmelkamp J1, Wolbers F, Andersson H, Dacosta RS, Wilson BC, Vermes I, et al. The potential of autofluorescence for the detection of single living cells for label-free cell sorting in microfluidic systems. Electrophoresis 2004;25:3740-45. [DOI] [PubMed] [Google Scholar]
- 65.Rigacci L, Alterini R, Bernabei PA, Ferrini PR, Agati G, Fusi F, Monici M. Multispectral imaging autofluorescence microscopy for the analysis of lymph-node tissues. Photochem Photobiol 2000;71:737-42. [DOI] [PubMed] [Google Scholar]
- 66.Kapadia CR, Cutruzzola FW, O’Brien KM, Stetz ML, Enriquez R, Deckelbaum LI. Laser-induced fluorescence spectroscopy of human colonic mucosa. Detection of adenomatous transformation. Gastroenterology 1990;99:150-7. [DOI] [PubMed] [Google Scholar]
- 67.Ramanujam N, Mitchell MF, Mahadevan A, Thomsen S, Malpica A, Wright T, et al. Development of a multivariate statistical algorithm to analyze human cervical tissue fluorescence spectra acquired in vivo. Lasers Surg Med 1996;19:46-62. [DOI] [PubMed] [Google Scholar]
- 68.Gupta PK, Majumder SK, Uppal A. Breast cancer diagnosis using N2 laser excited autofluorescence spectroscopy. Lasers Surg Med 1997;21:417-22. [DOI] [PubMed] [Google Scholar]
- 69.Eker C, Rydell R, Svanberg K, Andersson-Engels S. Multivariate analysis of laryngeal fluorescence spectra recorded in vivo. Lasers Surg Med 2001;28:259-66. [DOI] [PubMed] [Google Scholar]
- 70.Takehana S, Kaneko M, Mizuno H. Endoscopic diagnostic system using autofluorescence. Diagn Ther Endosc 1999;5:59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qu J, Macaulay C, Lam S, Palcic B. Optical properties of normal and carcinomatous bronchial tissue. Appl Opt 1994;33:7397-405. [DOI] [PubMed] [Google Scholar]
- 72.Welch AJ, Gardner C, Richards-Kortum R, Chan E, Criswell G, Pfefer J, et al. Propagation of fluorescent light. Lasers Surg Med 1997;21:166-78. [DOI] [PubMed] [Google Scholar]
- 73.Zeng H, MacAulay C, McLean DI, Palcic B. Reconstruction of in vivo skin autofluorescence spectrum from microscopic properties by Monte Carlo simulation. J Photochem Photobiol B 1997;38:234-40. [DOI] [PubMed] [Google Scholar]
- 74.Drezek R, Sokolov K, Utzinger U, Boiko I, Malpica A, Follen M, et al. Understanding the contributions of NADH and collagen to cervical tissue fluorescence spectra: modeling, measurements, and implications. J Biomed Opt 2001;6:385-96. [DOI] [PubMed] [Google Scholar]
- 75.Pavlova I, Weber CR, Schwarz RA, Williams MD, Gillenwater AM, Richards-Kortum R. Fluorescence spectroscopy of oral tissue: Monte Carlo modeling with site-specific tissue properties. Biomed Opt 2009;14: 014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Durr NJ, Weisspfennig CT, Holfeld BA, Ben-Yakar A. Maximum imaging depth of two-photon autofluorescence microscopy in epithelial tissues. J Biomed Opt 2011; 16:026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marchesini R, Fumagalli S, Pignoli E, Sichirollo A, Romatis S, Di Palma S, et al. Light-induced fluorescence of human colon tissue: dependence on histological and histochemical properties studied by means of a simplified model for simulation, p. 76-83 In: Cubeddu R., Marchesini R., Mordon S., Svanberg K., Rinneberg H., Wagnières G. (eds.), Optical biopsy and fluorescence spectroscopy and imaging. Proceedings of SPIE, Bellingham, WA, USA, 1994. [Google Scholar]
- 78.Bottiroli G, Croce AC, Locatelli D, Marchesini R, Pignoli E, Tomatis S, et al. Natural fluorescence of normal and neo-plastic human colon: a comprehensive “ex vivo” study. Lasers Surg Med 1995;16:48-60. [DOI] [PubMed] [Google Scholar]
- 79.Banerjee B, Miedema BE, Chandrasekhar HR Role of basement membrane collagen and elastin in the autofluorescence spectra of the colon. J Investig Med 1999;47: 326-32. [PubMed] [Google Scholar]
- 80.L’Heureux B, Gurden H, Pain F. Autofluorescence imaging of NADH and flavoproteins in the rat brain: insights from Monte Carlo simulations. Opt Express 2009;17:9477-90. [DOI] [PubMed] [Google Scholar]
- 81.De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol 2005;41:117-31. [DOI] [PubMed] [Google Scholar]
- 82.DaCosta RS, Andersson H, Wilson BC. Molecular fluorescence excitation-emission matrices relevant to tissue spectroscopy. Photochem Photobiol 2003;78:384-92. [DOI] [PubMed] [Google Scholar]
- 83.Subhash N, Mazzinghi P, Agati G, Fusi F, Lercari B . Analysis of laser-induced fluorescence line shape of intact leaves: application to uv stress detection Photochem Photobiol 1995;62:711-8. [Google Scholar]
- 84.Croce AC, Ferrigno A, Vairetti M, Bertone R, Freitas I, Bottiroli G. Autofluorescence properties of isolated rat hepatocytes under different metabolic conditions. Photochem Photobiol Sci 2004;3:920-6. [DOI] [PubMed] [Google Scholar]
- 85.Croce AC, Ferrigno A, Santin G, Vairetti M, Bottiroli G. Bilirubin: an autofluore-scence bile biomarker for liver functionality monitoring. J Biophotonics 2014;10:810-7. [DOI] [PubMed] [Google Scholar]
- 86.Croce AC, Ferrigno A, Santin G, Piccolini VM, Bottiroli G, Vairetti M. Autofluorescence of liver tissue and bile: organ functionality monitoring during ischemia and reoxygenation. Lasers Surg Med 2014;46:412-21. [DOI] [PubMed] [Google Scholar]
- 87.Croce AC, Ferrigno A, Piccolini VM, Tarantola E, Boncompagni E, Bertone V, et al. Integrated autofluorescence characterization of a modified-diet liver model with accumulation of lipids and oxidative stress. Biomed Res Int 2014;2014:803491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duysens LN, Kroneberg GH. The fluorescence spectrum of the complex of reduced phosphopyridine nucleotide and alcohol dehydrogenase from yeast. Biochim Biophys Acta 1957;26:437-8. [DOI] [PubMed] [Google Scholar]
- 89.Salmon JM, Kohen E, Viallet P, Hirschberg JG, Wouters AW, Kohen C, et al. Microspectrofluorometric approach to the study of free/bound NAD(P)H ratio as metabolic indicator in various cell types. Photochem Photobiol 1982;36:585-93. [DOI] [PubMed] [Google Scholar]
- 90.Kunz WS, Kunz W. Contribution of different enzymes to flavoproteins fluorescence of isolated rat liver mitochondria. Biochim Biophys Acta 1985;841:237-46. [DOI] [PubMed] [Google Scholar]
- 91.Kunz WS. Spectral properties of fluorescent flavoproteins of isolated rat liver mitochondria. FEBS Lett 1986;195:92-6. [DOI] [PubMed] [Google Scholar]
- 92.Reinert KC, Gao W, Chen G, Wang X, Peng YP, Ebner TJ.Cellular and metabolic origins of flavoprotein autofluorescence in the cerebellar cortex in vivo. Cerebellum 2011;10:585-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides-small molecules with a multitude of functions. Biochem J 2007;402:205-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakamura M, Bhatnagar A, Sadoshima J. Overview of pyridine nucleotides review series. Circ Res 2012;111:604-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Penzer GR. Molecular emission spectroscopy (Fluorescence and Phosphorescence), p. 70-114 In: Brown S.B. (ed.), An introduction to spectroscopy for biochemists. London, Academic Press, 1980. [Google Scholar]
- 96.Wolfbeiss OS. The fluorescence of organic natural products. In: Schulman S.G. editor. Molecular Fluorescence Spectroscopy. Methods and Applications; Part I. New York (NY, USA):John Wiley & Sons;1985. p. 167-370 [Google Scholar]
- 97.Hess B. Organization of glycolysis: oscillatory and stationary control. In: Davies D.B. (ed.), Rate control of biological processes. Symposia Society for Experimental Biology, vol. 27 Cambridge University Press, 1973. [PubMed] [Google Scholar]
- 98.Kosowski H, Schild L, Kunz D, Halangk W. Energy metabolism in rat pancreatic acinar cells during anoxia and reoxygenation. Biochim Biophys Acta 1998;1367:118-26. [DOI] [PubMed] [Google Scholar]
- 99.Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. J Biol Chem 2004;279:31780-7. [DOI] [PubMed] [Google Scholar]
- 100.Smelt MJ, Faas MM, de Haan BJ, de Vos P. Pancreatic beta-cell purification by altering FAD and NAD(P)H metabolism. Exp Diabetes Res 2008;2008:165360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun MY, Yoo E, Green BJ, Altamentova SM, Kilkenny DM, Rocheleau JV.Autofluorescence imaging of living pancreatic islets reveals fibroblast growth factor-21 (FGF21)-induced metabolism. Biophys J 2012;103:2379-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thorniley MS, Simpkin S, Fuller B, Jenabzadeh MZ, Green CJ. Monitoring of surface mitochondrial NADH levels as an indication of ischemia during liver isograft transplantation. Hepatology 1995;21: 1602-9. [PubMed] [Google Scholar]
- 103.Vollmar B, Burkhardt M, Minor T, Klauke H, Menger MD. High resolution microscopic determination of hepatic NADH fluorescence for in vivo monitoring of tissue oxygenation during hemorragic shock and resuscitation. Microvasc Res 1997;54:164-73. [DOI] [PubMed] [Google Scholar]
- 104.Coremans JM1, Ince C, Bruining HA, Puppels GJ. (Semi-)quantitative analysis of reduced nicotinamide adenine dinucleotide fluorescence images of bloodperfused rat heart. Biophys J 1997;72: 1849-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klauke H, Minor T, Vollmar B, Isselhard W, Menger MD. Microscopic analysis of NADH fluorescence during aerobic and anaerobic liver preservation conditions: a noninvasive technique for assessment of hepatic metabolism. Cryobiology 1998;36: 108-14. [DOI] [PubMed] [Google Scholar]
- 106.Croce AC, Ferrigno A, Vairetti M, Bertone R, Freitas I, Bottiroli G. Autofluorescence spectroscopy of rat liver during experimental transplantation procedure. An approach for hepatic metabolism assessment. Photochem Photobiol Sci 2005;4: 583-90. [DOI] [PubMed] [Google Scholar]
- 107.Raman RN, Pivetti CD, Matthews DL, Troppmann C, Demos SG. Quantification of in vivo autofluorescence dynamics during renal ischemia and reperfusion under 355 nm excitation. Optics Express 2008;16:4930-44. [DOI] [PubMed] [Google Scholar]
- 108.Croce AC, De Simone U, Vairetti M, Ferrigno A, Bottiroli G. Autofluorescence properties of rat liver under hypermetabolic conditions. Photochem Photobiol Sci. 2007;6:1202-9. [DOI] [PubMed] [Google Scholar]
- 109.Folmes CDL, Dzeja PP, Nelson TJ, Terzic A. Energy metabolism plasticity enables stemness programs. Ann N Y Acad Sci 2012;1254:82-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uchugonova A, König K. Two-photon autofluorescence and second-harmonic imaging of adult stem cells. J Biomed Opt 2008;13:054068. [DOI] [PubMed] [Google Scholar]
- 111.Stringari C, Cinquin A, Cinquin O, Digman MA, Donovan PJ, Gratton E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc Natl Acad Sci USA 2011;108:13582– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stringari C, Nourse JL, Flanagan LA, Gratton E. Phasor fluorescence lifetime microscopy of free and protein-bound NADH reveals neural stem cell differentiation potential. PLoS One 2012;7:e48014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Santin G, Paulis M, Vezzoni P, Pacchiana G, Bottiroli G, Croce AC. Autofluorescence properties of murine embryonic stem cells during spontaneous differentiation phases. Lasers Surg Med 2013. Nov;45:597-607. [DOI] [PubMed] [Google Scholar]
- 114.Warburg O. On the origin of cancer cells. Science 1956;123:309-14. [DOI] [PubMed] [Google Scholar]
- 115.Croce AC, Spano A, Locatelli D, Barni S, Sciola L, Bottiroli G. Dependence of fibroblast autofluorescence properties on normal and transformed conditions. Role of the metabolic activity. Photochem Photobiol 1999;69:364-74. [DOI] [PubMed] [Google Scholar]
- 116.Galeotti TGV, Van Rossum D, Mayer DH, Chance B. On the fluorescence of NAD(P)H in whole-cell preparation of tumours and normal tissues. Eur J Biochem 1970;17:485-96. [DOI] [PubMed] [Google Scholar]
- 117.Meixensberger J, Herting B, Roggendorf W, Reichmann H. Metabolic patterns in malignant gliomas. J Neuro-Oncol 1995;24:153-61. [DOI] [PubMed] [Google Scholar]
- 118.Villette S, Pigaglio-Deshayes S, Vever-Bizet C, Validire P, Bourg-Heckly G. Ultraviolet-induced autofluorescence characterization of normal and tumoral esophageal epithelium cells with quantitation of NAD(P)H. Photochem Photobiol Sci 2006;5:483-92. [DOI] [PubMed] [Google Scholar]
- 119.Viallet P, Kohen E, Schachtschabel DO, Marty A, Salmon LM, Kohen C, et al. The effect of atebrine and an acridine analog (BCMA)on the coenzyme fluorescence spectra of cultured melanoma and Ehrlich ascites (EL2)cells. Histochemistry 1978; 57:189-201. [DOI] [PubMed] [Google Scholar]
- 120.Bottiroli G, Croce AC, Locatelli D, Nano R, Giombelli E, Messina A, et al. Brain tissue autofluorescence: an aid for intraoperative delineation of tumor resection margins. Cancer Detect Prev 1998;22:330-9. [DOI] [PubMed] [Google Scholar]
- 121.Croce AC, Fiorani S, Locatelli D, Nano R, Ceroni M, Tancioni F, et al. Diagnostic potential of autofluorescence for an assisted intraoperative delineation of glioblastoma resection margins. Photochem Photobiol 2003;77:309-18. [DOI] [PubMed] [Google Scholar]
- 122.Perelman L, Backman V, Wallace W, Zonios G, Manoharan R, Nustrat A, et al. Observation of periodic fine structure in reflectance from biological tissue: a new technique for measuring nuclear size distribution. Phys Rev Lett 1998;80;627-30. [Google Scholar]
- 123.Morgan DC, Wilson JE, MacAulay CE, MacKinnon NB, Kenyon JA, Gerla PS, et al. New method for detection of heart allograft rejection: validation of sensitivity and reliability in a rat heterotopic allograft model. Circulation 1999;100:1236-41. [DOI] [PubMed] [Google Scholar]
- 124.Heintzelman DL, Lotan R, Richards-Kortum RR. Characterization of the auto-fluorescence of polymorphonuclear leukocytes, mononuclear leukocytes and cervical epithelial cancer cells for improved spectroscopic discrimination of inflammation from dysplasia. Photochem Photobiol 2000;71:327-32. [PubMed] [Google Scholar]
- 125.Wolman M. Lipid pigments (chromolipids): their origin, nature and significance. Pathobiol Ann 1980;10:253-67. [PubMed] [Google Scholar]
- 126.Rattan SIS, Keeler KD, Buchanan JH, Holliday R. Autofluorescence as an index of ageing in human fibroblastsin culture. Biosci Rep 1982;2:561-7. [DOI] [PubMed] [Google Scholar]
- 127.Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res 1992;275:395-403. [DOI] [PubMed] [Google Scholar]
- 128.Riga D, Riga S. Lipofuscin and ceroid pigments in aging and brain pathology. A review. I. Biochemical and morphological properties. Rom J Neurol Psychiatry 1995;33:121-36. [PubMed] [Google Scholar]
- 129.Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci 2007;1119: 97-111. [DOI] [PubMed] [Google Scholar]
- 130.Goebel HH, Müller HD. Storage diseases: diagnostic position. Ultrastruct Pathol 2013;37:19-22. [DOI] [PubMed] [Google Scholar]
- 131.Palmer DN, Oswald MJ, Westlake VJ, Kay GW. The origin of fluorescence in the neuronal ceroid lipofuscinoses (Batten disease) and neuron cultures from affected sheep for studies of neurodegeneration. Arch Gerontol Geriatr 2002;34:343-57. [DOI] [PubMed] [Google Scholar]
- 132.Croce AC, Pisu MB, Roda E, Avella D, Bernocchi G, Bottiroli G. Autofluorescence properties of rat cerebellum cortex during postnatal development. Lasers Surg Med 2006;38:598-607. [DOI] [PubMed] [Google Scholar]
- 133.Patková J, Vojtíšek M, Tůma J, Vožeh F, Knotková J, Santorová P, et al. Evaluation of lipofuscin-like pigments as an index of lead-induced oxidative damage in the brain. Exp Toxicol Pathol 2012;64:51-6. [DOI] [PubMed] [Google Scholar]
- 134.Fonnum F, Lock EA. Cerebellum as a target for toxic substances. Toxicol Lett 2000;112-113:9-16. [DOI] [PubMed] [Google Scholar]
- 135.Terman A, Gustafsson B, Brunk UT. Mitochondrial damage and intralysosomal degradation in cellular aging. Mol Aspects Med 2006;27:471-82. [DOI] [PubMed] [Google Scholar]
- 136.Grossi CE, Zaccheo D. Sulla fluorescenza delle granulazioni specifiche dei leucociti eosinofili. Boll Soc It Biol Sper 1963;39:421-3. [Google Scholar]
- 137.Mayeno AN, Hamann KJ, Gleich GJ. Granule-associated flavin adenine dinucleotide (FAD) is responsible for eosinophil autofluorescence. J Leukoc Biol 1992;51:172-5. [DOI] [PubMed] [Google Scholar]
- 138.Barnes D, Aggarwal S, Thomsen S, Fitzmaurice M, Richards-Kortum R. A characterization of the fluorescent properties of circulating human eosinophils. Photochem Photobiol 1993;58:297-303. [DOI] [PubMed] [Google Scholar]
- 139.Dorward DA, Lucas CD, Alessandri AL, Marwick JA, Rossi F, Dransfield I, Haslett C, Dhaliwal K, Rossi AG. Technical advance: autofluorescence-based sorting: rapid and nonperturbing isolation of ultrapure neutrophils to determine cytokine production. J Leukoc Biol 2013; 94:193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barni S, Bertone V, Croce AC, Bottiroli G, Bernini F, Gerzeli G. Increase in liver pigmentation during natural hibernation in some amphibians. J Anat 1999;195:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Oliveira C, Franco-Belussi l. Melanic pigmentation in ectothermic vertebrates: occurrence and functions, p. 213-5 In: Ma X.P., Sun X.X. (eds.), Melanin: biosynthesis, functions and health effects. Nova Science Publisher Inc., 2012. [Google Scholar]
- 142.Elleder M, Borovanský J. Autofluorescence of melanins induced by ultraviolet radiation and near ultraviolet light. A histochemical and biochemical study. Histochem J 2001;33:273-81. [DOI] [PubMed] [Google Scholar]
- 143.Nighswander-Rempel SP, Mahadevan IB, Rubinsztein-Dunlop H, Meredith P. Time-resolved and steady-state fluorescence spectroscopy of eumelanin and indolic polymers. Photochem Photobiol 2007;83: 1449-54. [DOI] [PubMed] [Google Scholar]
- 144.Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye 1995;9:763-71. [DOI] [PubMed] [Google Scholar]
- 145.Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med 2010;10:802-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nandakumar N, Buzney S, Weiter JJ. Lipofuscin and the principles of fundus autofluorescence: a review. Semin Ophthalmol 2012;27:197-201. [DOI] [PubMed] [Google Scholar]
- 147.Bottiroli G, Ramponi R, Croce AC. Quantitative analysis of intracellular behaviour of porphyrins. Photochem Photobiol 1987;46:663-7. [DOI] [PubMed] [Google Scholar]
- 148.Seherer J. Chemisch-physiologische untersuchungen. Liebihs Ann Chem 1841;40:1-64. [Google Scholar]
- 149.Meyer-Betz F. Untersuchungen uber die Biologische (photodynamische) Wirkung des Hamatoporphyrins und anderer Derivative des Blut- und Galenfarbstoffs. Dtsch Arch Klin Med 1913;112:476-503. [Google Scholar]
- 150.Auler H, Banzer G. [Untersuchungen uber die Rolle der Porphyrine bei geschwulstkranken Menschen und Tieren]. [Article in German]. Z Krebsforsch 1942;53:65-8. [Google Scholar]
- 151.Figge FHJ, Weiland GS, Morganiello LOJ. Cancer detection and therapy: affinity of neoplastic, embryonic, and traumatized tissues for porphyrins and metalloporphyrins. Proc Soc Exp Biol Med 68;1948:640-1. [DOI] [PubMed] [Google Scholar]
- 152.Lipson RL, Baldes EJ. The Photodynamic properties of a particular hematoporphyrin derivative. Arch Dermatol 1960;82: 508-16. [DOI] [PubMed] [Google Scholar]
- 153.Dougherty TJ, Henderson BW, Schwartz S, Winkelman IW, Lipson RL. Historical perspective, p. 1-15 In: Henderson B.W., Dougherty T.J. (eds.), Photodynamic therapy. New York, Marcel Dekker, 1992. [Google Scholar]
- 154.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Juzeniene A, Peng Q, Moan J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem Photobiol Sci 2007;6:1234-45. [DOI] [PubMed] [Google Scholar]
- 156.Berg K, Golab J, Korbelikc M, Russell D. Drug delivery technologies and immunological aspects of photodynamic therapy. Photochem Photobiol Sci 2011;10:647-8. [DOI] [PubMed] [Google Scholar]
- 157.Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. Photochem Photobiol B: Biol 1992;14:275-92. [DOI] [PubMed] [Google Scholar]
- 158.Gaullier JM, Berg K, Peng Q, Anholt H, Selbo PK, Ma LW, et al. Use of 5-aminolaevulinic acid esters to improve photodynamic therapy on cells in culture. Cancer Res 1997;57:1481-6. [PubMed] [Google Scholar]
- 159.Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci 2008;7:283-9. [DOI] [PubMed] [Google Scholar]
- 160.Peng Q, Warloe T, Berg K, Moan J, Kongshaug K, Giercksky KE, et al. 5-aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 1997;79:2282-308. [DOI] [PubMed] [Google Scholar]
- 161.Xu X, Meng J, Hou S. The characteristic fluorescence of the serum of cancer patients. J Lumin 1988;40-41:219-20. [Google Scholar]
- 162.Madhuri S, Vengadesan N, Aruna P, Koteeswaran D, Venkatesan P, Ganesan S. Native fluorescence spectroscopy of blood plasma in the characterization of oral malignance. Photochem Photobiol 2003; 78:197-204. [DOI] [PubMed] [Google Scholar]
- 163.Masilamani V, Al-Zhrani K, Al-Salhi M, Al-Diab A, Al-Ageily M. Cancer diagnosis by autofluorescence of blood components. J Lumin 2004;109:143-54. [Google Scholar]
- 164.Lualdi M, Battaglia L, Colombo A, Leo E, Morelli D, Poiasina E, et al. Colorectal cancer detection by means of optical fluoroscopy. A study on 494 subjects. Front Biosci 2010;E2:694-700. [DOI] [PubMed] [Google Scholar]
- 165.Devanesan S, Mohamad Saleh A, Ravikumar M, Perinbam K, Prasad S, Abbas HA, et al. Fluorescence spectral classification of iron deficiency anemia and thalassemia. J Biomed Opt 2014;19: 027008. [DOI] [PubMed] [Google Scholar]
- 166.Lucchini F, Sacco MG, Hu N, Villa A, Brown J, Cesano L L., et al. , Early and multifocal tumors in breast, salivary, harderian and epididymal tissues developed in MMTY-Neu transgenic mice. Cancer Lett 1992;64:203-9. [DOI] [PubMed] [Google Scholar]
- 167.Weagle G, Paterson PE, Kennedy J, Pottier R, The nature of the chromophore responsible for naturally occurring fluorescence in mouse skin. J Photochem Photobiol B 1988;2:313-20. [DOI] [PubMed] [Google Scholar]
- 168.Taketani S, Ishigaki M, Mizutani A, Uebayashi M, Numata M, Ohgari Y, Kitajima S. Heme synthase (ferrochelatase) catalyzes the removal of iron from heme and demetalation of metalloporphyrins. Biochemistry 2007;46:15054-61. [DOI] [PubMed] [Google Scholar]
- 169.Croce AC, Santamaria G, De Simone U, Lucchini F, Freitas I, Bottiroli G. Naturally-occurring porphyrins in a spontaneous-tumour bearing mouse model. Photochem Photobiol Sci 2011;10:1189-95. [DOI] [PubMed] [Google Scholar]
- 170.Freitas I, Boncompagni E, Vaccarone R, Fenoglio C, Barni S, Baronzio GF. Iron accumulation in mammary tumour suggests a tug of war between tumour and host for the microelement. Anticancer Res. 2007;27:3059–35. [PubMed] [Google Scholar]
- 171.Lillie RD, Daft FS, Sebrell WH Jr. Cirrhosis of the liver in rats on a deficient diet and the effect of alcohol. Pub Health Rep I94I;56:1255-8. [Google Scholar]
- 172.Popper H, Gyorgy P, Goldblatt H. Fluorescent material (ceroid) in experimental nutritional cirrhosis. Arch Path 1944; 37:161-8. [Google Scholar]
- 173.Chio KS, Reiss U, Fletcher B, Tappel AL. Peroxidation of subcellular organelles: formation of lipofuscin like fluorescent pigments. Science 1969;166:1535-6. [DOI] [PubMed] [Google Scholar]
- 174.Wang X, Liao Y, Li G, Yin D, Sheng SA comparative study of artificial ceroid/lipofuscin from different tissue materials of rats. Exp Aging Res 2008;34:282-95. [DOI] [PubMed] [Google Scholar]
- 175.Croce AC, De Simone U, Vairetti M, Ferrigno A, Boncompagni E, Freitas I, et al. Liver autofluorescence properties in animal model under altered nutritional conditions. Photochem Photobiol Sci 2008;7:1046-53. [DOI] [PubMed] [Google Scholar]
- 176.Grimaud JA, Druguet M, Peyrol S, Chevalier O, Herbage D, El Badrawy N. Collagen immunotyping in human liver: Light and electron microscope study. J Histochem Cytochem 1980;28:1145-56. [DOI] [PubMed] [Google Scholar]
- 177.Marcu L, Cohen D, Maarek JM, Grundfest WS. Characterization of type I, II, III, IV, and V collagens by time-resolved laserinduced fluorescence spectroscopy, p. 93-101 In: Alfano R.R. (ed.), Optical biopsy III. Proceedings of SPIE, Bellingham, WA, USA, 2000. [Google Scholar]
- 178.Vollmar B, Siegmund S, Menger MD. An intravital fluorescence microscopic study of hepatic microvascular and cellular derangements in developing cirrhosis in rats. Hepatology 1998;27:1544-53. [DOI] [PubMed] [Google Scholar]
- 179.Lee HS, Liu Y, Chen HC, Chiou LL, Huang GT, Lo W, et al. Optical biopsy of liver fibrosis by use of multiphoton microscopy. Opt Lett 2004;29:2614-16. [DOI] [PubMed] [Google Scholar]
- 180.Croce AC, De Simone U, Freitas I, Boncompagni E, Neri D, Cillo U, et al. Human liver autofluorescence: an intrinsic tissue parameter discriminating normal and diseased conditions. Lasers Surg Med 2010;42:371-8. [DOI] [PubMed] [Google Scholar]
- 181.Getz GS, Bartley W, Stirpe F, Notton BM, Renshaw A. The lipid composition of rat liver. Biochem J 1961;80:176-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Finkelstein SD, Gilfor D, Farber JL. Alterations in the metabolism of lipids in ischemia of the liver and kidney. J Lipid Res 1985;26:726-34. [PubMed] [Google Scholar]
- 183.Agati G, Fusi F. New trends in photobiology (invited review). Recent advances in bilirubin photophysics. J Photochem Photobiol B 1990;7:1-14. [DOI] [PubMed] [Google Scholar]
- 184.Amin SB, Lamola AA. Newborn jaundice technologies: unbound bilirubin and bilirubin binding capacity in neonates. Semin Perinatol 2011;35:134-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mazzoni M, Agati G, Troup GJ, Pratesi R. Analysis of the wavelength-dependent photoisomerisation quantum yields in bilirubins by fitting two exciton absorption bands. J Opt A-Pure Appl Op 2003; 5:S374. [Google Scholar]
- 186.Zonios GI, Cothren RM, Arendt JT, Wu J, Van Dam J, Crawford JM, et al. Morphological model of human colon tissue fluorescence IEEE Trans Biomed Eng 1996;43:113-22. [DOI] [PubMed] [Google Scholar]
- 187.Deckelbaum L, Lam JK, Cabin HS, Clubb KS, Long MB. Discrimination of normal and atherosclerotic aorta by laser-induced fluorescence. Lasers Surg Med 1987;7: 330-5. [DOI] [PubMed] [Google Scholar]
- 188.König K, Schenke-Layland K, Riemann I, Stock UA. Multiphoton autofluorescence imaging of intratissue elastic fibers. Biomaterials 2005;26:495-500. [DOI] [PubMed] [Google Scholar]
- 189.Schenke-Layland K, Riemann I, Damour O, Stock UA, König K. Two-photon microscopes and in vivo multiphoton tomographs- powerful diagnostic tools for tissue engineering and drug delivery. Adv Drug Deliv Rev 2006;58:878-96. [DOI] [PubMed] [Google Scholar]
- 190.Abraham T, Hirota JA, Wadsworth S, Knight DA. Minimally invasive multiphoton and harmonic generation imaging of extracellular matrix structures in lung airway and related diseases. Pulm Pharmacol Ther 2011;24:487-96. [DOI] [PubMed] [Google Scholar]
- 191.Birk JW, Tadros M, Moezardalan K, Nadyarnykh O, Forouhar F, Anderson J, et al. Second harmonic generation imaging distinguishes both high-grade dysplasia and cancer from normal colonic mucosa. Dig Dis Sci 2014;59:1529-34. [DOI] [PubMed] [Google Scholar]
- 192.Breunig HG, Studier H, König K. Multiphoton excitation characteristics of cellular fluorophores of human skin in vivo. Opt Express 2010;18:7857-71. [DOI] [PubMed] [Google Scholar]
- 193.Fritze O, Schleicher M, König K, Schenke-Layland K, Stock U, Harasztosi C. Facilitated noninvasive visualization of collagen and elastin in blood vessels. Tissue Eng Part C Methods 2010;16:705-10. [DOI] [PubMed] [Google Scholar]
- 194.Seidenari S, Arginelli F, Bassoli S, Cautela J, Cesinaro AM, Guanti M, et al. Diagnosis of BCC by multiphoton laser tomography. Skin Res Technol 2013; 19:e297-304. [DOI] [PubMed] [Google Scholar]
- 195.Pongor S, Ulrich PC, Bencsath FA, Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci USA 1984;81:2684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mulder DJ, Water TV, Lutgers HL, Graaff R, Gans RO, Zijlstra F, et al. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation end-products: an overview of current clinical studies, evidence, and limitations. Diabetes Technol Ther 2006;8:523-35. [DOI] [PubMed] [Google Scholar]
- 197.Tan W, Parpura V, Haydon PG, Yeung ES. Neurotransmitter imaging in living cells based on native fluorescence detection. Anal Chem 1995;67:2575-9. [DOI] [PubMed] [Google Scholar]
- 198.Michiaki M, Junichi S, Koichi O, Yoichi K, Shigeo F, Kazumi M. Time resolved fluorescence spectroscopy of dopamine in single cells. P Soc Photo-Opt Ins 2001;4252:27 doi:10.1117/12.426738. [Google Scholar]
- 199.Maiti S, Shear JB, Williams RM, Zipfel WR, Webb WW. Measuring serotonin distribution in live cells with three-photon excitation. Science 1997;275:530-2. [DOI] [PubMed] [Google Scholar]
- 200.Balaji J, Desai R, Kaushalya SK, Eaton MJ, Maiti S. Quantitative measurement of serotonin synthesis and sequestration in individual live neuronal cells. J Neurochem 2005;95:1217-26. [DOI] [PubMed] [Google Scholar]
- 201.Kaushalya SK, Maiti S. Quantitative imaging of serotonin autofluorescence with multiphoton microscopy, p. 1-18 In: Chattopadhyay A. (ed.), Frontiers in neuroscience. Serotonin receptors in neurobiology. Boca Raton, CRC Press, 2007. [PubMed] [Google Scholar]
- 202.Crespi F, Croce AC, Fiorani S, Masala B, Heidbreder C, Bottiroli G. In vivo autofluorescence spectrofluorometry of central serotonin. J Neurosci Methods 2004; 140:67-73. [DOI] [PubMed] [Google Scholar]
- 203.Crespi F, Croce AC, Fiorani S, Masala B, Heidbreder C, Bottiroli G. Autofluorescence spectrofluorometry of central nervous system (CNS) neuromediators. Lasers Surg Med 2004;34:39-47. [DOI] [PubMed] [Google Scholar]
- 204.Xylas J, Varone A, Quinn KP, Pouli D, McLaughlin-Drubin ME, Thieu HT, et al. Noninvasive assessment of mitochondrial organization in three-dimensional tissues reveals changes associated with cancer development. Int J Cancer 2015; 136:322-32. [DOI] [PMC free article] [PubMed] [Google Scholar]


