Abstract
Background
There has never been a single case report of any parasitic zoonosis in Ile-Ife while just a case of human Acanthocephalan infection in Nigeria is available.
Methods
Fifty (house–rats) Rattus rattus (Linnaeus, 1758) were caught in houses and raw food sellers’ stalls in a market in Ile-Ife. A caught rat was removed from the cage and sacrificed by cervical jerking. A rat was weighed, measured, quickly following which thick and thin blood films on microscope slides were made from blood collected from the tail vein. The rat was examined for ectoparasites then dissected to check for endoparasites.
Results
Two ectoparasites (Xenopsylla cheopis and Laelaptid mite) were recovered from 19 (38.0%) of the rats. Five genera of helminthes (Moniliformis, Hymenolepis, Taenia, Trichuris and Trichinella) were recovered from 29 (58.0%) of the rats while seven genera of protozoa organisms (Amoeba, Dientamoeba, Entamoeba, Retortamonas, Trichomonas, Chilomastix and Trypanosoma) were recovered from 48 (96.0%) of them. There was no correlation (Spearman’s correlation coefficient = -0.111) between the weight of the individual rat and the total number of alimentary canal acquired parasites.
Conclusion
In relation to human health, implications of the rats serving as reservoir hosts for the different pathogens are highlighted. In view of the possibility of unexpected zoonosis arising from the parasites found in the peridomestic rats in this investigation and others not found, and in view of the difficulties that may be associated with diagnosing such ailment, especially by a clinician who trained locally, this report should be like raising awareness to these salient facts.
Keywords: Endoparasites, Ectoparasites, Peridomestic, House-rats, Health
Introduction
Among the rats that commonly patronize human dwellings are Rattus rattus (Linnaeus, 1758) and R. norvegicus. The former is black and has a tail which is longer than the rest of the body axis (head- thorax-abdomen) while the latter is brownish and having a tail that is shorter than the rest of the body axis. Text books of parasitology contain reports showing house dwelling animals (domesticated and non- domesticated) serving as various kinds of hosts to parasites. Prevalence studies on parasitic infection of house rats had been conducted in several parts of the world (1-6).
In most of the under-developed countries (which happen to be in the tropical and sub- tropical regions), the climatic factors, socio-economic status political instability and, occasionally, natural disaster, have combined to enhance human abodes to be not only frequently and easily visited, but shared with non-domesticated or peri- domesticated rodents. Animals in these groups share foods with humans, often feed on human faeces plus other dead lower animals and or their waste products and quite often get their excrements mixing (albeit accidentally) with human food items (7, 8).
This study is an attempt to determine the kinds of parasites that may be originating from house rats and that may constitute a risk to human health in Ile-Ife, an ancient but recently rapidly developing town in Nigeria. There has never been a single case report of any parasitic zoonosis in Ile-Ife. Nevertheless, this does not mean there are none. As a matter of fact, only a single reported case of human Acanthocephalan infection in Nigeria is available (9).
Materials and Methods
The study Area
Ile-Ife is a rapidly developing town in Osun State, western Nigeria, it is on latitude 70 301 N and longitude 40 311 E. It has an estimated population of 356,000 inhabitants (10).With respect to basic social amenities, it can boast of two universities (Oduduwa and Obafemi Awolowo) and the teaching hospital of the later and, incidentally, much more bigger university. Many new houses are springing up in the bushes- often with no access roads. Water system facility is lacking in virtually all these newly erected houses just like in several of those erected long ago. Hence, the environment looks very conducive for intermingling or high frequency visitation by bush and/ or peridomestic rats.
Rats and sources
Wire-cage traps were set up in arbitrarily selected houses and raw food sellers’ shops in a major market in Ile-Ife within a period of six months. Trapping was done during the dry season, i.e. between the months of November of one year and April of the following year. A caught rat was carefully removed from the cage and then killed by cervical jerking. The killed rat was weighed, whole length and tail length were measured following which the rat was bled from the tail vain and thick and thin blood films were made on microscope slides. Some of these were viewed wet under x10 microscope objective lens while others were left to dry and stained.
Rats were differentiated on the basis of their color, length of tail, body shape, size of the ears, eyes and shape of their snout as suggested by Vinogradov and Argyropulo (11).
Ectoparasites
Killed rats were examined for ectoparasites. Available ectoparasites were gently removed with the aid of an artist paint brush and dissecting needle.
Endoparasites
A rat was opened up longitudinally at the mid-ventral line, from the lower jaw to the cloaca. The thoracic and abdominal cavities and the surfaces of the visceral organs were examined for spots, nodules or life parasites.
The heart, lungs, liver, gall bladder, spleen and kidneys were removed separately into petri-dishes. They were examined macroscopically for parasites and then carefully teased in physiological saline.
The alimentary canal was then severed at the two extremities, completely freed from all connective tissues and placed in physiological saline (8.5 gm NaCl in 1.0 liter of distilled water) in dissecting trays. One after the other and beginning from the esophagus, each portion of the tract (esophagus, stomach, small intestine, large intestine and the caecum) was slitted. Macroscopical examination for available parasites and removal of such into beakers of physiological saline was done. This was followed by a microscopical examination of the portions contents and mucosal scrapings under a dissecting microscope. Using a sharp scapel blade, thin sheets of striated muscles from the thoracic region, thighs, diaphragm and the heart were sliced and pressed between two microscope slides, then examined under the compound microscope. Smears of fecal samples in normal saline were made and observed under a compound microscope. Other smears were left to dry, stained and microscopically examined. In summary, preparations utilized are strictly the methods suggested by Cable (12) for ectoparasites, blood parasites, helminths, protozoa and ova of helminths in faeces. The various parasitic worms were identified on the basis of either the morphology of whole worm (or segments of them) using as guidelines information in Cable (12) or morphology of eggs, using as guidelines information (13). A maximum of four rats were processed per day. On days when more than four rats were caught, the unprocessed ones were kept in the laboratory and fed on commercial food pellets.
Results
A total of 50 rats, 35 (70%) males and 15 (30%) females were caught within the six months. All the rats belonged to the genus Rattus and specie rattus. The weight of the male rats ranged between 40 gm and 215 gm, with a mean of 100.8 gm. The female weights ranged between 40 gm and 145 gm and had a mean of 94.6 gm.
Ectoparasites were found on 19 (38%) rats. The flea Xenopsylla cheopis was found on 15 (42.9%) male rats and 3(20%) female rats. Laelaptid mite was found on 1 (2.9%) male rat.
Five genera of helminthes were recovered from the rats. These include Moniliformis moniliformis, Hymenolepis diminuta, Taenia taeniformis, Trichuris muris and Trichinella spiralis. Sex distribution of the helminths among the rats is shown in Table 1.
Table 1.
Sex prevalence of helminth infection among infected house rats (Rattus rattus) in Ile-Ife
| Parasites | (No and % of Parasites) | |
|---|---|---|
| Male | Female | |
| Moniliformis moniliformis | 12 (34.38) | 6 (40) |
| Hymenolepis diminuta | 6 (17.1) | 2 (13.3) |
| Taenia taeniformis | 4 (11.4) | 4 (26.7) |
| Trichuris muris | 21 (8.5) | 0 (0) |
| Trichinella spiralis | 5 (13.4) | 1 (6.7) |
While the anatomical distribution of the available helminths among the male and female rats is shown in Table 2. Nine genera and one genus of protozoa organisms were recovered from the gastro-intestinal tract and blood, respectively, of the rats. They include Amoeba sp., Dientamoeba fragilis, Entamoeba sp., Entamoeba coli, Entamoeba muris, Retortamonas intestinalis, Trichomonas muris, Chilomastix bettencourti, Chilomastix intestinalis (in the alimentary canal) and Trypanosoma lewisi (in the blood). Sex distribution of the protozoa organisms among the rats is shown in Table 3.
Table 2.
Sex prevalence and anatomical distribution of helminthes in the parasitized rats
| (Anatomical organ/intestinal portion) Number of parasites present in | ||||
|---|---|---|---|---|
| Helminth | Liver | Stomach | Ileum | Caecum |
| Moniformis moniliformis | ||||
| Male | 0 | 0 | 260 | 0 |
| Female | 0 | 0 | 53 | 0 |
| Total | 0 | 0 | 313 | 0 |
| Hymenolepis diminuta | ||||
| Male | 0 | 0 | 20 | 0 |
| Female | 0 | 0 | 14 | 0 |
| Total | 0 | 0 | 34 | 0 |
| Taenia taeniformis | ||||
| Male | 5 | 0 | 0 | 0 |
| Female | 8 | 0 | 0 | 0 |
| Total | 13 | 0 | 0 | 0 |
| Trichuris muris | ||||
| Male | 0 | 0 | 0 | 12 |
| Female | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 0 | 12 |
Table 3.
Sex prevalence of protozoa parasites among infected house rats (Rattus rattus)
| No and (%) of parasites | ||
|---|---|---|
| Parasites | Male | Female |
| Amoeba sp | 17 (48.6) | 2 (13.3) |
| Dientamoeba fragilis | 1 (2.9) | 1 (6.7) |
| Entamoeba sp | 2 (5.7) | 2 (13.3) |
| Entamoeba coli | 0 (0) | 1 (6.7) |
| Entamoeba muris | 1 (2.9) | 0 (0) |
| Retortamonas intestinalis | 26 (74.3) | 10 (66.7) |
| Trichomonas muris | 11 (31.4) | 2 (13.3) |
| Chilomastix bettencourti | 10 (28.6) | 5 (33.3) |
| Chilomastix intestinalis | 0 (0) | 1 (6.7) |
| Trypanosoma lewisi | 8 (22.9) | 1 (6.7) |
Discussion
Rats were weighed and the parasites in those that were positive for them were counted. The number of worms per positive rats was then plotted against the weight of the rats. Considering the total number of parasites harbored by some rats, it should be expected that the parasite burden will influence the weight of a highly parasitized host. However, for helminth infected rats in this investigation there was no correlation between their weights and the parasites harbored (Spearman’s correlation coefficient r = -0.111), (Fig 1).
Fig 1.
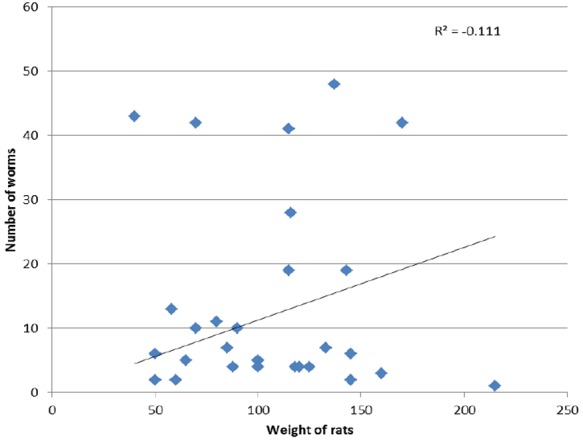
Worm burden of Rattus rattus in the study
The tropical setting has the climate and vegetation that is all the year round conducive to habitation of many and various lives- rodents, parasites and humans inclusive. In many occasions human residential areas could easily be visited by not only peridomestic but also wild or bush rodents. Then on occasions whereby good or adequate environmental sanitation is either breached or compromised, the frequency of deliberate or non- deliberate contact between humans and such rodents increases. Such rodents that may be harboring parasite that are natural to them may accidentally contaminate human foods or waters in their search for food or shelter. In several of the developing countries, such observations are notable and may increase cases of zoonosis by parasites that were found in the peridomestic rats in this study.
Among the parasitized rats, in respect of the anatomical habitat of the worms, majority of them are found in the ileum. Incidentally these are acanthocephalan and cestodes. These are the group of organisms that are devoid of digestive, circulatory, skeletal and respiratory systems. They have large surface area with a large and efficient absorptive capacity. The ileum portion of the small intestine is where digested food is absorbed into circulatory system, being the portion housing the villi. Hence for M. moniliformis and H. diminuta, they are in the right place for their morphological built. One wonders if this could have been evolutionary adaptation. All the 13 T. taeniformis helminths (also cestodes) were concealed in the superficial layers of the liver lobes. Since digested food materials are not expected to be stored in such sites, it is not clear how this worm derives its nutrient. However, it is known that among other functions, the liver stores digested and absorbed food macromolecules. It is therefore speculated that energy for its metabolism is absorbed at the macromolecular level. Derivation of nutrients from this source (the liver) places the organism at a higher level of advantage compared with other cestodes inhabiting the ileum.
T. muris is a nematode with very thick cuticular covering. It has an alimentary canal and a complete digestive tract, but it is not certain if it takes in undigested or semi digested food per oral. Hence it could feel comfortable in either the stomach or the caecum.
This study did not investigate the occurrence of Lassa fever virus; however, R. rattus was one of the rodents harboring strains of Lassa virus according to Wulff et al., (8). It is not known if there could have been misdiagnosis of diseases in this part of the world since signs and symptoms of Lassa fever may be difficult to distinguish from diseases that are common in the tropics (14).
Human infection with any of the Acanthocephalan worms is rare but is most commonly caused by infection with M. moniliformis and Macracanthor hyncus hirudinaeceus (15). The first case of human infection with M. moniliformis in Iran was reported in 1970 (16). Since then, human infection had been reported in the United States of America (17), Australia (18). While a wide range of symptoms including diarrhea, weight loss, irritability, edema and body weakness had been associated with the infection (19, 20), there has also been cases of asymptomatic passage of worms (17, 21). Beetle or cockroach serves as intermediate host. R. rattus had also been implicated in the transmission of pathogens causing bubonic plague.
Apart from the M. moniliformis parasite found, T. spiralis is a nematode parasite that can use any mammal as the definitive host or the intermediate host. In this part of the world, it is not uncommon to trap or kill rats for human consumption. In such times, killed rats are mostly cooked, but occasionally merely roasted. Consumption of improperly cooked or roasted infected rat meat may bring about human infection.
In respect of the reportedly found worms that are egg producers, i.e. H. diminuta, T. taeniformis, Ascaris sp. and T. muris, faeces of infected rats is very likely to accidentally contaminate grains sold in markets. This scenario is even more likely to occur in respect of a local food item named “Gaari”. Gaari is a product of peeled, grated, fermented and fried cassava. Such is merely soaked in cold (neither hot nor boiled) water and eaten. Human infection by T. taeniformis and H. diminuta will result in cysticercosis although cases are rather rare, while, by Ascaris sp. and T. muris will induce visceral larva migrans. All these infections may not be readily diagnosed. It is not unlikely that zoonosis does occur in Ile-Ife; however, definitively diagnosing the condition may not be straight forward. The aforementioned are the “implication on human health”.
Conclusion
In this part of the world, diagnostic criteria of diseases are yet to reach the advanced stage. Hence in making differential diagnosis, a clinician here who trained locally will require some knowledge of tropical diseases to think along the line of those that could be acquired from these non-human parasites but that could induce pathologies in humans. And as said earlier on, zoonosis may be occurring in Ile-Ife but remain either unnoticed or diagnosed.
Acknowledgments
The authors declare that there is no conflict of interests.
References
- 1.Price BW, Chitwood BG. Incidence of internal parasites in wild rats in Washington D.C. J Parasitol. 1931;18:55. [Google Scholar]
- 2.Tsuchiya H, Rector LB. Studies on intestinal parasites among wild rats caught in St. Louis. Am J Trop Med. 1956;16:705–714. [Google Scholar]
- 3.Mishera GS, Gonzalez JP. Results of a study on the endoparasites of the rat Rattus norvegicus in Tunisia. Archives de I’ Institut Pasteur de Tunis. 1975;52(1/2):71–87. [Google Scholar]
- 4.Tolani MT, Gandhi NM, Freitas YM. Microbial Flora of the alimentary tract of Rattus rattus and Rattus norvegicus from Bombay. Indian J Microbiol. 1977;11(1-2):13–14. [Google Scholar]
- 5.Kamiya M, Kanda T. Helminth parasites of rats from Ishigaki Island, South western Japan. Japanese J Parasitol. 1977;26(4):271–275. [Google Scholar]
- 6.Balachandra DV, Ranade DR. A preliminary survey of helminth parasites of rats from Poona city. India Vet J. 1978;55(2):175–176. [Google Scholar]
- 7.Monath TP, Maher M, Casals J, Kissling RE, Cacciapuoti A. Lassa fever in Eastern Province of Sierra Leone 11. Clin and Virological Studies in selected hospitalized patients. Am J Trop Med Hyg. 1974;23:1140–1149. doi: 10.4269/ajtmh.1974.23.1140. [DOI] [PubMed] [Google Scholar]
- 8.Wulff H, Lange J. Indirect immunofluorescence for diagnosis of Lassa fever infection. Bull WHO. 1975;52:429–436. [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeh EI, Anosike JC, Okon E. Acanthocephalan infection in man in Northern Nigeria. J Helminthol. 1992;66(3):241–2. doi: 10.1017/s0022149x00014632. [DOI] [PubMed] [Google Scholar]
- 10.Nigerian Population Commission. 2006:282–283. [Google Scholar]
- 11.Vinogradov BS, Argyropulo AI. Fauna of the USSR. Mammals, Mammals, Key to the Rodents. 2. Moscow: Leningrad Pub; 1941. [Google Scholar]
- 12.Cable RM. An illustrated Laboratory manual of Parasitology. 4. Minneapolis, Minn: Burgess Publishing Company; 1958. [Google Scholar]
- 13.Thienpont D, Rochette F, Vanparijs OFJ. Diagnosing Helminthiasis by Corprological Examination. 2. Beerse: Janssen Research Foundation; 1968. (2nd). [Google Scholar]
- 14.World Health Organization News letter. Lassa fever. 2005 [Google Scholar]
- 15.Neafie R, Marty AM. Unusual Infections in Humans. Clin Infect Diseas. 1993;39:173–178. doi: 10.1128/cmr.6.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahba GH, Arfaa F, Rastegar M. Human infection with Moniliformis dubius (Acanthocephala) (Meyer 1932) (Syn Moniliformis moniliformis) (Bremser 1811) (Travassos 1915) in Iran. Trans Roy Soc Trop Med Hyg. 1970;64:284–286. doi: 10.1016/0035-9203(70)90137-9. [DOI] [PubMed] [Google Scholar]
- 17.Counselman K, Field C, Lea G. Moniliformis moniliformis from a child in Florida. Am J Trop Med Hyg. 1989;41:88–90. [PubMed] [Google Scholar]
- 18.Prociv P, Walker J, Crompton LJ. First record of human acanthocephalan infection in Australia. Med J Aust. 1990;152:215–216. doi: 10.5694/j.1326-5377.1990.tb125152.x. [DOI] [PubMed] [Google Scholar]
- 19.Berenji F, Abdolmajid F, Hosseininjad Z. A case of Moniliformis moniliformis (Acanthocephalan) infection in Iran. Korean J Parasitol. 2007;45:145–148. doi: 10.3347/kjp.2007.45.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salehabadi A, Mowlavi G, Sadjjadi SM. Human infections with Moniliformis moniliformis (Bremser1811) (Travasso 1915) in Iran: another case report after three decades. Vector Borne Zoonotic Disease. 2008;8:101–103. doi: 10.1089/vbz.2007.0150. [DOI] [PubMed] [Google Scholar]
- 21.Sahar MM, Madani TA, Al Moshen IZ. A child with an acanthocephalan infection. Ann Saudi Med. 2006;26:321–324. doi: 10.5144/0256-4947.2006.321. [DOI] [PMC free article] [PubMed] [Google Scholar]


