Abstract
Background
The aim of this study was to investigate the effects of Leishmania major infection on the induction of oxidative stress in skin and lung of female mice.
Methods
BALB/c mice were randomly divided into the control and experimental groups. The experimental groups were subcutaneously infected with inoculums promastigotes of L. major. The animals were sacrificed at 20, 40, 60, 90 and 120 days post-infection, and tissues were isolated and analyzed.
Results
Superoxide dismutase activity, percent of DNA fragmentation and superoxide anion production levels were increased in skin and lung of infected mice. Lung catalase activity and skin malondialdehyde level were also increased. The decreased glutathione level was observed in both tissues. The highest alteration in these parameters in both tissues was observed at 90 days post-infection.
Conclusion
L. major infection induces the production of free radicals and oxidative stress in a time-dependent manner in mice skin and lung by depletion of glutathione and increasing lipid peroxidation. The elevated DNA fragmentation may be related with increased oxidative stress. The skin is more sensitive to the effects of L. major infection on oxidative stress induction compared to the lung.
Keywords: Leishmania major, Oxidative stress, BALB/c mice, Skin, Lung
Introduction
Reactive oxygen species (ROS), such as superoxide anion (O2-•), hydrogen peroxide (H2O2) and the highly reactive hydroxyl radical radicals are produced during normal cellular function, which leads to the oxidation of lipids, proteins, and nucleic acids. ROS generation is controlled by the cellular antioxidant defense system. A variety of internal or external pathological factors such as viral and bacterial infections may disrupt the pro-oxidant/antioxidant balance, which leads to oxidative stress (1-2).
Protozoan parasites of the genus Leishmania are responsible for visceral, cutaneous, or mucosal forms of human leishmaniasis, a spectrum of vector-borne diseases endemic in tropical and subtropical countries. This disease affects 12 million people and threatens an additional 350 million people worldwide, with almost 2 million new infections every year (3). Cutaneous leishmaniasis (CL) is the most common form of disease, which causes skin sores. The skin sores of CL may heal on their own, but this can take months or even years. Leishmania (L.) major is the principal agent of disease in Isfahan, Dehloran and Kashan of Iran (4). The clinical expression of the resulting leishmaniasis depends on both the parasite species and the response and immune status of the host (5). Leishmania lives extracellularly as flagellated promastigotes in the gut and salivary glands of the sandfly vector and intracellularly as amastigotes in the vertebrate host macro-phages (6). Exposure of macro-phages to Leishmania lead to the formation of ROS and reactive nitrogen species (RNS), which are responsible for parasite killing in macrophages (7-9). Several studies have clearly demonstrated that both ROS and RNS contribute to the killing of a number of Leishmania species in both human and murine macrophages (9-11). Oxidative events against Leishmania infection are not well elucidated in animals. There are very limited reports on the time-dependent effects of Leishmania infection on induction of oxidative stress in various tissues of the host (11-13). However, studies of oxidative stress as a possible mechanism of skin and lung damage in CL have not been performed. The aim of the present study was to evaluate the oxidative effects of L. major infection on important biomarkers of oxidative stress including malondialdehyde (MDA) content as a marker of lipid peroxidation (LPO) and antioxidant defense parameters such as superoxide anion and glutathione (GSH) levels, activities of superoxide dismutase (SOD) and catalase (CAT) and percent of DNA fragmentation in skin and lung of BALB/c mice. BALB/c mice were selected, because they are very sensitive to L. major infection (14).
Materials and Methods
Animals
This experimental study was performed in 2011-2012. Female BALB/c mice with 20-30 g weight at the age of 6-8 week were obtained from Pastour Institute (Tehran, Iran) and acclimated for at least 2 weeks prior to experimental use. All the animals were fed a standard mouse chow and water ad libitum and maintained under standard conditions of temperature at 20-22 °C and 60±10% relative humidity with an alternating cycle of 12 h light and dark. All procedures were in accordance with the standards for animal care established by the Ethical Committee of the Baqiyatallah University of Medical Sciences.
Parasite culture
MRHO/IR/75/ER of L. major as a prevalent strain of CL in Iran was maintained in BALB/c mice. Amastigotes were isolated from mice spleens, and then transformed to promastigotes in Novy- MacNeal- Nicolle (NNN) medium supplemented with penicillin (100 U/ml), streptomycin (100μg/ml) and 20% heat-inactivated fetal calf serum or FCS (Gibco, UK) at 22±1°C. Subsequently the third passage promastigotes from NNN medium were progressively adopted to RPMI 1640 media (Gibco, UK) supplemented with antibiotics, L-glutamine (30 mg/L) and FCS (15).
Iinfection of mice with L. major promastigotes
BALB/c mice were randomly divided into control (n=42) and experimental (n=52) groups. Infected untreated control groups were received RPMI 1640 medium. Eexperimental groups were subcutaneously infected in the base of the tail with inoculums of 2×106 promastigotes of L. major at stationary phase in 0.1 ml culture medium. The body weight and the mortality of the animals were recorded up to 120 days after inoculation.
Tissue preparation
Seven mice in each group were anesthetized with inhalation of diethyl ether at 20, 40, 60, 90 and 120 days after inoculation. Then, skin and lung tissues were quickly removed and washed in ice-cold phosphate buffer saline (PBS). Washed tissues were immediately immersed in liquid nitrogen and stored at −70°C until biochemical analysis. On the day of use, frozen tissue samples were quickly weighed and homogenized 1:10 in ice-cold PBS. The homogenates were then centrifuged at 16000×g for 15 min at 4°C. The supernatants were separated and used for enzyme activities assays and protein determination
SOD activity assay
The activity of SOD was determined according to Paoletti and Mocali (16). For assay, TDB buffer (triethanolamine – diethanolamine (0.1M each) – 1.38% HCl buffer at pH 7.4) was added into cuvettes, followed by 0.27 mM NADH, 5 mM EDTA, 2.5 mM MnCl2 and 0.1 ml of sample. The samples were read on a Genesys 10 UV spectrophotometer at 340 nm for 5 min to obtain a stable baseline reading, then the reaction was started with 3.75 mM 2-mercaptoethanol and was read again after 10 min. One unit of activity is defined as the amount of enzyme that inhibits the oxidation of NADH by 50% at 25°C and results were expressed as U/mg protein.
CAT activity assay
CAT activity was measured in tissues homogenates by the method of Aebi (17). Reaction mixture containing 0.85 ml potassium phosphate buffer 50 mM, pH 7.0 and 0.1 ml homogenate was incubated at room temperature for 10 min. Reaction was initiated by addition of 0.05 ml H2O2 (30 mM prepared in potassium phosphate buffer, pH 7.0) and the decrease in absorbance was recorded for 3 min at 240 nm. Specific activity is expressed as 1μmole H2O2 decomposed min-1 mg-1 protein.
Determination of GSH level
GSH level were determination by the method of Tietz (18). Cellular protein was precipitated by addition of 5% sulfosalicylic acid and removed by centrifugation at 2000 g for 10 min. GSH in the supernatant was assayed as follows: 100 μl of the protein-free supernatant of the cell lysate, 800 μl of 0.3 mM Na2HPO4 and 100 μl of 0.04% 5, 5′-dithiobis 2-nitrobenzoic acid (DTNB) in 0.1% sodium citrate. The absorbance of DTNB was monitored at 412 nm for 5 min. A standard curve of GSH was performed and sensitivity of measurement was determinated to be between 1 to 100 μM. The level of GSH was expressed as nmol/mg protein.
Determination of MDA level
Skin and lung LPO was determined by measuring the level of MDA (19). One hundred μl of tissue homogenate was added to 50 μl of 8.1% sodium dodecyl sulfate, vortexed and incubated for 10 min at room temperature. Three hundred and seventy-five μl of 20% acetic acid and 375 μl of thiobarbituric acid (0.6%) were added and placed in a boiling water bath in sealed tubes for 60 min. The samples were allowed to cool at room temperature. 1.25 ml of butanol: pyridine (15:1) was added, vortexed and centrifuged at 2000 g for 5 min. Five hundred μl of the colored pink layer was measured at 532 nm on a spectrophotometer using 1, 1, 3, 3-tetra-ethoxypropane as standard. MDA concentration was expressed as nmol/mg protein.
Protein level assay
The total protein concentrations in the cytosols were measured by Bradford’s method (20) using bovine serum albumin as standard.
Assay for superoxide anion production level
Tissue homogenates were centrifuged at 2000g for 15 min at 4 °C. The packed cells were washed twice with PBS and then to each sample 200 μl cytochrome C (160 μM), 200 μl phorbol 12-myristate 13-acetate (10-6 M) was added. Besides to the control was added 17 μl of superoxide dismutase (1mg /ml; 60 units). The samples were incubated at 37°C for 15 min and then centrifuged at 6000 g for 10 min at 4°C. Superoxide anion production was determined from absorbance reading at 550 nm (21).
Quantitative analysis of DNA fragmentation
A small piece (<1 cm3) of frozen tissue sample was quickly weighed and powdered in the liquid nitrogen. The powdered tissues were lysed in 0.5 ml of lysis buffer containing 10 mM Tris–HC1 (pH 8), 1 mM EDTA, 0.2% Triton X-100 and centrifuged at 6000×g for 20 min at 4°C. The pellets were resuspended in 0.5 ml of lysis buffer. To the pellets (P) and the supernatants (S), 0.5 ml of 25% trichloroacetic acid (TCA) was added and incubated at 4°C for 24 h. The samples were centrifuged for 20 min at 6000×g at 4 °C and the pellets were suspended in 80 μl of 5% TCA, followed by incubation at 83°C for 20 min. Then to each sample 160 μl of diphenylamine (DPA) solution (150 mg DPA in 10 ml glacial acetic acid with 150 μl of sulfuric acid and 50 μl acetaldehyde 16 mg/ml) were added and incubated at room temperature for 24 h (22). The proportion of fragmented DNA was calculated from absorbance reading at 600 nm using the following formula:
Statistical analysis
All calculations were performed using INSTAT statistical software. For the time-dependent studies, the data were statistically analysed using analysis of variance (ANOVA) followed by Tukey post hoc multiple comparison test. P-values less than 0.05 were considered statistically significant. Results were expressed as means±SD, with n denoting the number of experiments performed.
Results
Percent of DNA fragmentation and O2-• production level
Effect of L. major infection on skin and lung superoxide anion production level and percent of DNA fragmentation in control and experimental mice is summarized in Table 1. Percent of DNA fragmentation was increased in skin (>40 days, p<0.001 at 90 days) and lung (at 90 days, P<0.05) of infected mice comparing with the control. The increased DNA fragmentation in the skin was higher than the lung. Superoxide anion production level was increased 18.72 (P<0.05), 36.59 (P<0.001), and 26.54 (P<0.01) % in skin and 11.17, 22.35 (P<0.01), and 16.2 (P<0.05) % in lung at 60, 90, and 120 days post-infection.
Table 1.
Effect of L. major infection on skin and lung superoxide anion production level and percent of DNA fragmentation in control and experimental mice at different times. Values are expressed as mean ± SD. (n=7)
| Time (day) | O2•- (nmol/mg protein) |
% DNA Fragmentation |
||
|---|---|---|---|---|
| Skin | Lung | Skin | Lung | |
| Control | 0.358±0.023 | 0.179±0.019 | 9.512±1.247 | 8.642±1.126 |
| 20 | 0.369±0.035 | 0.182±0.016 | 10.542±0.221 | 8.983±0.925 |
| 40 | 0.396±0.038 | 0.188±0.014 | 11.114±0.235 | 9.126±1.039 |
| 60 | 0.425±0.045* | 0.199±0.019 | 12.062±0.254** | 9.891±1.186 |
| 90 | 0.489±0.041***,# | 0.219±0.023**,# | 15.235±0.287***,# | 10.524±1.126* |
| 120 | 0.453±0.032**,# | 0.206±0.018* | 14.947±0.298***,# | 10.245±1.027 |
Control is mean of the control groups at different time intervals.
P<0.05,
P<0.01 and
P<0.001 vs. control.
P<0.05 vs. other times in infected mice
GSH and MDA levels
Mean skin and lung GSH and MDA levels in control and experimental groups at different times post-infection are depicted in Table 2. MDA level was increased 18.86 (P<0.05), 38.9 (P<0.001) and 23.15 (P<0.01) % in skin and 7.9, 14.45 and 11.19% in lung at 60, 90, and 120 days post-infection. GSH level was decreased 11.87, 20.56 (P<0.001) and 17.04 (P<0.01) % in skin and 6.18, 9.25 (P<0.05) and 8.46% in lung at 60, 90, and 120 days post-infection. There was no significant change observed in MDA level in lung of infected mice.
Table 2.
Effect of L. major infection on skin and lung GSH and MDA levels in control and experimental mice at different times. Values are expressed as mean ± SD. (n=7)
| Time (day) | GSH (nmol/mg protein) |
MDA (nmol/mg protein) |
||
|---|---|---|---|---|
| Skin | Lung | Skin | Lung | |
| Control | 16.542±1.458 | 74.124±5.478 | 2.543±0.135 | 4.354±0.415 |
| 20 | 15.875±1.254 | 73.257±4.463 | 2.789±0.221 | 4.456±0.368 |
| 40 | 15.245±1.045 | 71.261±6.527 | 2.913±0.235 | 4.574±0.425 |
| 60 | 14.578±1.062 | 69.542±5.572 | 3.019±0.254* | 4.698±0.548 |
| 90 | 13.141±1.235***,# | 67.853±6.258* | 3.528±0.287***,# | 4.983±0.421 |
| 120 | 13.723±1.123**,# | 67.269±6.465 | 3.128±0.308**,# | 4.841±0.358 |
Control is mean of the control groups at different time intervals.
P<0.05,
P<0.01 and
P<0.001 vs. control.
P<0.05 vs. other times in infected mice
Antioxidant enzyme activities
Figure 1 and 2 shows the alteration of SOD and CAT activities in control and infected mice. SOD activity was significantly increased in skin (>40 days, P<0.001 at 90 days) and lung (at 90 days, P<0.01) of infected mice comparing with the control.
Fig 1.
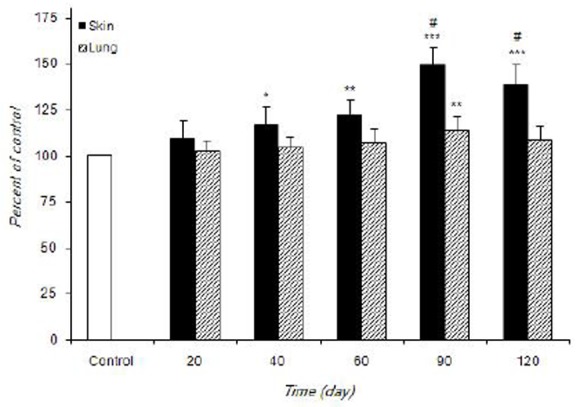
The alteration of SOD activity in control and infected mice during experimental period. Values are expressed as percentage of control (100%)±S.D. (n=7). *P<0.05, **P<0.01 and ***P<0.001 vs control. #P<0.05 vs other times in infected mice
Fig 2.
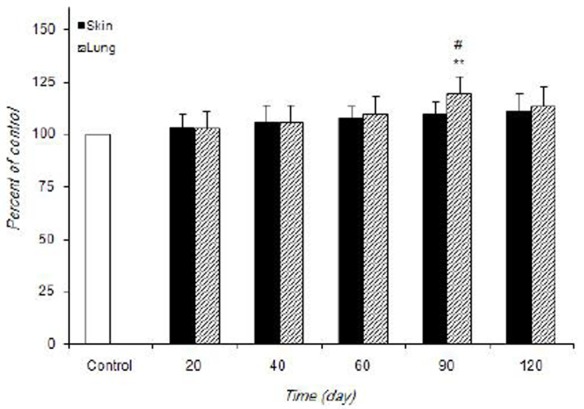
The alteration of CAT activity in control and infected mice at different times during experimentation. Values are expressed as percentage of control (100%)±S.D. (n=7). **P<0.01 vs control. #P<0.05 vs other times in infected mice
The increased CAT activity in lung was observed only at 90 days post-infection (P<0.01). L. major infection did not cause any noticeable increase in skin CAT activity at different times post-infection.
Discussion
Upon infection, macrophages undergo respiratory burst, producing ROS and RNS as part of an oxygen dependent mechanism to destroy invading parasites. Many parasites are susceptible to being killed by ROS and RNS and their relative susceptibilities may vary between species and life cycle stages (23-24). Promastigotes of L. donovani were shown to be more susceptible to ROS than amastigotes (23).
The accumulation of ROS in the cells is capable of damaging lipids of membranes, which are probably the most susceptible if not controlled by appropriate antioxidant scavenging system. MDA as an end-product of LPO, can be used as an index for measuring the damage that occurs in membranes of tissues as a result of free radical generation (1, 25). The present study showed that MDA level was significantly increased in the skin at 60, 90 and 120 days post-infection. L. major infection was caused the highest accumulation of MDA at 90 days in the skin of mice. The enhanced LPO shows that Leishmania infection-induced ROS are not totally scavenged by the antioxidant enzymes. Increased level of erythrocytes MDA has been described in visceral leishmaniasis (VL) in Hamsters (13, 26), dogs (27) and human (25). Heidarpour et al. (28) showed that a significant increase in serum MDA level observed in dog liver and kidney infected with L. infantum. LPO level of the patients with active CL was significantly higher (29, 30).
GSH as an important antioxidant plays a crucial role in protecting cells against ROS and RNS and serves as a substrate for glutathione peroxidase and glutathione S-transferase (GST) during the detoxification of hydrogen peroxide, lipid hydroperoxides and electrophilic compounds. In addition, GSH is involved in protein and DNA synthesis and maintenance of other antioxidants, such as ascorbate and α-tocopherol (31). A significant depletion of GSH was noted in the present study in time dependent manner in mice skin (>60 days) and lung (at 90 days) tissues infected with L. major that is due to high oxidative stress and over utilization of GSH by cells. Depletion of GSH leads to produce oxidized GSH (GSSG) and finally decreased the GSH/GSSG ratio in tissues of infected mice, which is an index of tissue oxidative stress (32). Infection with malarial parasite has been found to produce depletion of GSH in host erythrocytes which may contribute to cell lysis (13). Our finding is in agreement with the results of the previous reports that Infection with parasites depleted GSH in host erythrocytes and whole blood (13, 25, 27). However, the increased GSH level in erythrocytes of CL patients was shown by other studies (25, 29).
Excessive generation of ROS in the tissues affects the integrity of DNA in the nucleus. DNA bases are susceptible to oxidative damage resulting in base modification, strand breaks, and chromatin cross-linking (33). In this study, quantitative DNA fragmentation analysis of skin and lung in infected mice, using diphenylamine reaction procedure, revealed that the fragmentation of DNA was increased after 40 to 120 days in skin and at 90 days of inoculation in lung when compared to the control groups. The maximal effect was observed at 90 days, produced 15 and 10% of DNA fragmentation in skin and lung, respectively. Similar results were observed in spleen and liver of mice infection by L. major (34). Kocyigit et al (35) showed the increased DNA damage and oxidative stress in patients with CL.
Superoxide anions play a key role in protecting the animal from infectious organisms. (36). In the present study, the increased superoxide anion production level in skin and lung were observed in infected mice. Peroxynitrite anion (ONOO) produces from NO and superoxide anion in macrophages, which constitutes a harmful agent against a diverse variety of pathogens such as L. major, and L. amazoniensis (37). It is also able to rapidly penetrate membranes and diffuse across cells thus reacting with various components eventually leading to DNA fragmentation or lipid peroxidation and protein nitration (38). In L. amazonensis, there is evidence of enhanced levels of NO in the blood of infected mice and of peroxynitrite aassociated with their lesions (14).
Mammalian cells have developed complex antioxidant systems to counteract ROS. Several antioxidant enzymes such as SOD and CAT exist that convert ROS into harmless products (2). SOD catalyzes the dismutation of superoxide anion to hydrogen peroxide (H2O2). H2O2 can be transformed into H2O and O2 by CAT. The activities of antioxidant enzymes may be increased or inhibited under infection depending on the duration of the infection (1-2). In present study, the increased SOD activity in skin and lung and CAT activity in lung were observed in infected mice as compared to controls. The increased activities of SOD and CAT are due to the adaptive response to the increased ROS generation in tissues, indicating the failure of the total antioxidant defense mechanism to protect the tissues from mechanical damage caused by L. major infection, as evidenced by lipid peroxidation (Table 2). The increased SOD activity without change in skin CAT activity leads to the accumulation of H2O2 in this tissue, which may be the cause of the induction of oxidative stress (26). This pattern is similar to the obtained results from mice spleen tissue infected with L. major (39). Several studies reported reduction of catalase, and SOD activities of erythrocytes in hamsters (13) and humans (40) and a significant decrease in serum total antioxidant status in dog liver and kidney infected with L. infantum (28).
The increased MDA, and depleted GSH levels in skin of the infected mice, in the present study, were higher than lung. The highest alteration in these parameters in both tissues was observed at 90 days post-infection. The skin is the largest organ in the human body that plays a key role in protecting the body against pathogens (41). It is a major target for Leishmania, where it initially produces cutaneous lesions after a sandfly bite (6). The observed differences in L. major infection-induced responses among various tissues may depend on several factors such as oxygen consumption, metabolic activity rate, susceptibility to oxidants and many more (42).
Understanding the molecular and cellular pathways activated in response to infection with a variety of intracellular protozoan parasites is important for the development of pharmacological intervention to alleviate toxicity. For protection of BALB/c mice against cutaneous L. major infection, it was associated with activation of Th2 cells secreting interleukine (IL)-4, IL-5, IL-6 and IL-10 and the production of ROS and RNS, especially NO which contribute to the regulation of the inflammatory response (12, 43). High concentrations of ROS and RNS have pro-apoptotic effects and prevent the development of parasite (44). In this study, L. major infection in skin and lung induces oxidative stress through the depletion of GSH and generation of ROS, which may lead to increase of intracellular Ca2+ level, lipid peroxidation, DNA damage, alteration of cell antioxidant defense systems and cell death (12, 45). The presence of apoptosis has also been reported in Leishmania and host T cells other unicellular organisms (44, 46, 47). Other studies with L. donovani promastigotes have clearly indicated the involvement of the single mitochondrion of the parasite in precipitating oxidative stress-induced apoptosis through a Ca2+-mediated mechanism (48, 49). However, further studies are required to investigate the effects of L. major infection on induction of cell death in variety of cell types using an in vivo system.
Conclusion
Leishmania major infection induces the production of ROS and oxidative stress in a time-dependent manner in mice skin and lung by increased superoxide anion production, depletion of GSH and increasing lipid peroxidation. Increased ROS not only kill the parasites but also damage the cells and release MDA as a secondary marker of tissue damage. The elevated DNA damage may be related with increased oxidative stress. The skin is found to be more sensitive to the effects of L. major infection on oxidative stress induction compared to the lung.
Acknowledgments
The authors would like to thank H. Mahdavi and J. Rasouli for their assistance. This work was supported by a grant from Molecular Biology Research Center of Baqiyatallah University of Medical Sciences. The authors declare that there is no conflict of interest.
References
- 1.Limon-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Oter S, Jin S, Cucullo L, Dorman HJD. Oxidants and antioxidants: friends or foes? Oxid Antioxid Med Sci. 2012;1:1–4. doi: 10.5455/oams.080612.ed.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Control of the leishmaniases, Technical Report Series 949; Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases; Geneva. 2010. pp. 22–26. [Google Scholar]
- 4.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. East Mediterr Health J. Vol. 10. Republic of Iran: 2004. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran; pp. 591–599. [PubMed] [Google Scholar]
- 5.Yaghoobi R, Maraghi S, Bagherani N. Cutaneous leishmaniasis with unusual presentation. Iranian J Parasitol. 2009;4:67–69. [Google Scholar]
- 6.Ramos CS, Yokoyama-Yasunaka JKU, Guerra-Giraldez C, Price HP, Mortara RA, Smith DF, Uliana SRB. Leishmania amazonensis META2 protein confers protection against heat shock and oxidative stress. Exp Parasitol. 2011;127:228–237. doi: 10.1016/j.exppara.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Kavoosi Gh, Ardestania SK, Kariminia A, Abolhassani M, Turco SJ. Leishmania major: Reactive oxygen species and interferon gamma induction by soluble lipophosphoglycan of stationary phase promastigotes. Exp Parasitol. 2006;114:323–328. doi: 10.1016/j.exppara.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Zamani Sorkhroodi F, Alavi Naeini AM, Zahraei Ramazani AR, Aghaye Ghazvini MR, Mohebali M, Keshavarz SA. Therapeutic effect of sodium selenite and zinc sulphate as supplementary with meglumine antimoniate (glucantime®) against cutaneous leishmaniasis in BALB/C mice. Iranian J Parasitol. 2010;5:11–19. [PMC free article] [PubMed] [Google Scholar]
- 9.Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P. Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med. 2011;51:337–351. doi: 10.1016/j.freeradbiomed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Moreira W, Leblanc E, Ouellette M. The role of reduced pterins in resistance to reactive oxygen and nitrogen intermediates in the protozoan parasite Leishmania. Free Radic Biol Med. 2009;46:367–375. doi: 10.1016/j.freeradbiomed.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Reybier K, Ribauta C, Costea A, Launay J, Fabre PL, Nepveu F. Characterization of oxidative stress in Leishmaniasis-infected or LPS-stimulated macrophages using electrochemical impedance spectroscopy. Biosens Bioelectron. 2010;25:2566–2572. doi: 10.1016/j.bios.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Biswas T, Pal JK, Naskar Kh, Kumar Ghosh D, Ghosal J. Lipid peroxidation of erythrocytes during anemia of the hamsters infected with Leishmania donovani. Mol Cell Biochem. 1995;146:99–105. doi: 10.1007/BF00944601. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira FJ, Cecchini R. Oxidative stress of liver in hamsters infected with Leishmania (L.) chagasi. J Parasitol. 2000;86:1067–1072. doi: 10.1645/0022-3395(2000)086[1067:OSOLIH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Shirbazou Sh, Jafari M. The multiple forms of Leishmania major in BALB/C mice lung in iran. Iranian J Parasitol. 2012;6:99–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrani H, Mahmoodzadeh A. Immunological effects of Leishmania major secretory and excretory products on cutaneous leishmaniasis in BALB/c Mice. Iranian J Parasitol. 2007;2:9–19. [Google Scholar]
- 16.Paoletti F, Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD (P) H oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- 17.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 18.Tietz F. Enzymic method for quantitative determination of nanogram amount of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagl K. Assay for lipid peroxides in animal tissues by thiobarbitoric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Mayo LA, Curnutte JT. Kinetic microplate assay for superoxide production by neutrophils and other phagocytic cells. Methods Enzymol. 1990;186:567–575. doi: 10.1016/0076-6879(90)86151-k. [DOI] [PubMed] [Google Scholar]
- 22.Burton K. The study of the conditions and mechanisms of the diphenylamine reaction for the calorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:615–623. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Channon JY, Blackwell JM. A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. I. Differences in susceptibility correlate with parasite removal of hydrogen peroxide. Parasitology. 1985;91:197–204. doi: 10.1017/s0031182000057309. [DOI] [PubMed] [Google Scholar]
- 24.Mkoji GM, Smith JM, Prichard RK. Antioxidant systems in Schistosoma mansoni: Correlation between susceptibility to oxidant killing and the levels of scavengers of hydrogen peroxide and oxygen free radicals. Int J Parasitol. 1988;18:661–666. doi: 10.1016/0020-7519(88)90101-4. [DOI] [PubMed] [Google Scholar]
- 25.Neupane DP, Majhi S, Chandra L, Rijal S, Baral N. Erythrocyte glutathione status in human visceral leishmaniasis. Indian J Clin Biochem. 2008;23:95–97. doi: 10.1007/s12291-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen G, Mukhopadhyay R, Ghosal J, Biswas T. Oxidative damage of erythrocytes: a possible mechanism for premature hemolysis in experimental Visceral Leishmaniasis in hamsters. Ann Hematol. 2001;80:32–37. doi: 10.1007/s002770000240. [DOI] [PubMed] [Google Scholar]
- 27.Bildik A, Kargin F, Seyrek K, Pasa S, Ozensoy S. Oxidative stress and non-enzymatic antioxidative status in dogs with visceral leishmaniasis. Res Vet Sci. 2004;77:63–66. doi: 10.1016/j.rvsc.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Heidarpour M, Soltani S, Mohri M, Khoshnegah J. Canine visceral leishmaniasis: relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol Res. 2012;111:1491–1496. doi: 10.1007/s00436-012-2985-8. [DOI] [PubMed] [Google Scholar]
- 29.Vural H, Aksoy N, Ozbilge H. Alterations of oxidative-antioxidative status in human cutaneous leishmaniasis. Cell Biochem Funct. 2004;22:153–156. doi: 10.1002/cbf.1066. [DOI] [PubMed] [Google Scholar]
- 30.Serarslan G, Ylmaz HR, Sugut S. Serum antioxidant activities, malondialdehyde and nitric oxide levels in human cutaneous leishmaniasis. Clin Exp Dermatol. 2005;30:267–271. doi: 10.1111/j.1365-2230.2005.01758.x. [DOI] [PubMed] [Google Scholar]
- 31.Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, Hajigholamali M. The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol Mech Methods. 2012;22:638–647. doi: 10.3109/15376516.2012.716090. [DOI] [PubMed] [Google Scholar]
- 32.Werner P, Cohen G. Glutathione disulfide (GSSG) as a marker of oxidative injury to brain mitochondria. Ann NY Acad Sci. 1993;679:363–369. doi: 10.1111/j.1749-6632.1993.tb18323.x. [DOI] [PubMed] [Google Scholar]
- 33.Cocuzza M, Sikka SC, Athayde KS, Agarwal A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int Braz J Urol. 2007;33:603–621. doi: 10.1590/s1677-55382007000500002. [DOI] [PubMed] [Google Scholar]
- 34.Jafari M, Sherbazo Sh, Mahmodzadeh A, Rezaie R, Norozi M. Study of apoptosis in leishmania infected liver cells. Clin Chem Lab Med. 2007;45(Supple. 1):S128–S147. [Google Scholar]
- 35.Kocyigit A, Keles H, Selek S, Guzel S, Celik A, Erel O, Increased DNA. damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat Res. 2005;585:71–78. doi: 10.1016/j.mrgentox.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 37.Augusto O, Linares E, Giorgio S. Possible roles of nitric oxide and peroxynitrite in murine leishmaniasis. Braz J Med Biol Res. 1996;29:853–862. [PubMed] [Google Scholar]
- 38.Auxiliadora Dea-Ayuela M, Ordonez-Gutierrez L, Bolas-Fernandez F. Changes in the proteome and infectivity of Leishmania infantum induced by in vitro exposure to a nitric oxide donor. Int J Med Microbiol. 2009;299:221–232. doi: 10.1016/j.ijmm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Jafari M, Sherbazo Sh, Norozi M. Study of stress oxidative in leishmania infected spleen cells. FEBS J. 2008;275(Supple. 1):S99–S437. [Google Scholar]
- 40.Biswas T, Ghose DK, Mukherjee N, Ghosal J. Lipid peroxidation of erythrocytes in visceral leishmaniasis. J Parasitol. 1997;83:151–152. [PubMed] [Google Scholar]
- 41.Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 42.Kaushik S, Kaur J. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin Chim Acta. 2003;333:69–77. doi: 10.1016/s0009-8981(03)00171-2. [DOI] [PubMed] [Google Scholar]
- 43.Amini M, Nahrevanian H, Farahmand M. Pathogenicity variations of susceptibility and resistance to Leishmania major MRHO /IR /75/ER strain in BALB/c and C57BL/6 mice. Iranian J Parasitol. 2008;3:51–59. [Google Scholar]
- 44.Pinheiro RO, Pinto EF, Benedito EB, Lopes UG, Rossi-Bergmann B. The T-cell energy induced by Leishmania amazonensis antigens is related with defective antigen presentation and apoptosis. Ann Braz Acad Sci. 2004;76:519–527. doi: 10.1590/s0001-37652004000300006. [DOI] [PubMed] [Google Scholar]
- 45.Shaha C. Apoptosis in Leishmania species & its relevance to disease pathogenesis. Indian J Med Res. 2006;123:233–244. [PubMed] [Google Scholar]
- 46.Bertho AL, Santiago MA, Da Cruz AM, Coutinho SG. Detection of early apoptosis and cell death in T CD4+ and CD8+ cells from lesions of patients with localized cutaneous leishmaniasis. Braz J Med Biol Res. 2000;33:317–325. doi: 10.1590/s0100-879x2000000300010. [DOI] [PubMed] [Google Scholar]
- 47.Zangger H, Mottram JC, Fasel N. Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ. 2002;9:1126–1139. doi: 10.1038/sj.cdd.4401071. [DOI] [PubMed] [Google Scholar]
- 48.Mehta A, Shaha C. Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J Biol Chem. 2004;279:11798–11813. doi: 10.1074/jbc.M309341200. [DOI] [PubMed] [Google Scholar]
- 49.Sudhandiran G, Shaha C. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J Biol Chem. 2003;278:25120–25132. doi: 10.1074/jbc.M301975200. [DOI] [PubMed] [Google Scholar]


