Abstract
Background
The present study was carried out to investigate the accurate status of ovine Theileria infection in sheep from Ahvaz and surrounding region, a tropical area southwest Iran.
Methods
A PCR-RFLP method based on 18S ribosomal RNA gene was designed which could detect and differentiate Theileria and Babesia spp. and also differentiate main Theileria species in sheep at the same time. 119 sheep blood samples were collected from Ahvaz and surroundings.
Results
Microscopic examination of blood smears revealed 69.7% (83/119) infection with Theileria spp. Of the total samples subjected to PCR, 89% (106/119) were found to be positive, all of which were identified as Theileria by RFLP analysis using enzyme Hind II. In enzymatic digestion of PCR products by Vsp I, 91.5% (97/106) of Theileria positive samples were identified as T. ovis while mixed Theileria infections were found in 9 samples. The samples with mixed infections were analyzed with an additional nested PCR-RFLP method, by HpaII enzyme digestion. 3 samples with T. lestoquardi infection, 1 sample with T. ovis and T. annulata, 1 sample with T. lestoquardi and T. annulata, and 4 samples with T. ovis, T. lestoquardi and T. annulata mixed infections were detected.
Conclusion
Ovine theileriosis caused by T. ovis is highly prevalent in southwest Iran while T. lestoquardi and T. annulata infection can be detected in a lesser propor-tion of sheep in this region. The new PCR-RFLP method that was designed in this study, can serve as a beneficial diagnostic tool, especially in T. ovis prevalent re-gions.
Keywords: Theileria spp.Babesia, PCR-RFLP, 18S rRNA gene, Sheep, Iran
Introduction
Tick-borne parasites of the genus Theileria infect wild and domestic animals in the tropical and subtropical areas of the world (1). While cattle theileriosis has been extensively studied, not much is known about Theileria infection in sheep (2).
Ovine theileriosis, an important hemoprotozoal disease of sheep and goats (3), is caused by several species of Theileria, of which, T. lestoquardi (syn. T. hirci) and the newly described Theileria spp. china 1 are considered highly pathogenic. The other species such as T. ovis and T. separata cause subclinical infections in small ruminants (1).
T. ovis and T. lestoquardi are believed to cause ovine theileriosis in Iran, according to the clinical observations (4). Theileria lestoquardi, which is a causal agent of malignant ovine theileriosis (5) is distributed in south, south-west and south-east regions (4,6-9), and T. ovis infection is widespread all over the country (4). However, the epidemiological aspects of ovine theileriosis in Iran are poorly understood and further investigations are required to facilitate the development of improved control measures against this tick-borne disease (10).
The diagnosis of small ruminant piroplasmosis is based on clinical symptoms and morphological examination of blood smears (11). Although these methods are practical for the detection of acute disease, they are not reliable for species differentiation due to morphological similarity among these organisms (1).
Serological tests, frequently employed in determining subclinical infections, are commonly associated with false positive and negative results due to cross-reactions or weakening in specific immune response (1).
Recently, PCR has been the preferred method of diagnosis of bovine and ovine theileriosis in epidemiological studies since this technique is more sensitive and specific than other conventional methods and allows the detection of piroplasms at low parasitaemia, discrimination between Babesia and Theileria and that of different Theileria species (12-14).
PCR analysis based on 18S rRNA gene has been successfully applied to identify several Theileria as well as Babesia parasites (15).There have been limited molecular studies on ovine theileriosis in Iran (6,8,9).
Ahvaz, the capital city of Khuzestan Province, is a tropical area southwest Iran which is of great importance in livestock industry. As the hot and humid weather is a predisposing factor, parasitic infections and tick-borne diseases including theileriosis are highly prevalent in this region (9,16).
This study was carried out to investigate the accurate status of Theileria and Babesia infection and also differentiate Theileria species in sheep from Ahvaz and surrounding region, by two PCR-RFLP methods.
Materials and Methods
Parasite isolate
Theileria spp. piroplasms were diagnosed microscopically in sheep blood samples at our laboratory (Department of Clinical Pathology, Faculty of Veterinary Medicine, University of Tehran, Iran). Genomic DNAs extracted from these blood samples were confirmed for T.ovis or T.lestoquardi by 18S rRNA gene PCR and sequence analysis (HeidarpourBami et al. 2009) and used as positive controls for T. ovis and T. lestoquardi specific PCR (17). A venous blood sample, taken from a healthy sheep without previous contact with ticks, was used as negative control.
Collection of blood samples
This study was carried out in Ahvaz, the capital city of Khuzestan Province, and outskirt, which is situated in southwest of Iran, the tropical endemic area of ovine tick-borne diseases. Sampling was performed during the tick activity season, July to September 2011, from eight different areas of Ahvaz and the villages in north, northwest, and south of the city with a history of tick infestation and ovine theileriosis. According to Khuze-stan meteorological organization data, the temperature and humidity of this area in the mentioned period ranges between 26.3° to 47.3° C, and 10% to 48%, respectively. Blood was collected from jugular vein of 119 sheep (50 male and 69 female) into sterile tubes with anticoagulant (EDTA). The animals used in this study ranged in age from 3 months to 9 years. The blood samples were used to prepare thin blood smears for microscopic examination and the remaining was stored at -20° C until performing DNA extraction for PCR analysis.
Microscopic examination
Blood smears were prepared and fixed with methanol for 5 min and stained with 5% Giemsa solution for 30 min and then examined for the presence of piroplasms infection under oil immersion lens (1000×). Parasitemia was assessed by counting the number of infected red blood cells by examination of 200 microscopic fields (approximately 100,000 cells).
DNA extraction
DNA extraction was performed, using molecular biological system transfer kit (MBST Iran), based on the manufacturer’s instructions.
DNA yields were determined with an Eppendorf Biophotometer (Germany), and typical nucleic acids concentration values ranged between 15 and 25 ng/μl. DNA was stored at -20°C until subsequent analysis.
Primer design and PCR amplification
One pair of oligonucleotide primers was designed based on the 18S ribosomal RNA gene sequence of Theileria and Babesia spp. The accession numbers of genes used in this study were AY260171.1 for T. ovis, AF081135.1 for T. lestoquardi, AY260178.1 for B.ovis and AY260180.1 for B.motasi.
Primers designed were forward strand primer FThBab 5′-GCATTCGTATTTAACTGTCAGAGG-3′ and reverse strand primer RThBab 5′- GATAAGGTTCACAAAACTTCCCTAG-3′.
A PCR method was used to detect Theileria and Babesia spp. The PCR was performed in a total reaction volume of 30 μl containing 3 μl 10 X reaction buffer [100 mM Tris-HCl (pH =9), 500 mM KCl, 1% Triton X-100], 1.5 mM MgCl2, 250 μM of each of the four deoxynucleotide triphosphates, 1.5 unit of Taq DNA polymerase (Fermentas) and 20 picomol of each primer. 2 μl of DNA suspension (30-50ng) was used as the template in the PCR.
The amplification was performed in an automated thermocycler (Corbett Research, Australia) under following program: an initial denaturation step at 94°C for 5 min followed by 30 cycles at 94°C for 30 s (denaturing step), 59°C for 60 s (annealing step) and 72°C for 60 s (extension step) with a final extension step of 72°C for 5 min.
Then, 10-μl aliquots of the PCR products were stained with cyber green solution and electrophoresed through a 1.5% agarose gel. After electrophoresis, results were visualized by UV transilluminator. Expected PCR products for the Theileria and Babesia spp. were 861 bp.
RFLP of PCR products
To differentiate Theileria and Babesia and also various Theileria species which infect sheep (T. ovis and T. lestoquardi) RFLP of PCR products of the 18S rRNA gene was done. The PCR amplified a DNA fragment of 861 bp size, which was sequenced and analyzed for the presence of restriction sites. The Hind II enzyme was found to discriminate between Theileria and Babesia, and VspI enzyme to differentiate T.ovis and T.lestoquardi.
The amplified products were digested with restriction enzymes (Fermentas) as described by the supplier. The digestion reaction was set up in 20 μl volumes within 500 μl PCR tubes. The enzymatic digestion was carried out in 20 μl reaction mixtures consisting of 10 μl PCR amplicons, 2 μl 10X corresponding buffer, and 0.1 μl (1 U) restriction enzyme made up to 20 μl with autoclaved triple-distilled water. The digestion mixture was incubated at 37°C for 2 h., electrophoresed on 3% agarose gel and analyzed by SYBR green staining under UV condition. The restriction analysis patterns are listed in Table 1.
Table 1.
The DNA fragments size expected for PCR- RFLP of different Theileria species using HindII and VspI enzymes
| Species | Hind II | Vsp I |
|---|---|---|
| Babesia spp. | 170, 242, 439 bp | |
| Theileria spp. | 418, 443 bp | |
| T. ovis | 86, 171, 605 bp | |
| T. lestoquardi | 86, 776 bp | |
| T. annulata | 86,776 bp |
Detection of mixed infections
The developed PCR–RFLP assay cannot differentiate T. lestoquardi from T. annulata. Therefore, a nested PCR-RFLP method using HpaII enzyme digestion was employed to identify these species (17).
Results
Microscopic examination of 119 Giemsa-stained blood smears obtained from eight different areas of Ahvaz and surrounding region, revealed that 69.7% (83/119) sheep were infected with Theileria spp. piroplasms. No Babesia spp. piroplasms were detected in the blood smears.
In order to assess the true status of Theileria infection in sheep from Ahvaz region, the samples were analyzed by PCR, to detect any amplification.
Of the total samples subjected to PCR, 89% (106/119) were positive as revealed by the amplification of a 861-bp product, all of which were identified as Theileria by RFLP analysis using Hind II enzyme (Figs 1 and 2). One Babesia ovis DNA sample which was also subjected to this method, as positive control, resulted in expected fragments in RFLP analysis (Fig.3), differentiating it from Theileria spp.
Fig 1.
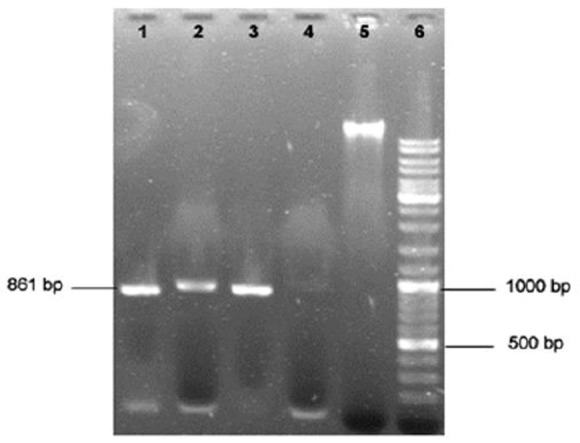
Agarose gel electrophoresis of Theileria/Babesia 18SrRNA gene PCR product. Lanes 1 to 3 positive samples; lane 4 negative sample; lane 5 extracted genomic DNA; lane 6, 100 bp DNA ladder
Fig 2.
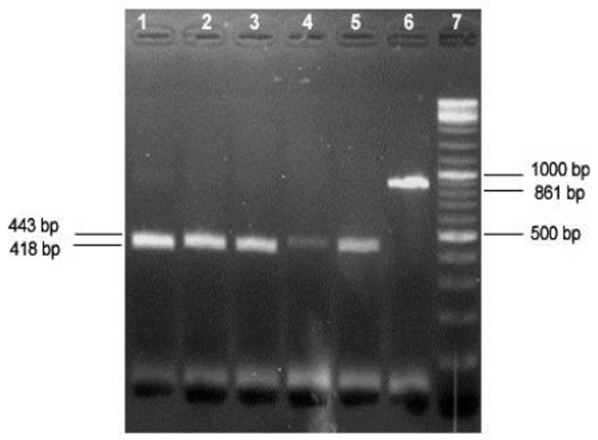
RFLP analysis of PCR products of Theileria 18SrRNA gene by HindII restriction enzyme. Lanes 1 to 5, digested Theileria PCR products; Lane 6, undigested PCR product; Lane 7, 100 bp DNA ladder
Fig 3.
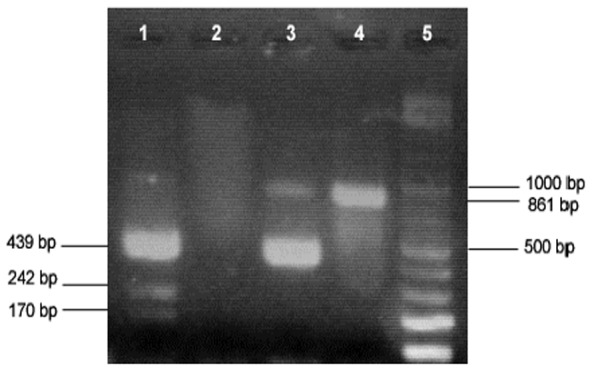
RFLP analysis of PCR products of Babesia and Theileria 18SrRNA gene by HindII restriction enzyme. Lane 1, Babesia digestion pattern; Lane 2, Negative control; Lane 3, Theileria digestion pattern; Lane 4, undigested PCR product; Lane 5, 100 bp DNA ladder
All microscopically positive samples were confirmed by PCR. No Theileria piroplasms were seen on blood smears of samples that were negative in PCR.
However there were 23 PCR positive samples which were negative in microscopic examination (Table 2).
Table 2.
Comparison of microscopic examination and PCR analysis results in Theileria infection diagnosis in sheep
| PCR analysis | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Microscopic examination | Positive | 83 | 0 | 83 |
| Negative | 23 | 13 | 36 | |
| Total | 106 | 13 | 119 | |
In enzymatic digestion of PCR products by VspI, to differentiate various Theileria species in sheep, 97 out of 106 (91.5%) Theileria positive samples were identified as T.ovis (Fig. 4).
Fig 4.
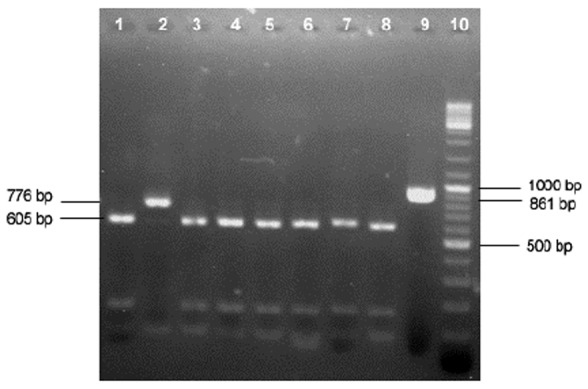
RFLP analysis of PCR products of Theileria 18SrRNA gene by VspI restriction enzyme. Lanes 1, 3 to 8, T. ovis; Lane 2, T. lestoquardi/T. annulata; Lane 9, undigested PCR product; Lane 10, 100 bp DNA ladder
Mixed Theileria infections were diagnosed in 9 blood samples (8.5%) of the total 106 Theileria positive animals. The results demonstrated that 3 samples of these were infected with T. lestoquardi. 1 sample with T. ovis and T. annulata, 1 sample with T. lestoquardi and T. annulata, and 4 samples with T. ovis, T. lestoquardi and T. annulata mixed infections were also detected(Table 3).
Table 3.
Theileria spp. infections detected in sheep blood samples by PCR-RFLP
| Species | Number |
|---|---|
| T. ovis | 97 |
| T. lestoquardi | 3 |
| T. ovis, T. annulata | 1 |
| T. annulata, T. lestoquardi | 1 |
| T. ovis, T. annulata, T.lestoquardi | 4 |
| Total | 106 |
Discussion
Theileriosis and babesiosis are important diseases in small ruminants which cause high economic losses in Iran (13).
Diagnosis of ovine theileriosis is traditionally made through microscopic examination of thin blood smears and many studies about piroplasms infections have been reported in sheep and goats from different regions of Iran (4, 5, 18). However, a wide-ranging survey regarding Theileria infection rate in sheep has not been done so far in the southwest of Iran which is an endemic area.
In the present study, microscopic examination of sheep blood smears obtained from different areas of Ahvaz and surroundings revealed Theileria spp. infection in 69.7% (83/119) of sampled sheep.
Ovine theileriosis have been reported in various parts of Iran (6, 18) and neighboring countries including Iraq (16, 19), Turkey (1) and Pakistan (20), using microscopic blood smear examination.
Theileria species cannot be reliably detected and differentiated according to their piroplasms structure by microscopy, especially in subclinical infections (6, 18). To identify the role of each species in the epidemiology of ovine theileriosis, sensitive and specific diagnostic tests, such as polymerase chain reaction (PCR), are required to be used (1,3,17).
It has been proved that some Theileria and Babesia spp. share the same vector, and in most endemic areas sheep are infected with both Theileria and Babesia. Thus, it would be practical to make use of a method that is able to simultaneously detect these two genera (13). Shayan and Rahbari (2005) showed that a common primer derived from hyper variable region V4 of 18S rRNA can be used for simultaneous differentiation of Theileria from Babesia by PCR.
In the current study, the DNA samples of sheep from Ahvaz and outskirt were subjected to PCR, using one pair of primers designed based on 18S ribosomal RNA gene sequence of Theileria and Babesia spp. followed by two enzymatic digestions.
Overall, 89% of examined samples were piroplasm positive, and all of which were identified as Theileria by RFLP analysis. In enzymatic digestion of PCR products, the majority (91.5%) of Theileria positive samples were identified as T.ovis while mixed Theileria infections were also detected in 9 samples (8.5%).
Nested PCR-RFLP analysis of mixed infections revealed that 3 samples were infected with T. lestoquardi, 1 sample with T. ovis and T. annulata, 1 sample with T. lestoquardi and T. annulata, and 4 samples with T. ovis, T. lestoquardi and T. annulata. Our data was comparable to the previous studies on prevalence of Theileria spp. infection in sheep in Iran and other countries.
Theileria species involved in ovine theileriosis in eastern half of Iran were determined by Heidarpour Bami et al. (2010). Of the total collected blood samples subjected to nested-PCR, 60% were positive for Theileria spp., of which, 55.3% were identified asT. lestoquardi and 44.7% were T. ovis, in RFLP analysis (6). Zaeemi et al. (2011) carried out a nested PCR–RFLP to identify Theileria species in sheep in some area in western half of Iran including Ahvaz. In nested PCR assessment,Theileria infection was found in 32.8% of samples, out of them, 54.8% and 40.2% were determined as T. lestoquardi and T. ovis, respectively. Mixed infections of T. annulata and T. lestoquardi were also detected in 4.8% of cases (9). In their study using PCR-RFLP, the highest rate of infection, 60% (30/50), was observed in Ahvaz, which was consisted of T. lestoquardi (86.6%) and mixed infections with T. annulata and T. lestoquardi (13.3%). It is evident that these results differ from our findings on the species involved in ovine theileriosis in Ahvaz region. This may be attributed to different areas selected for sampling in this endemic region.
T. annulata commonly found and pathogenic in cattle was detected in mixed infections in sheep both in the current study and in a previous one in Ahvaz (9). Since, Khuzestan Province is a known endemic site for both bovine and ovine theileriosis and these animals are raised together in some parts of this region, our findings regarding mixed infections of T. annulata in sheep are reasonable. The presence of antibodies against T. annulata was demonstrated earlier in naturally infected sheep (21,22).
Ovine theileriosis was reported in neighbors of Iran like Turkey and Pakistan, using molecular methods. In a survey of sheep Theileria parasites in eastern Turkey, 41.2% of blood samples were found positive for Theileria spp. in PCR analysis, whereas none were amplified by Theileria lestoquardi-specific primers (1). Prevalence of ovine theileriosis in district Lahore, Pakistan was determined by PCR, 35%, out of which 79% were positive for T. ovis and 21% for T. lestoquardi (11).
Ovine theileriosis prevalence, in microscopic and PCR assessment, was higher in our results compared to previous studies in Iran and neighboring countries. This may be related to the environmental status in Ahvaz region which is favorable for tick vectors propagation and transmission and other predisposing factors and underlying diseases that increase animals’ susceptibility to infection.
Conclusion
The results obtained from the present study demonstrated that ovine theileriosis is present and highly prevalent in Ahvaz and surrounding region, southwest Iran. T. ovis was the dominant causative agent in this region but the evidence of T. lestoquardi and T. annulata infection of sheep in a few cases were noted, as well. The possibility of natural infection of sheep with T. annulata may complicate epidemiology of bovine theileriosis. In addition, the PCR-RFLP method that was designed and successfully employed in this study, with at least one step less than other available approaches, is more economical and efficient in detecting and differentiating T.ovis and T. lestoquardi infection in sheep even at low levels and can serve as a beneficial diagnostic tool, especially in T. ovis prevalent regions.
Acknowledgments
This study was supported by the Faculty of Veterinary Medicine, University of Tehran, Iran. The authors would like to acknowledge all veterinarians and technicians in Veterinary Organization of Ahvaz and Faculty of veterinary Medicine, Shahid Chamran University of Ahvaz who helped in sample collection. We also thank the staff in Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran, for excellent technical assistance. The authors declare that there is no conflict of interest.
References
- 1.Aktas M, Altay K, Dumanli N. Survey of Theileria parasites of sheep in eastern Turkey using polymerase chain reaction. Small Rum Res. 2005;60:289–293. [Google Scholar]
- 2.Gao YL, Yin H, Luo JX, Ouyang WQ, Bao HM, Guan GQ, Zhang QC, Lu WS, Ma ML. Development of an enzyme-linked immunosorbent assay for the diagnosis of Theileria sp. infection in sheep. Parasitol Res. 2002;88:8–10. doi: 10.1007/s00436-001-0560-9. [DOI] [PubMed] [Google Scholar]
- 3.Altay K, Aktas M, Dumanli N. Theileria infections in small ruminants in the East and Southeast Anatolia. Türkiye Parazitoloji Dergisi. 2007;31:268–271. [PubMed] [Google Scholar]
- 4.Hashemi-Fesharaki R. Tick-borne diseases of sheep and goats and their related vectors in Iran. Parasitologia. 1997;39:115–117. [PubMed] [Google Scholar]
- 5.Hooshmand-Rad P, Hawa NJ. Malignant theileriosis of sheep and goats. Trop Anim Health Prod. 1973;5:97–102. [Google Scholar]
- 6.HeidarpourBami M, Khazraiinia P, Haddadzadeh HR, Kazemi B. Identification of Theileria species in sheep in the eastern half of Iran using nested PCR-RFLP and microscopic techniques. Iranian J Vet Res. 2010;11:262–266. [Google Scholar]
- 7.Razmi GR, Hossieni M, Aslani MR. Identification of tick vectors of ovine theileriosis in an endemic region of Iran. Vet Parasitol Res. 2003;116:1–6. doi: 10.1016/s0304-4017(03)00254-1. [DOI] [PubMed] [Google Scholar]
- 8.Spitalska E, Namavari MM, Hosseini MH, Shad-del F, Amrabadi OR, Sparagano OAE. Molecular surveillance of tick-borne diseases in Iranian small ruminants. Small Rum Res. 2005;57:245–248. [Google Scholar]
- 9.Zaeemi M, Haddadzadeh H, Khazraiinia P, Kazemi B, Bandehpour M. Identification of different Theileria species (Theileria lestoquardi Theileria ovis, and Theileria annulata) in naturally infected sheep using nested PCR-RFLP. Parasitol Res. 2011;108:837–843. doi: 10.1007/s00436-010-2119-0. [DOI] [PubMed] [Google Scholar]
- 10.Haddadzadeh HR, Rahbari S, Khazraiinia P, Nabian S. New concepts on limiting factors of ovine and caprine malignant theileriosis (OCMT) in Iran. Iranian J Vet Res. 2004;5:43–46. [Google Scholar]
- 11.Inci A, Ica A, Yildirim A, Duzlu O. Identification of Babesia and Theileria species in small ruminants in Central Anatolia (Turkey) via reverse line blotting. Turk J Vet Anim Sci. 2010;34:205–210. [Google Scholar]
- 12.Altay K, Dumanlia N, Holmanb PJ, Aktas M. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet Parasitol. 2005;127:99–104. doi: 10.1016/j.vetpar.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Shayan P, Rahbari S. Simultaneous differentiation between Theileria spp. and Babesia spp. on stained blood smear using PCR. Parasitol Res. 2005;97:281–286. doi: 10.1007/s00436-005-1434-3. [DOI] [PubMed] [Google Scholar]
- 14.Kirvar E, Ilhan T, Katzer F, Wilkie G, Hooshmand-Rad P, Brown D. Detection of Theileria lestoquardi (hirci) in ticks, sheep, and goats using the polymerase chain reaction. Ann NY Acad Sci. 1998;849:52–62. doi: 10.1111/j.1749-6632.1998.tb11033.x. [DOI] [PubMed] [Google Scholar]
- 15.Gubbels MJ, Hong Y, Van Der Weide M, Qi B, Nijman IJ, Guangyuan L, Jongejan F. Molecular characterisation of the Theileria buffeli/ orientalis group. Int J Parasitol. 2002;33:943–952. doi: 10.1016/s0020-7519(00)00074-6. [DOI] [PubMed] [Google Scholar]
- 16.Al-Amery MAY, Hasso SA. Laboratory diagnosis of novel species of Theileria hirci, Eimeria caprovina and Eimeria pallida in goats in Iraq. Small Rum Res. 2002;44:163–166. [Google Scholar]
- 17.HeidarpourBami M, Haddadzadeh HR, Kazemi B, Khazraiinia P, Bandehpour M, Aktas M. Molecular identification of ovine Theileria species by a new PCR-RFLP method. Vet Parasitol. 2009;161:171–177. doi: 10.1016/j.vetpar.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Razmi GR, Eshrati H, Rashtibaf M. Prevalence of Theileria spp. infection in sheep in South Khorasan province Iran. Vet Parasitol. 2006;140:239–243. doi: 10.1016/j.vetpar.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Al-Alousi TL, Hayatee ZG, Latif BMA. Incidence of Theileriasis in sheep of Mosul area in Iraq. J Vet Parasitol. 1988;2:149–150. [Google Scholar]
- 20.Durrani AZ, Younus M, Kamal N, Mehmood N, Shakoori AR. Prevalence of Ovine Theileria Species in District Lahore Pakistan. Pakistan J Zool. 2011;43:57–60. [Google Scholar]
- 21.Salih DA, El Hussein AM, Hayat M, Taha KM. Survey of Theileria lestoquardi antibodies among Sudanese sheep. Vet Parasitol. 2003;111:361–367. doi: 10.1016/s0304-4017(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 22.Leemans I, Brown D, Hooshmand-Rad P, Kirvar E, Uggla A. Infectivity and cross-immunity studies of Theileria lestoquardi and Theileria annulata in sheep and cattle: I. In vivo responses. Vet Parasitol. 1999;82:179–192. doi: 10.1016/S0304-4017(99)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


