Abstract
Fusarium wilt is caused by the infection and growth of the fungus Fusarium oxysporum in the xylem of host plants. The physiological responses of cucumbers that are infected with Fusarium oxysporum f. sp. cucumerinum (FOC) was studied in pot and hydroponic experiments in a greenhouse. The results showed that although water absorption and stem hydraulic conductance decreased markedly in infected plants, large amounts of red ink accumulated in the leaves of infected cucumber plants. The transpiration rate (E) and stomatal conductance (gs) of the infected plants were significantly reduced, but the E/gs was higher than healthy plants. We further found that there was a positive correlation between leaf membrane injury and E/gs, indicating that the leaf cell membrane injury increased the non-stomatal water loss from infected plants. The fusaric acid (FA), which was detected in the infected plant, resulted in damage to the leaf cell membranes and an increase in E/gs, suggesting that FA plays an important role in non-stomatal water loss. In conclusion, leaf cell membrane injury in the soil-borne Fusarium wilt of cucumber plants induced uncontrolled water loss from damaged cells. FA plays a critical role in accelerating the development of Fusarium wilt in cucumber plants.
Fusarium wilt, which is caused by Fusarium oxysporum, causes yield losses and poor quality of plants1. The visible symptoms of Fusarium wilt include leaf yellowing, wilting, vascular tissue damage, and ultimately plant death2,3. Fusarium oxysporum infects the host plant via the roots or stem, especially through root wounds, and invades the vascular system4. The chlamydospores can survive for many years in infested field soil and are difficult to eliminate5.
The water relations of host plants that are infected with Fusarium oxysporum have been widely illustrated6,7. The major physiological processes of wilting in plants that are infected with Fusarium oxysporum include a disturbance in the water balance, which can be attributed to factors such as reducing root water uptake8, increasing resistance to water flow through xylem elements9,10, and increasing leaf non-stomatal water loss from damaged cells11. Pathogen infection affects the physiological processes of host plants in different aspects7,10,12,13. The water status and water loss pathways during Fusarium oxysporum infection are extremely important for illustrating the mechanism underlying plant wilt.
The mechanism of the pathological wilting of higher plants is generally attributed to vessel plugging and/or systemic toxicity14. The plugging theory indicates that the vessels of infected plants are plugged by fungal hyphae15, thus limiting water transport in the xylem. Generally, pathogen-infected plants form vascular occlusions of callose16, tylose17, or gel18 to inhibit the intrusion and spread of the pathogen19. Vascular blockage reduces the diameter of the conductive elements and increases resistance to water movement9,10, ultimately resulting in leaf wilt due to water deficiency20.
The systemic toxin theory considers that the toxins that are produced by pathogens disturb the metabolism of the infected plant, resulting in leaf wilt21. The toxins reduce the stem hydraulic conductance and leaf water potential22, regulate stomatal opening23, and induce membrane injury, leading to water leakage24. Fusaric acid (FA, 5-n-butyl-2-pyridine carboxylic acid), which is the main fungal toxin that is produced by Fusarium oxysporum, plays an important role in the infection of higher plants7,25. Fusaric acid, whose production depends on the virulence of Fusarium oxysporum isolates26, can be isolated in diseased plant tissues that are inoculated with Fusarium pathogens7,27. Fusaric acid causes the early hyperpolarisation of the root membrane electrical potential, which acidifies the extracellular medium and increases membrane permeability28,29, with a consequent reduction in root growth, leading to apoptosis, necrosis, and even death28,30,31,32.
Previous studies have focused on identifying the physiological mechanism of plant wilt via the plugging and toxin theories; however, the pathological mechanism of Fusarium wilt is still unclear due to the complicated interactions between water loss and plant wilt. This study aimed to further characterise the mechanism by which Fusarium oxysporum f. sp. Cucumerinum (FOC, the causal agent of cucumber Fusarium wilt) affects the water relations and membrane damage of cucumber plants that are grown in a controlled environmental greenhouse, with the ultimate goal of providing direct evidence as to whether the ability of water absorption and transportation is associated with plant wilt.
Results
Effects of FOC infection on photosynthesis and the water relations of cucumber plants
The plant biomass, leaf net photosynthetic rate, stomatal conductance and intercellular CO2 concentration of Fusarium oxysporum f. sp. cucumerinum (FOC)-infected cucumber plants were significantly lower than those of healthy plants (Table 1). Both leaf transpiration rate and plant water uptake were markedly reduced after FOC infection, by which the transpiration rate was reduced by 46%, and the water uptake was reduced by only 23%. To evaluate the effects of pathogen infection on plant water transport, shoot hydraulic conductance was determined. FOC infection significantly reduced the hydraulic conductance of cucumber plants to 3.07 × 10−6 kg s−1 MPa−1, approximately 1/7 of the healthy plants. Although the stomatal conductance was reduced in the infected plants, which resulted in a lower leaf transpiration rate (E), the ratio of the transpiration rate to stomatal conductance (E/gs) was markedly increased in infected plants. These data indicate that water was lost from a non-stomatal pathway in infected plants. The leaf water content of the infected plants was significantly lower than that of the healthy cucumber plants (Table 1).
Table 1. Effects of FOC infection on the plant biomass, net photosynthetic rate (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (E), water uptake, hydraulic conductance, ratio of transpiration rate to stomatal conductance (E/gs) and leaf water content of cucumber plants.
| Parameters | Healthy | Infected |
|---|---|---|
| Plant biomass (g DW plant−1) | 1.67 ± 0.35 a | 0.89 ± 0.15 b |
| A (μmol CO2 m−2 s−1) | 17.3 ± 1.9 a | 11.8 ± 1.2 b |
| gs (mol H2O m−2 s−1) | 0.71 ± 0.09 a | 0.22 ± 0.05 b |
| Ci (μmol L−1) | 351 ± 13 a | 293 ± 24 b |
| E (mmol H2O m−2 s−1) | 8.00 ± 0.48 a | 4.08 ± 0.56 b |
| Water uptake (g plant−1 day−1) | 115 ± 10 a | 89 ± 7 b |
| Hydraulic conductance (10−6 kg s−1 MPa−1) | 20.9 ± 8.3 a | 3.07 ± 0.7 b |
| E/gs (×10−3) | 11.4 ± 0.9 b | 18.6 ± 1.6 a |
| Leaf water content (%) | 87.8 ± 0.8 a | 80.8 ± 2.3 b |
Note: The ratio of the transpiration rate to stomatal conductance (E/gs) was calculated to indicate the water loss outside of the stomata. The data are shown as the mean ± SD of five replicates. The significant differences (P < 0.05) among the treatments are indicated by different letters.
The red ink absorption of healthy and FOC-infected cucumber plants is illustrated in Fig. 1. Both the veins and the mesophyll cells of the infected leaves stained red, whereas only the veins of healthy leaves stained red. Compared to the healthy cucumber plants, the infected plants accumulated more red ink in the leaves.
Figure 1. Microscopic observation of cucumber leaves that were stained with red ink.
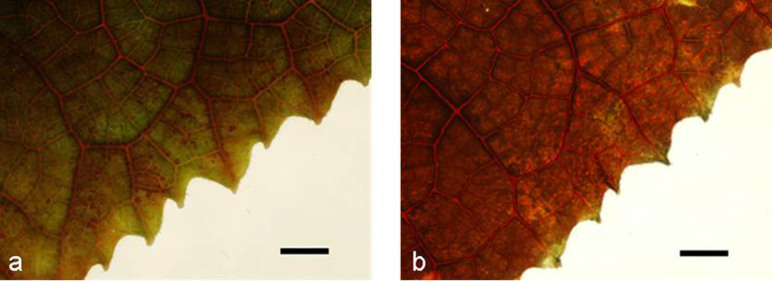
The red ink is mainly distributed in the veins of the healthy leaves (a) but is uniformly distributed in the veins and mesophyll cells of the FOC-infected cucumber leaves (b). The roots of the plant were immersed in red ink for 12 h. For analysis, the leaf directly above a fully expanded leaf was used. Scale bar = 5 mm.
Leaf membrane injury caused by FOC infection
The leaf membrane injury of the infected plants was approximately 45% higher than that of the healthy plants (Table 2). The electrical conductance of the apoplastic sap in the infected plants (107.7 μS cm−1) was significantly higher than that of the healthy plants (52.3 μS cm−1), indicating severe membrane injury (Table 2). Similar results of electrical conductance were observed regarding the apoplastic soluble sugar content (Table 2), in which apoplastic soluble sugar content was greater in the infected plants (49.0 μg) compared to that of the control plants (18.4 μg).
Table 2. Membrane injury, electrical conductance and soluble sugar content of the apoplastic sap in the leaves of healthy and FOC-infected plants.
| Treatments | Membrane injury (%) | Electrical conductance of apoplastic sap (μS cm−1) | Soluble sugar content of apoplastic sap (μg) |
|---|---|---|---|
| Healthy | 12.1 ± 1.1 b | 52.3 ± 3.7 b | 18.4 ± 1.8 b |
| Infected | 21.9 ± 3.8 a | 107.7 ± 20.3 a | 49.0 ± 6.3 a |
Note: The membrane injury was calculated as the percentage of the total electrolyte content. The apoplastic sap was collected by centrifugation. The electrical conductance was measured directly using a micro-electrical conductivity meter. The soluble sugar content was measured by anthrone colourimetry. The data are shown as the mean ± SD of four replicates. The significant differences (P < 0.05) among the treatments are indicated by different letters.
The effect of FOC infection on the ultrastructure of the leaf mesophyll cell plasma membrane in cucumber seedlings was analysed by transmission electron microscopy (TEM). The plasma membrane of healthy plants was intact and smooth, with starch granules clearly appearing in the chloroplasts (Fig. 2a). In contrast, the plasma membranes of the infected plants were ruptured, and more cell inclusions were present in the infected cells, suggesting cell membrane leakage (Fig. 2b).
Figure 2. Transmission electron micrographs of the mesophyll cells in the leaves of healthy and FOC-infected cucumber plants.
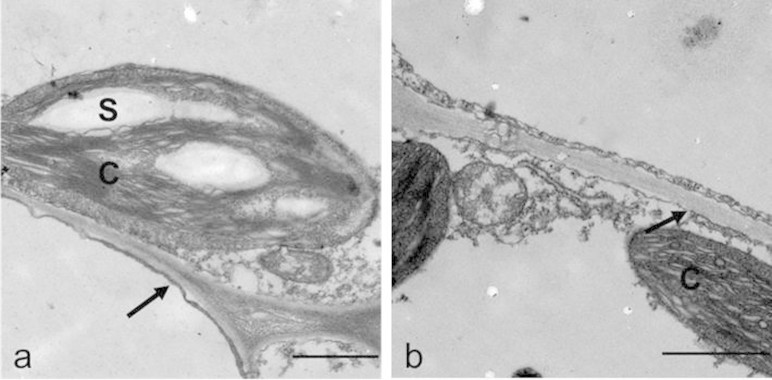
(a) The plasma membrane of the healthy plants leaf was intact, and the starch granule was visible in the chloroplast. Scale bar = 1 μm. (b) The membrane in the FOC-infected plants was broken, and the starch granule was not observed in the chloroplast. Scale bar = 1 μm. S, starch granule; C, chloroplast. The arrows point to the plasma membrane.
Non-stomatal water loss induced by leaf cell membrane injury
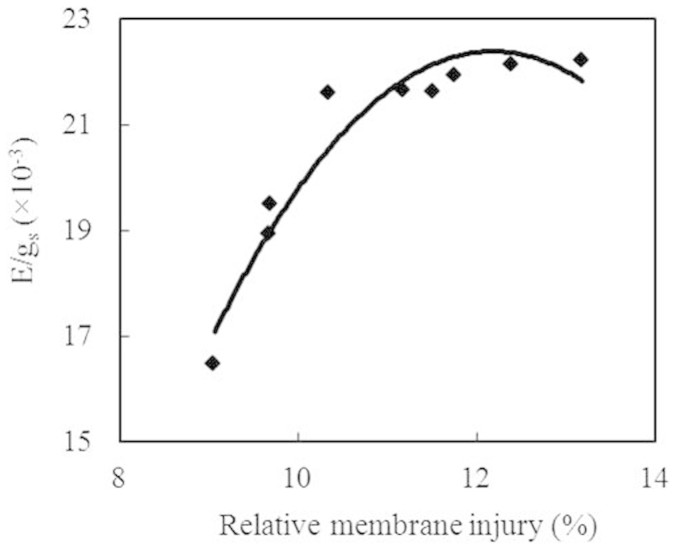
There was a significant positive correlation (P < 0.01) between the leaf membrane injury and E/gs, suggesting that the non-stomatal water loss in the leaves of the infected plants was induced by leaf cell membrane injury (Fig. 3).
Figure 3. Correlation between membrane injury and non-stomatal water loss (E/gs).
(y = −0.5464x2 + 13.31x − 58.666, R2 = 0.925, P < 0.01, n = 9).
Response of leaf temperature to light intensity and FOC infection
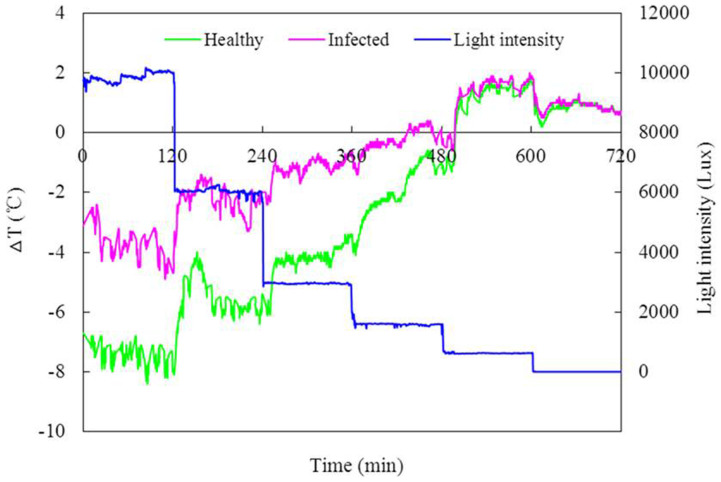
The leaf temperature of both the healthy and FOC-infected plants increased as the light intensity decreased (Fig. 4). When the light intensity ranged from 10,000 Lux to 2000 Lux, the leaf temperature of the infected plants was significantly higher than that of the healthy plants (P < 0.05). The leaf temperature response to the light intensity of the infected plants was less sensitive compared to that of the healthy plants, suggesting a decrease in the regulation ability of the stomata.
Figure 4. Response of the leaf temperature to the light intensity in the healthy and FOC-infected cucumber plants.
The light intensity changed from 10,000 Lux to 0 Lux. The leaf temperature was recorded by digital infrared thermography. ΔT represents the temperature difference between the leaf temperature and the environmental temperature.
FA content in cucumber seedlings after FOC infection
In the FOC-infected plants, FA accumulated in both the leaves and the stem (Table 3). The FA content in the leaves of the infected plants was approximately 2-fold of that in the stem, whereas FA was not detectable in both the leaves and the stem of the healthy plants.
Table 3. Effect of FOC infection on the leaves and stem FA contents of cucumber plants.
Note: The data are shown as the mean ± SD of four replicates. The significant differences (P < 0.05) among the treatments are indicated by different letters.
aND indicates not detected.
Effects of FA on cucumber seedlings
Both the veins and mesophyll cells of the FA (100 ppm)-treated plants stained deep red after red ink absorption (Fig. 5b), while the red stain was difficult to observe in the control plants (Fig. 5a). The amount of red stain (the absorbance at 515 nm) in 100-ppm-FA-treated leaves was approximately 9-fold of that in the control leaves (Table 4). The leaf conductivity was significantly higher in the FA-treated plants compared to that of the control, indicating leaf membrane injury (Table 4). Compared to those of the control plants, the leaf stomatal conductance and transpiration rate were markedly reduced in the FA-treated plants. The ratio of the E/gs increased approximately 35% under FA treatment.
Figure 5. Microscopic observation of FA-treated cucumber leaves that were stained with red ink.

The roots of the cucumber were immersed in red ink solution after being exposed to 0 ppm (a) and 100 ppm FA (b) for 9 h. The red ink was distributed throughout the whole leaves of the plants that were treated with 100 ppm FA (b), while less red colour was observed in the leaves of the control plants (a). The roots of the plant were immersed in red ink for 10 h. For analysis, the leaf directly above the fully expanded leaf was used. Scale bar = 5 mm.
Table 4. Effects of FA on the absorbance of red ink, membrane injury, transpiration rate (E), stomatal conductance (gs) and the ratio of transpiration rate to stomatal conductance (E/gs) of cucumber leaves.
| Treatments | Absorbance of red ink | membrane injury (%) | E (mmol H2O m−2 s−1) | gs (mol H2O m−2 s−1) | E/gs (×10−3) |
|---|---|---|---|---|---|
| 0 ppm | 0.077 ± 0.007 b | 9.46 ± 1.39 b | 7.06 ± 0.65 a | 0.302 ± 0.057 a | 23.7 ± 2.46 b |
| 100 ppm | 0.689 ± 0.082 a | 25.59 ± 5.86 a | 3.49 ± 0.17 b | 0.110 ± 0.009 b | 31.9 ± 1.26 a |
Note: The roots of the cucumber plants were treated with 0 ppm and 100 ppm FA for 9 h. For red ink absorption, the roots were immersed in red ink solution for another 10 h. The data represent the mean ± SD of five replicates. The significant differences (P < 0.05) among the treatments are indicated by different letters.
Discussion
Stomatal water loss and water transport decreased in FOC-infected plants
Pathogen infection can affect the photosynthesis and water physiology of higher plants10,12,20,33. Decreased net photosynthetic rate has been attributed to stomatal closure induced by pathogen infection and a disruption in the metabolic pathways of photosynthesis, such as a reduction in the mesophyll conductance and the Rubisco activity13,34,35. In tomato plants that were infected with the soil-borne pathogen Fusarium oxysporum f. sp. lycopersici race 1, the decreased photosynthetic capacity of the tomato leaves was accompanied by a reduction in both the carboxylation efficiency and the regeneration of RuBP12. In the present study, the photosynthetic rate of cucumber plants was markedly reduced after FOC infection (Table 1), and both the stomatal conductance and the intercellular CO2 concentration significantly decreased after FOC infection, which could suppress the photosynthetic rate of cucumber seedlings, leading to reduced biomass production.
The leaves of the infected cucumber plants exhibited a decrease in the stomatal conductance and transpiration rate (Table 1), resulting in a reduction in water loss. The same results were obtained in tomato plants that were infected with Fusarium oxysporum34, potato plants that were infected with Verticillium dahliae36, and cherry plants that were infected with Blumeriella jaapii37. The transpiration rate was negatively correlated with the leaf temperature38,39, there was an increase in the leaf temperature of cucumber plants after FOC infection (Fig. 4), and the leaf temperature difference between the healthy and infected plants was reduced as the light intensity decreased, indicating that the response of the stomata in the infected plant was less sensitive to the light intensity.
To prevent Fusarium oxysporum invasion through the xylem, infected plants form callose barriers and tylose-like structures40,41; these materials and the fungal mycelium in the xylem reduce the water movement and eventually result in dehydration10. A similar phenomenon was described by Street and Cooper42, in which the xylem of Verticillium-infected tomato was plugged, and the water flow rate was significantly reduced before visible wilt symptoms were observed in the leaves. The hydraulic conductivity of infected pepper stems was reduced 80% compared to that of non-infected pepper plants9. Our study showed that the hydraulic conductance of the stem was significantly reduced in FOC-infected plants (Table 1), suggesting that the stem was blocked by the abovementioned materials (i.e., callose, tylose, and gels), reducing the water transport of the cucumber plants.
Interestingly, in the red ink absorption experiment, the red staining was significantly greater in the infected plants than in the healthy plants (Fig. 1), indicating that although the water uptake and transport were reduced after FOC infection (Table 1), water and red ink (large particle) were transported to the leaves of the infected plants. The great red ink accumulation in the infected plants was attributed to (i) the leaf cell membrane being seriously injured in infected plants, resulting in a greater amount of red ink accumulation (Table 2) and (ii) the roots being damaged in infected plants by FOC infection43,44. Thus, the ability of root selective absorption was reduced, and the red ink particle was easily taken up by the roots and transported to the leaves. The transpiration rate was reduced by 46%, while the water uptake was reduced by only 23% after FOC infection (Table 1), indicating that the water balance of the cucumber plant was disturbed by FOC infection. Based on the red ink assays, we can speculate that the leaf membranes of the infected plants were injured, inducing uncontrolled water loss from damaged cells.
FOC infection induced uncontrolled water loss from damaged cells
Injuries of the leaf cell membranes caused by air-borne pathogenic infections have been described in previous studies39,45,46,47. The pathogenesis of Pseudoperonospora cubensis causing downy mildew of cucumber induced membrane injury at the infection site of leaves39. In our study, the ultrastructure of the mesophyll cells showed that the plasma membrane of the infected plants was damaged and degraded (Fig. 2). Meanwhile, the leaf electrolyte leakage, electrical conductance and soluble sugar content of the leaf apoplastic sap of the infected plants increased, demonstrating that the leaf plasma membrane was injured (Table 2). Similar results were illustrated in pea powdery mildew, which showed an increase in the glucose, sucrose, and fructose contents of apoplastic sap in infected plants48; these phenomenon are caused by cell membrane injury, which allows soluble sugars to penetrate into the apoplastic space.
The membrane injury of infected plant leaves may result in uncontrolled water loss from damaged cells, and the leaf water balance could be affected11. In cucumber plants that are infected with Pseudoperonospora cubensis, which is the causal agent of downy mildew, the appearance of chlorosis is associated with an initial temperature decrease due to the abnormal opening of the stomata or the loss of cell membrane integrity38,39. In our previous studies, we monitored water loss in the dark and found that the leaf water loss of wilted plants in the dark was significantly higher than that of healthy plants11. In the present study, the infected plant leaves exhibited an increase in the ratio of E/gs under light (Table 1), indicating that an increased percentage of water was evaporated outside of the stomata7,11, and we further found that there was a positive correlation between the leaf membrane injury and E/gs (Fig. 3), demonstrating that the uncontrolled water loss from a leaf was induced by leaf cell membrane injury after FOC infection. Because no pathogen was detected in the infected plant leaves even at 8 days post inoculation (unpublished data), we speculated that a metabolite from FOC that was produced by the interaction between FOC and cucumber plants was a potential candidate for leaf membrane injury.
FA played a critical role in accelerating the development of Fusarium wilt in cucumber plants
Several studies have described the damage to plants caused by toxins that are produced by pathogens49,50. Botta et al.51 reported that potato leaves that were treated with a filtrate of Fusarium eumartii increased electrolyte leakage, as the absence of the pathogen in infected leaflets suggested that a phytotoxin was the cause of the leakage. As far as has been documented, FA is the main toxin that is produced by Fusarium oxysporum and has a direct effect on membranes28,52. We detected FA in the infected plants after FOC infection (Table 3) and found that the FA content in the leaves of the infected plants was higher than that in the stem, possibly because FA was transported to the leaves by the xylem transpiration stream and accumulated in the leaves53.
The toxic effects of FA on cucumber seedlings were investigated in a hydroponic experiment. The results indicate that the leaves of FA treated plants accumulated more red ink and had a higher relative electronic conductance (Fig. 5, Table 4), in agreement with our previous study with transmission electron microscopy (TEM), in which the leaf mesophyll cell membrane was seriously injured after treatment with FA54. The ratio of the transpiration rate to the stomatal conductance (E/gs) was increased in the FA-treated plants, indicating that water had evaporated from damaged cells, which is caused by FA movement from the infection site to the leaf.
In conclusion, FOC infection reduced the stomatal conductance and transpiration rate of cucumber plants, but the ratio of the transpiration rate to stomatal conductance (E/gs) increased, indicating that non-stomatal water loss occurred after infection. The leaf cell membrane injury was induced by FOC infection, which was caused by FA that was produced by the pathogen in host plants. We concluded that the leaf cell membrane was damaged in the soil-borne Fusarium wilt of cucumber plants, resulting in uncontrolled water loss from damaged cells, and this phenomenon was partly caused by FA.
Methods
Plant material and growth conditions
Cucumber seeds (cultivar ‘Jingyan 4’, which is susceptible to Fusarium wilt and was supplied by Vegetable Research Institute of Tianjin, China) were germinated in steam-sterile quartz sand, and the seedlings were transplanted to a mixture of sterile soil and vermiculite (1:6, v/v) when the first leaf emerged. The seedlings were watered daily with half-strength Hoagland's solution. The plants were grown in a greenhouse at 30/25°C (day/night) with a relative humidity (RH) of 70 ± 10% and a photoperiod of 14 h day−1 (>300 μmol m−2 s−1).
Pathogen incubation and infection
Fusarium oxysporum f. sp. cucumerinum (FOC) was isolated from infected cucumber plants that were provided by the Laboratory of Plant-Microbe Interactions, Nanjing Agricultural University, China. The FOC isolates were first incubated on potato dextrose agar (PDA) medium in Petri dishes in the dark at 25°C for 7 days. Then, the dishes were drenched with 20 ml of sterile distilled water, and the spores were carefully freed from the culture surface with a fine artist's brush. A conidial suspension was obtained by filtering through three layers of sterile cheesecloth to eliminate mycelial fragments. For inoculation, the roots of four-week-old plants were immersed in FOC conidial suspension (106 conidia ml−1) for 2 h. The roots of the control plants were immersed in sterilised water. After inoculation, the plants were grown in sterile soil as described above. The number of FOC in infected plants was approximately 107 to 109 copies g−1 FW in the stem and roots at 8 days post inoculation (unpublished data), indicating that the inoculated cucumber plants were actually infected. Furthermore, disease symptoms, such as leaf yellowing and wilting, were also observed in the inoculated plants.
Gas exchange measurements
To determine if pathogen infection could affect the photosynthesis of cucumber plants, gas exchange measurements were collected from fully expanded leaves using a Li-Cor 6400 portable photosynthesis open system. The leaf temperature during the measurements was maintained at 25°C, with a photosynthetic photon flux density (PPFD) of 1000 μmol photons m−2 s−1 and a relative humidity of 45%. The data were recorded after equilibration to a steady state (approximately 15 min).
Water uptake measurement
Water uptake was determined by weighing the pots. The surfaces of the pots were sealed with plastic film to inhibit evaporation. After 8 days post inoculation, the pots and plants were weighed once every 24 h, and the weight loss indicated water uptake.
Shoot hydraulic conductance
The shoot hydraulic conductance was measured using a high-pressure flow meter (HPFM, Dynamax Inc., Houston, TX, USA) as described by Tyree et al.55. The transient measurement mode, which records water flow entering the root or shoot systems as a series of applied pressures, was used on the excised shoots. The stem was cut 30 to 50 mm above the soil surface underwater to prevent embolism formation, immediately connected to the HPFM and perfused with water at a constant pressure of 0.3 MPa for approximately 15 min until the flow rate stabilised. The pressure was then released and continually increased from 0.1 to 0.3 MPa at a rate of 4 to 5 kPa s−1, with flow and pressure being recorded every 2 s. The slope of the flow versus the applied pressure indicated the hydraulic conductance of the root or shoot.
Leaf water content
To investigate the leaf water status of the cucumber plants, the leaf water content of the healthy and FOC infected plants was measured at 8 days post inoculation. The leaf water content was determined by drying at 105°C for 30 min and then at 70°C until constant weight. After cooling to room temperature, the samples were reweighed, and the leaf water content (WC) was calculated as follows:
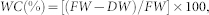 |
where FW is the fresh weight, and DW is the dry weight.
Red ink absorption of plant
The cucumber seedlings were gently removed from the soil, and the soil particles that adhered to the roots were shaken off. The roots were then washed with distilled water and immersed in dilute red ink for 12 h. The leaves were examined under an Olympus MVX10 light microscope that was equipped with an Olympus DP71 microscope digital camera system.
The quantitative measurement of red ink absorption was described by Dong et al.7 and Wang et al.54. The absorption spectrum of red ink solution was measured in the wavelength range 400–800 nm using an ultraviolet-visible (UV-Vis) spectrophotometer (SHIMADZU, UV-1601 PC, Japan). The absorptive peak of red ink solution occurs at 515 nm (data not shown). After red ink absorption, the healthy and FOC infected cucumber leaves were washed with distilled water and cut into small pieces (5 mm × 5 mm). Twenty leaf pieces were added to a test tube containing 20 ml of distilled water and boiled in a water bath for 30 min. After cooling to room temperature, the samples were filtrated, and the clear filtrates were read at 515 nm with a spectrophotometer (T6, Beijing Purkinje General Instrument Co., Ltd., Beijing, China).
Membrane injury
Twenty leaf discs (10 mm in diameter) without midrib were cut from fully expanded leaves and washed with distilled water to remove any electrolytes adhering to the leaves or in the cut ends of the tissues. The segments were infiltrated with distilled water, and the vacuum was slowly released. The conductivity of the diffusate was directly measured using an electrical conductivity meter (DDS-11A, LEICI Instrument Co., Shanghai, China). Subsequently, the leaf tissues were killed by boiling in a 100°C water bath for 20 min. A second conductivity reading was collected after the sample cooled to room temperature. The membrane injury was expressed as the relative injury (%), which is the ratio between the first and second conductivity measurements.
Apoplastic sap extraction and analysis
The apoplastic sap of the cucumber leaves was extracted using the vacuum-infiltration technique as described by Speer and Kaiser56. Briefly, leaf discs that were 15 mm in diameter were cut using a stainless steel punch and washed with distilled water. Twenty discs were vacuum-infiltrated three or four times with distilled water for 30 s to ensure full infiltration. The infiltrated discs were blotted dry and packed vertically into a 20-ml plastic syringe barrel with holes at the bottom. The barrel was transferred to a centrifuge tube and centrifuged at 2,500 g for 15 min at 4°C. The apoplastic sap was collected, and the volume was brought to 3 ml for further measurement. Malate dehydrogenase (MDH; EC 1.1.1.37) was used as a cytosolic contamination marker for apoplastic sap. The MDH assay mix contained 0.1 mM NADH, 0.4 mM oxalacetate, and 46.5 mM Tris-HCl (pH 9.5). NADH oxidation was followed at 340 nm57. MDH activity was related to the total bulk leaf activity as described by López-Millán et al.58. The activity of malate dehydrogenase (MDH) in apoplastic sap was less than 1% of the total activity in the leaves and showed little damage to cells during centrifugation (Data not shown).
The electrical conductivity of the apoplastic sap was measured directly using a micro-electrical conductivity meter (DDS-11A, LEICI Instrument Co., Shanghai, China). The soluble sugar content of the apoplastic sap was measured using anthrone colourimetry59. The reaction consisted of mixing 2 ml of sap with 0.5 ml of anthrone reagent (dissolving 1 g of anthrone in 50 ml of ethyl acetate) and 5 ml of H2SO4 and then heating in a boiling water bath for 1 min. After cooling to room temperature, the samples were read at an absorbance of 630 nm using a spectrophotometer. A calibration curve with sucrose was used as a standard.
Transmission electron microscopy
The leaves of healthy and infected plants were cut into pieces and fixed with 3% (v/v) glutaraldehyde in phosphate buffer (50 mM, pH 6.8) for 4 h at 4°C. The samples were then post-fixed with 1% (w/v) osmium tetroxide in the same buffer for 2 h at 4°C, after which they were dehydrated in a graded ethanol series, followed by propylene oxide, and embedded in Spurs epoxy resin. Ultrathin sections were cut with a diamond knife and collected on copper-supported grids for observation using an H-7650 transmission electron microscope (TEM; Hitachi) at 80 kV. The TEM analyses were repeated three times. Five leaf slides of each treatment were checked each time, and approximately five cells were observed in each slide. The ratio of the injured membranes in the infected plants was approximately 94%. Abnormal structures in the healthy plants that were most likely caused by operations were approximately 3%.
Leaf temperature measurements
Leaf temperature was measured using a digital infrared thermograph. Infrared images were obtained using an infrared camera (SC 620, FLIR Systems, Inc., USA) with a spectral sensitivity ranging from 7.5 μm to 13 μm and a spatial resolution of 0.65 mrad. The SC 620 camera has a 640 × 480 pixel focal plane array uncooled microbolometer and a 24° × 18°-field of view lens with a minimum focus distance of approximately 0.3 m. The thermal resolution of the camera is 0.065°C at an ambient temperature of 30°C. Digital thermograms were analysed with the ThermaCAM Researcher Professional 2.9 software (FLIR Systems). To detect the leaf responses to light intensity, thermal images were recorded when the light intensity decreased from 10,000 Lux to 0 Lux.
FA extraction and analysis
FA was extracted as described by Smith60. A 20-g sample of cucumber seedlings was ground in a homogeniser with 100 ml of 1:1 methanol-1% KH2PO4 (pH 3.0). The ground samples were centrifuged for 20 min at 20,000 g, and the pH of the supernatant was adjusted to 3.0 with 2 M HCl. The acidified supernatant was extracted sequentially three times with methylene chloride. The methylene chloride extracts were pooled and evaporated to dryness under a vacuum at 40°C on a rotary evaporator. The residue was resuspended in 2 ml of methanol and analysed by high-performance liquid chromatography (HPLC).
The HPLC analysis was performed in an Agilent 1200 Series HPLC system (Agilent Technologies, USA) that was equipped with an Agilent of Zorbax Eclipse XDB-C18 column (4.6 × 250 mm, 5 μm) and set at 50°C. The samples (10 μl) were eluted with methanol/0.43% o-phosphoric (68%:32%) for 15 min. The retention time was approximately 5.8 min, with a mobile-phase flow rate of 1 ml min−1. FA was detected by monitoring the UV A271. The samples were quantified against a standard curve of synthetic FA (Sigma). The experiment was performed twice with similar results.
Assessment of FA on cucumber seedlings
To investigate the effects of FA on cucumber plants, the seedlings were directly transplanted to plastic cups (9.0-cm and 5.5-cm top and bottom diameters, respectively, and 12.5-cm height) containing 500 ml of aerated half-strength nutrient solution when the first leaf emerged. The composition of the nutrient solution was as follows: 2.5 mM (NH4)2SO4 or Ca(NO3)2, 2.5 mM K2SO4, 1.0 mM KH2PO4, 2.0 mM MgSO4, 35.8 μM Fe-EDTA, 57.8 μM H3BO3, 11.4 μM MnCl2, 0.96 μM ZnSO4, 0.4 μM CuSO4, and 0.48 μM H2MoO4. A nitrification inhibitor (DCD) was added to each nutrition solution to prevent the oxidation of the ammonium. After two weeks, the cucumber seedlings were treated with 0 and 100 ppm FA (from Sigma) that was diluted with nutrient solution according to our previous study54. The effects of FA on the leaf red ink absorption, membrane injury and gas exchange were investigated using the abovementioned methods.
Statistical analysis
The experiments were repeated three times. One-way analysis of variance (ANOVA) was applied using SPSS 18.0 software to assess the differences in each parameter among the treatments. The mean and standard deviation were calculated from three independent experiments. The significant differences (P < 0.05) between the treatments are indicated by different letters.
Author Contributions
M.W. and S.G. designed the experiments. M.W., Y.S., X.L., G.S. and L.Z. performed the experiments. M.W., Y.S., X.L. and S.G. analyzed the data. M.W. and S.G. wrote the main manuscript text. Q.S. and S.G. commented and improved the manuscript. All authors have read and approved the manuscript.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (2013CB127403 and 2015CB150505) and the National Natural Science Foundation of China (31172020 and 31401941). We thank Prof. Dr. Z. Zhong, Nanjing Agricultural University, China, for helpful discussion and critical comments of this work.
References
- Owen J. H. Fusarium wilt of cucumber. Phytopathology 45, 435–439 (1955). [Google Scholar]
- Zhang S. et al. Control of Fusarium wilt disease of cucumber plants with the application of a bioorganic fertilizer. Biol. Fertil. Soils 44, 1073–1080 (2008). [Google Scholar]
- Ahn P., Chung H. S. & Lee Y. H. Vegetative compatibility groups and pathogenicity among isolates of Fusarium oxysporum f. sp. cucumerinum. Plant Dis. 82, 244–246 (1998). [DOI] [PubMed] [Google Scholar]
- Mace M. E., Bell A. A. & Beckman C. H. in Water Relations (eds Hall, R. & MacHardy, W. E.) 255–298 (Academic Press, New York, 1981). [Google Scholar]
- Ogura H. & Ma J. Persistence of Fusarium oxysporum f. sp. cucumerinum in continuous cropping fields. Ann. Phytopathol. Soc. Jpn. 58, 671–676 (1992). [Google Scholar]
- Duniway J. M. Water relations of Fusarium wilt in tomato. Physiol. Plant Pathol. 1, 537–546 (1971). [Google Scholar]
- Dong X., Ling N., Wang M., Shen Q. & Guo S. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in Fusarium-infected banana plants. Plant Physiol. Bioch. 60, 171–179 (2012). [DOI] [PubMed] [Google Scholar]
- Sant D. et al. Effect of Trichoderma asperellum strain T34 on Fusarium wilt and water usage in carnation grown on compost-based growth medium. Biol. Control 53, 291–296 (2010). [Google Scholar]
- Aguirreolea J., Irigoyen J., Sanchez-Diaz M. & Salaverri J. Physiological alterations in pepper during wilt induced by Phytophthora capsici and soil water deficit. Plant Pathol. 44, 587–596 (1995). [Google Scholar]
- Pshibytko N. L., Zenevich L. A. & Kabashnikova L. F. Changes in the photosynthetic apparatus during Fusarium wilt of tomato. Russ. J. Plant Physiol. 53, 25–31 (2006). [Google Scholar]
- Wang M. et al. Thermographic visualization of leaf response in cucumber plants infected with the soil-borne pathogen Fusarium oxysporum f. sp. cucumerinum. Plant Physiol. Bioch. 61, 153–161 (2012). [DOI] [PubMed] [Google Scholar]
- Nogués S., Cotxarrera L., Alegre L. & Trillas M. I. Limitations to photosynthesis in tomato leaves induced by Fusarium wilt. New Phytol. 154, 461–470 (2002). [DOI] [PubMed] [Google Scholar]
- Saeed I. A. M., MacGuidwin A. E., Rouse D. I. & Sharkey T. D. Limitation to photosynthesis in Pratylenchus penetrans- and Verticillium dahliae-infected potato. Crop Sci. 39, 1340–1346 (1999). [Google Scholar]
- Lakshminarayanan K. Mechanism of fusarium wilts of plants. P. Plant Sci. 38, 161–164 (1953). [Google Scholar]
- Jaroszuk-Scisel J., Kurek E., Winiarczyk K., Baturo A. & Lukanowski A. Colonization of root tissues and protection against Fusarium wilt of rye (Secale cereale) by nonpathogenic rhizosphere strains of Fusarium culmorum. Biol. Control 45, 297–307 (2008). [Google Scholar]
- Gonzalez J. et al. Arabidopsis thaliana: a model host plant to study plant-pathogen interaction using Chilean field isolates of Botrytis cinerea. Biol. Res. 39, 221–228 (2006). [DOI] [PubMed] [Google Scholar]
- Krivanek A. F., Stevenson J. F. & Walker M. A. Development and comparison of symptom indices for quantifying grapevine resistance to pierce's disease. Phytopathology 95, 36–43 (2005). [DOI] [PubMed] [Google Scholar]
- Baayen R. P. Regeneration of vascular tissues in relation to Fusarium wilt resistance of carnation. Eur. J. Plant Pathol. 92, 273–285 (1986). [Google Scholar]
- Beckman C. H. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 57, 101–110 (2000). [Google Scholar]
- Mepsted R., Flood J. & Cooper R. M. Fusarium wilt of oil palm II. Stunting as a mechanism to reduce water stress. Physiol. Mol. Plant Pathol. 46, 373–387 (1995). [Google Scholar]
- Wu H. S. et al. Effect of fungal fusaric acid on the root and leaf physiology of watermelon (Citrullus lanatus) seedlings. Plant Soil 308, 255–266 (2008). [Google Scholar]
- Van Alfen N. K. & Turner N. C. Influence of a Ceratocystis ulmi toxin on water relations of Elm (Ulmus americana). Plant Physiol. 55, 312–316 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Tucker E. B., Crain R. C. & Lee Y. Stomatal opening is induced in epidermal peels of Commelina communis L. by GTP analogs or pertussis toxin. Plant Physiol. 102, 95–100 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alfen N. K. & Turner N. C. Changes in alfalfa stem conductance induced by corynebacterium insidiosum toxin. Plant Physiol. 55, 559–561 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon C. W., Porter J. K., Norred W. P. & Leslie J. F. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 62, 4039–4043 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter S. L. & Steyn P. J. Correlation between fusaric acid production and virulence of isolates of Fusarium oxysporum that causes potato dry rot in South Africa. Potato Research 41, 289–294 (1998). [Google Scholar]
- Curir P., Guglieri L., Dolci M., Capponi A. & G A. Fusaric acid production by Fusarium oxysporum f. sp. lilii and its role in the lily basal rot disease. Eur. J. Plant Pathol. 106, 849–856 (2000). [Google Scholar]
- Bouizgarne B. et al. A putative role for fusaric acid in biocontrol of the parasitic angiosperm Orobanche ramosa. Mol. Plant Microbe. Interact. 19, 550–556 (2006). [DOI] [PubMed] [Google Scholar]
- Gapillout I., Milat M. L. & Blein J. P. Effects of fusaric acid on cells from tomato cultivars resistant or susceptible to Fusarium oxysporum f. sp. Lycopersici. Eur. J. Plant Pathol. 102, 127–132 (1996). [Google Scholar]
- Kuźniak E. Effects of fusaric acid on reactive oxygen species and antioxidants in tomato cell cultures. J. Phytopathology 149, 575–582 (2001). [Google Scholar]
- Bouizgarne B. et al. Early physiological responses of Arabidopsis thaliana cells to fusaric acid: toxic and signalling effects. New Phytol. 169, 209–218 (2006). [DOI] [PubMed] [Google Scholar]
- Samadi L., Shahsavan B. & Behboodi Fusaric acid induces apoptosis in saffron root-tip cells: roles of caspase-like activity, cytochrome c, and H2O2. Planta 255, 223–234 (2006). [DOI] [PubMed] [Google Scholar]
- Ye S. F., Yu J. Q., Peng Y. H., Zheng J. H. & Zou L. Y. Incidence of Fusarium wilt in Cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates. Plant Soil 263, 143–150 (2004). [Google Scholar]
- Lorenzini G., Guidi L., Nali C., Ciompi S. & Soldatini G. F. Photosynthetic response of tomato plants to vascular wilt diseases. Plant Sci. 124, 143–152 (1997). [Google Scholar]
- Santos L., Lucio J., Odair J., Carneiro M. L. & Alberto C. Symptomless infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytol. 147, 609–615 (2000). [DOI] [PubMed] [Google Scholar]
- Bowden R. L., Rouse D. I. & Sharkey T. D. Mechanism of photosynthesis decrease by Verticillium dahliae in potato. Plant Physiol. 94, 1048–1055 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederleitner S. & Knoppik D. Effects of the cherry leaf spot pathogen Blumeriella jaapiion gas exchange before and after expression of symptoms on cherry leaves. Physiol. Mol. Plant Pathol. 51, 145–153 (1997). [Google Scholar]
- Lindenthal M., Steiner U., Dehne H. W. & Oerke E. C. Effect of downy mildew development on transpiration of cucumber leaves visualized by digital infrared thermography. Phytopathology 95, 233–240 (2005). [DOI] [PubMed] [Google Scholar]
- Oerke E. C., Steiner U., Dehne H. W. & Lindenthal M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 57, 2121–2132 (2006). [DOI] [PubMed] [Google Scholar]
- Mueller W. C., Morgham A. T. & Roberts E. M. Immunocytochemical localization of callose in the vascular tissue of tomato and cotton plants infected with Fusarium oxysporum. Can. J. Bot. 72, 505–509 (1994). [Google Scholar]
- Trillas M. I., Cotxarrera L., Casanova E. & Cortadellas N. Ultrastructural changes and localization of chitin and callose in compatible and incompatible interactions between carnation callus and Fusarium oxysporum. Physiol. Mol. Plant Pathol. 56, 107–116 (2000). [Google Scholar]
- Street P. F. S. & Cooper R. M. Quantitative measurement of vascular flow in petioles of healthy and Verticillium-infected tomato. Plant Pathol. 33, 483–492 (1984). [Google Scholar]
- Persson L., Bødker L. & Larsson-Wikström M. Prevalence and pathogenicity of foot and root rot pathogens of pea in southern Scandinavia. Plant Dis. 81, 171–174 (1997). [DOI] [PubMed] [Google Scholar]
- McPhee K. E., Tullu A., Kraft J. M. & Muehlbauer F. J. Resistance to Fusarium wilt race 2 in the Pisum core collection. J. AM Soc. Hortic. Sci. 124, 28–31 (1999). [Google Scholar]
- Harrach B., Fodor J., Pogány M., Preuss J. & Barna B. Antioxidant, ethylene and membrane leakage responses to powdery mildew infection of near-isogenic barley lines with various types of resistance. Eur. J. Plant Pathol. 121, 21–33 (2008). [Google Scholar]
- Chaerle L., De Boever F., Van Montagu M. & Van Der Straeten D. Thermographic visualization of cell death in tobacco and Arabidopsis. Plant Cell Environ. 24, 15–25 (2001). [Google Scholar]
- Seo S. et al. Reduced levels of chloroplast FtsH protein in tobacco mosaic virus–infected tobacco leaves accelerate the hypersensitive reaction. Plant Cell 12, 917–932 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aked B. J. & Hall J. L. Effect of powdery mildew infection on concentrations of apoplastic sugars in pea leaves. New Phytol. 123, 283–288 (1993). [Google Scholar]
- Abbas H. K., Tanaka T. & Duke S. O. Pathogenicity of Alternaria alternata and Fusarium moniliforme and phytotoxicity of AAL-toxin and Fumonisin B1 on tomato cultivars. J. Phytopathology 143, 329–334 (1995). [Google Scholar]
- Barna B., Fodor J., Pogány M. & Király Z. Role of reactive oxygen species and antioxidants in plant disease resistance. Pest Manag. Sci. 59, 459–464 (2003). [DOI] [PubMed] [Google Scholar]
- Botta G. L., Dimarco M. P., Melegari A. L., Huarte M. A. & Barassi C. A. Potential of a Fusarium eumartii culture filtrate on the screening for wilting resistance in potato. Euphytica 80, 63–69 (1994). [Google Scholar]
- D'Alton A. & Etherton B. Effects of fusaric acid on tomato root hair membrane potentials and ATP levels. Plant Physiol. 74, 39–42 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. et al. Detection of the dynamic response of cucumber leaves to fusaric acid using thermal imaging. Plant Physiol. Bioch. 66, 68–76 (2013). [DOI] [PubMed] [Google Scholar]
- Wang M. et al. Effect of fusaric acid on the leaf physiology of cucumber seedlings. Eur. J. Plant Pathol. 138, 103–112 (2014). [Google Scholar]
- Tyree M. T., Patiño S., Bennink J. & Alexander J. Dynamic measurements of roots hydraulic conductance using a high-pressure flowmeter in the laboratory and field. J. Exp. Bot. 46, 83–94 (1995). [Google Scholar]
- Speer M. & Kaiser W. M. Ion relations of symplastic and apoplastic space in leaves from Spinacia oleracea L. and Pisum sativum L. under salinity. Plant Physiol. 97, 990–997 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannel F., Pfeffer H. & Marschner H. Isolation of apoplasmic fluid from sunflower leaves and its use for studies on influence of nitrogen supply on apoplasmic pH. J. plant physiol. 50, 208–213 (1995). [Google Scholar]
- López-Millán A. F. et al. Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant Physiol. 124, 885–898 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kojima K., Ide Y. & Sasaki S. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tiss. Org. 63, 199–206 (2000). [Google Scholar]
- Smith T. K. Fusaric acid content of swine feedstuffs. J. Agr. Food Chem. 41, 2296 (1993). [Google Scholar]