Synopsis
Background
Optimal treatment regimens for infections caused by Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae are not well defined.
Objectives
This study describes the treatment and outcomes of patients with urinary tract infection (UTI) caused by KPC-producing Enterobacteriaceae.
Methods
Retrospective cohort study of adult inpatients with bacteriuria caused by KPC-positive organisms at Barnes-Jewish Hospital from June 1, 2006 to February 1, 2008. KPC-positive isolates were identified utilizing disk diffusion susceptibility testing and confirmed to contain blaKPC via molecular methods.
Results
Twenty-one patients met inclusion criteria and all were classified as having symptomatic UTI. The majority of patients were female (15 of 21 – 71%) with a mean age of 62.4 years (SD ± 15.2). Successful clinical and microbiologic responses were observed in 16 patients (76%) for both outcomes. Patients with urinary catheters had them removed or replaced in 9 of 15 cases (60%). Antibiotics active against the isolated pathogen were provided in 14 of 21 cases (67%), often after considerable delay (median: 72.5 hours, range: 4–312 hours). All seven patients receiving aminoglycoside therapy had successful clinical and microbiological responses, and in vitro testing of an extended antibiotic panel revealed high susceptibility rates for tigecycline (28 of 29 – 97%), minocycline (22 of 29 – 76%), and fosfomycin (25 of 29 – 86%) against the KPC-positive isolates.
Conclusions
Although delays to receipt of appropriate therapy were often experienced, clinical outcomes investigated revealed high rates of successful response in this limited group of patients. Therapy with aminoglycosides and tetracycline derivatives suggest therapeutic promise in the treatment of KPC-producing Enterobacteriaceae UTI.
Keywords: Urinary tract infection, Carbapenemase, KPC, Treatment Outcome
Introduction
A recent Infectious Diseases Society of America white paper crafted the acronym “ESKAPE” to identify pathogens for which our current antimicrobial armamentarium is being threatened by resistance [1]. One of these organisms is Klebsiella pneumoniae, in which Klebsiella pneumoniae carbapenemase (KPC) production is described with increasing frequency worldwide [2]. The gene coding for this carbapenemase is termed blaKPC. blaKPC is plasmid-mediated and transmissible, has also been reported with other Gram-negative organisms [3], and can be associated with additional antibiotic resistance genes and multi-drug resistant phenotypes [4,5]. Treatment of infections caused by KPC-producing bacteria is problematic, with options chosen on a case-bycase basis utilizing susceptibility results. There is very little published literature on urinary tract infection (UTI) due to KPC-producing organisms, which is among the most common sites of infection [6,7]. We report the clinical and microbiological outcomes of 21 hospitalized patients treated for KPC-producing Enterobacteriaceae urinary tract infections.
Methods
This single-center, retrospective cohort study evaluated patients who were hospitalized between June 1, 2006 and February 1, 2008 at Barnes-Jewish Hospital (BJH), a 1,250-bed tertiary-care medical center in St. Louis, MO. All inpatients admitted and found to have bacteriuria caused by carbapenem-non-susceptible bacteria were identified through a query of our medical informatics database. Only the initial isolate and associated infection for each patient identified during the study period was described clinically. Isolates were identified phenotypically using the VITEK-2 identification system (bioMérieux, Inc., Marcy, France). Antimicrobial susceptibility testing was performed using disk diffusion according to CLSI guidelines at the time of study initiation [8,9]. In addition, minimum inhibitory concentrations (MICs) of ertapenem, meropenem, imipenem, doripenem, fosfomycin, chloramphenicol, tetracycline, tigecycline, and minocycline were determined using E-test (AB Biodisk, Solna, Sweden). Detection of blaKPC required extraction of total (chromosomal and plasmid) DNA from overnight cultures of carbapenem-resistant isolates using the QIAamp DNA mini extraction kit according to manufacturer recommendations (Qiagen, Hilden, Germany). The presence of blaKPC was verified using a SybrGreen® (BioRad, Hercules, CA) real-time PCR with primers specific for blaKPC as described previously [10,11]. Epidemiologic analysis of all isolates available for testing during the study period was performed on total isolated DNA using repetitive sequence PCR with the RW3A primer set, as described previously [12,13]. Analysis of amplified fragments was conducted using the Agilent 2100 Bioanalyzer (Diversilab, Athens, GA) according to manufacturer procedures. A similarity index of 85% or greater was used as a threshold to determine epidemiological relatedness between isolates [12,13].
Patient data on demographics, residential status, medical history, initial presentation, clinical course, treatments, and outcomes were abstracted from medical records for the entire duration of their hospital admission. Criteria for defining types of UTI were in accordance with previously established IDSA guidelines [14]. Definitions of clinical treatment response were defined as “positive”, “negative”, or “uncertain”, as described elsewhere [15]. Microbiologic response was defined as “positive” (KPC-producing organism eradication from a repeat urine culture); “presumed” (patients judged clinical responders with no follow-up cultures obtained to verify KPC-producing organism eradication and having no readmission within 30 days with a positive culture for the same KPC strain); “negative” (persistence of the KPC-producing organism despite at least three days of appropriate antibiotics); or “not documented”. Crude mortality during hospitalization was also recorded. Hospital-acquired infection was defined as having occurred ≥48 hours following hospital admission. Community-acquired infection was defined as both not meeting the criteria for hospital-acquired infection and not having been a long-term care facility (LTCF) resident at the time of admission. Patients with greater than 10 white blood cells per high power field on urinalysis were considered to have pyuria. Active antibiotics were defined as agents for which in vitro susceptibility was determined to be either susceptible or intermediate, as it was assumed that moderate drug resistance may be overcome in vivo by high urinary drug concentrations. For patients receiving active antibiotic therapy, time to appropriate antibiotics was defined as hours between KPC-positive specimen collection and initial active antibiotic administration. Definitions for immunosuppression and other factors complicating urinary tract infections were utilized as described previously [16]. This study was approved by the Human Research Protection Office at Washington University in St. Louis. Since this study was retrospective, written informed consent was not required.
Results
Twenty-five patients were identified through the database when screening for KPC-producing urinary tract isolates during the defined study period. All were confirmed to be KPC-positive by PCR. Four of these patients were excluded because they received outpatient care only and detailed analysis of their treatment courses and clinical outcomes were not readily available. Thus, 21 patients made up the study cohort (Table 1). The majority of the patients were female (15 of 21 – 71%). The mean age was 62.4 years (SD ± 15.2). Patients had a median of three UTI-complicating factors (range 2–8).
Table 1.
Characteristics and outcomes for 21 patients treated for KPC-producing UTIs
| Patient | Age (years) |
Sex | ICU Patient |
Transplant or Immuno- suppressed Patient |
LTCF Patient |
Hospital- Acquired Infection |
Complicating Factors a(N) |
Total LOS (days) |
Causative Organism |
Antibiotic Susceptibility | KPC Bacteremia |
Polymicrobial Culture |
Pyuria | Urinary Infection Type |
Active Antibiotics Administered |
Time to Appropriate Antibiotics (hours) |
Urinary Catheter Changed or Removed |
Prior Antibiotics During Admission |
Response | Survived Hospitalization |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical | Microbiologic | ||||||||||||||||||||
| 1 | 61 | F | Yes | Yes | No | Yes | 5 | 23 | KPN | S=GEN R=AMP, CEF, CFZ, CIP, CTX, MEM, NTF, T-S, P-T |
No | No | Yes | CC | None | N/A | No | VAN, CEF | Uncertain | Positive | No |
| 2 | 69 | F | No | No | Yes | Yes | 4 | 10 | KPN | S=AMK, CST, DOX, GEN, IMI, TIG I=MIN, TOB R=AMP, A-S, AZT, CFZ, CEF, CIP, CLF, MEM, NTF, P-T, T-C, T-S |
No | Yes, E. cloacae |
Yes | CC | None | N/A | Yes | VAN, CIP, MET |
Negative | Not Documented |
No |
| 3 | 76 | M | Yes | No | No | Yes | 4 | 115 | KPN | I=GEN R=AMP, CFZ, CEF, CTX, CIP, MEM, NTF, P-T, T-S |
Yes | Yes, P. aeruginosa |
Yes | CC | None | N/A | No | MEM, P-T | Positive | Presumed | Yes |
| 4 | 90 | F | No | No | No | Yes | 4 | 41 | KPN | S=AMK, CST, GEN, IMI, NTF, TOB I=CEF, MIN R=AMP, AZT, CFZ, CLF, CTX, CIP, MEM, P-T, T-C, T-S |
No | No | Yes | CC | None | N/A | Yes | VAN, CEF, LNZ, CIP |
Uncertain | Not Documented |
Yes |
| 5 | 61 | F | No | Yes | No | No | 2 | 6 | CFR | S=CIP, GEN, NTF R=IMI, MEM, T-S |
No | No | Yes | CC | CIP 250mg PO BID × 10d |
4 | Yes | None | Positive | Presumed | Yes |
| 6 | 48 | F | No | No | Yes | Yes | 3 | 10 | KPN | S=AMK, CST, GEN, TOB I=MIN R=AMP, AZT, CFZ, CTX, CEF, CLF, CIP, IMI, MEM, NTF, P-T, T-C, T-S |
No | No | Yes | CC | None | N/A | No | LNZ, CIP | Uncertain | Not Documented |
Yes |
| 7 | 73 | F | Yes | No | Yes | No | 3 | 8 | KPN | S=AMK, CST, MIN I=GEN R=AMP, AZT, CFZ, CTX, CEF, CLF, CIP, IMI, MEM, NTF, P-T, T-C, TOB, T-S |
No | Yes, E. faecalis |
Yes | CP | DOX 100mg PO BID × 5d |
157 | No | VAN, CEF, MET, LNZ |
Positive | Presumed | Yes |
| 8 | 63 | F | No | No | Yes | No | 3 | 5 | KPN | S=GEN, IMI I=CEF, CIP R=AMP, AZT, CFZ, CTX, MEM, NTF, P-T, T-S |
No | No | Yes | CP | CIP 250mg PO BID × 7d |
76 | Yes | T-S | Positive | Presumed | Yes |
| 9 | 55 | M | No | No | Yes | No | 4 | 24 | KPN | S=AMK, CST, DOX, GEN, MIN, TIG, TOB I=CEF, CIP, IMI, NTF R=A-S, AZT, CFZ, CTX, CLF, MEM, P-T, T-C, T-S |
Yes | No | No | CC |
|
312 | Yes | MEM | Positive | Positive | Yes |
| 10 | 56 | M | No | No | Yes | No | 8 | 12 | KPN | S=AMK, CST, IMI, TIG I=GEN R=AMP, AZT, CFZ, CEF, CTX, CLF, CIP, MEM, MIN, NTF, P-T, T-C, TOB, T-S |
No | No | Yes | CP | None | N/A | Yes | CEF, CIP, MEM, IMI |
Negative | Not Documented |
Yes |
| 11 | 72 | F | No | No | No | No | 4 | 5 | KPN | S=AMK, CST, GEN, MIN, NTF, TIG I=CEF, CIP. TOB R=AMP, AZT, CFZ, CTX, CLF, IMI, MEM, P-T, T-C, T-S |
No | No | Yes | CP | GEN 200mg IV daily × 14d |
8 | N/A | None | Positive | Presumed | Yes |
| 12 | 20 | M | No | No | Yes | Yes | 3 | 11 | KPN | S=GEN R=AMP, AZT, CEF, CIP, CFX, CFZ, CTX, IMI, NTF, P-T, T-S |
No | No | Yes | CP | GEN 160mg IV daily × 7d |
69 | No | LNZ, CEF, MET |
Positive | Presumed | Yes |
| 13 | 55 | F | No | No | No | Yes | 3 | 42 | KPN | S=AMK, CST, TIG I=CEF, CIP, GEN, MIN, NTF, TOB R=AMP, AZT, CFZ, CTX, CLF, IMI, MEM, P-T, T-C, T-S |
No | No | Yes | CP | CIP 400mg IV BID × 3d |
20 | Yes | VAN, CEF, MET, P-T |
Positive | Presumed | Yes |
| 14 | 52 | F | No | Yes | No | No | 3 | 4 | KPN | S=AMK, CST, GEN, MIN I=TOB R=AMP, AZT, CFZ, CEF, CTX, CLF, CIP, IMI, MEM, NTF, P-T, T-C, T-S |
No | No | Yes | CP | None | N/A | N/A | CEF | Positive | Positive | Yes |
| 15 | 52 | M | No | No | No | No | 2 | 10 | KPN | S=AMK, CST, GEN, TOB I=CEF, MIN R=AMP, AZT, CFZ, CTX, CLF, CIP, IMI, MEM, NTF, P-T, T-C, T-S |
No | No | Yes | CC | GEN 325mg IV daily × 5d |
84 | N/A | AMO, VAN, CIP, A-S |
Positive | Presumed | Yes |
| 16 | 65 | F | No | Yes | No | Yes | 2 | 166 | KPN | S=GEN R=AMP, CFZ, CFX, CEF, CIP, CTX, IMI, NTF, P-T, T-S |
No | No | Yes | CC | GEN 250mg IV daily × 3d |
106 | N/A | VAN, CIP, CEF, LNZ, MET, IMI, CLI, T-S, CFX |
Positive | Positive | No |
| 17 | 85 | F | No | No | Yes | No | 2 | 14 | KPN | S=AMK, CST, GEN, TOB, MIN, NTF, TIG I=CEF, CIP R=AMP, AZT, CFZ, CTX, CLF, IMI, MEM, P-T, T-C, T-S |
No | Yes, A. urinae |
Yes | CC | NTF 100mg PO BID × 10d |
94 | Yes | MOX, MET | Positive | Positive | Yes |
| 18 | 71 | F | No | No | Yes | No | 2 | 10 | KPN | S=GEN I=NTF R=AMP, CFZ, CEF, CTX, CIP, MEM, P-T, T-S |
No | No | Yes | CP |
|
22 | N/A | None | Positive | Presumed | Yes |
| 19 | 78 | M | No | No | No | No | 3 | 4 | KPN | S=AMK, GEN, MIN, TOB I=CEF, CIP, IMI, NTF R=AMP, AZT, CFZ, CTX, CLF, MEM, P-T, T-C, T-S |
No | Yes, P. mirabilis |
Yes | CC | CIP 250mg PO BID × 2d |
16 | N/A | None | Positive | Presumed | Yes |
| 20 | 42 | F | No | No | No | No | 4 | 17 | KPN | S=AMK, CST, DOX, GEN, MIN, TIG, TOB R=AMP, A-S, AZT, CFZ, CEF, CTX, CLF, CIP, IMI, MEM, NTF, P-T, T-C, T-S |
Yes | Yes, A. baumannii |
Yes | CP |
|
170 | Yes | CIP, NTF | Positive | Negative | Yes |
| 21 | 66 | F | No | No | Yes | No | 3 | 18 | KPN | S=GEN I=CIP, NTF R=AMP, CFZ, CEF, CTX, MEM, P-T, T-C |
No | No | Yes | CC | GEN 350mg IV daily × 8d |
21 | No | LNZ, P-T | Positive | Positive | No |
Abbreviation: AMO, amoxicillin; AMK, amikacin; AMP, ampicillin; A-S, ampicillin-sulbactam; AZT, aztreonam; CC, complicated cystitis; CEF, cefepime; CFR, Citrobacter freundii; CFX, cefoxitin; CFZ, cefazolin; CIP, ciprofloxacin; CLI, clindamycin; CLF, chloramphenicol; CP, complicated pyelonephritis; CST, colistin; CTX, ceftriaxone; DOX, doxycycline; GEN, gentamicin; ICU, intensive care unit; IMI, imipenem; KPN, Klebsiella pneumoniae; LNZ, linezolid; LOS, length-of-stay; MEM, meropenem; MET, metronidazole; MIN, minocycline; MOX, moxifloxacin; N/A, not applicable; NTF, nitrofurantoin; P-T, piperacillin-tazobactam; T-C, ticarcillin-clavulanate; TIG, tigecycline; TOB, tobramycin; T-S, trimethoprim-sulfamethoxazole; VAN, vancomycin
Complicating Factors are as defined by Neal [16]: Indwelling urinary catheter ≥ 48hrs, urinary tract obstruction, male sex, age < 12 years, diabetes mellitus, CrCL < 30mL/min, immunosuppression, urolithiasis, urinary tract surgery, voiding dysfunction, urethral valves, vesicoureteral reflux, pregnancy, and nosocomial acquisition (> 48 hours after admission or direct admission from a long-term care facility)
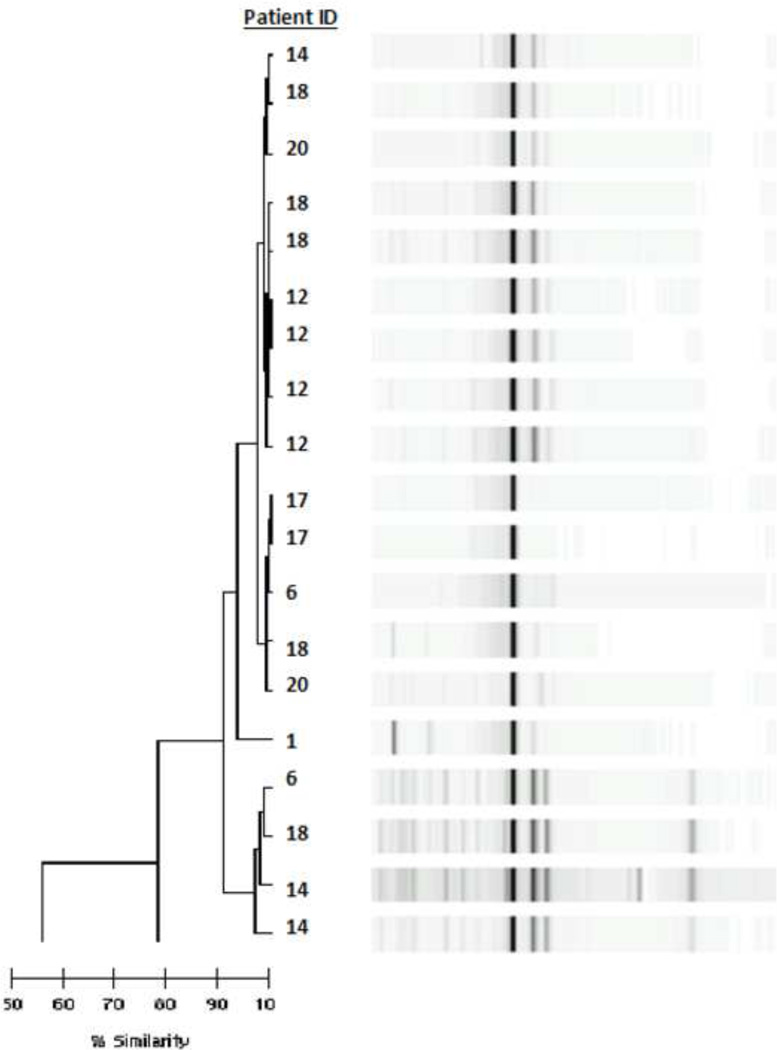
All but one of the isolates identified were K. pneumoniae, the other being Citrobacter freundii. Nineteen K. pneumoniae isolates from seven unique patients were tested for clonality and showed genotypic similarity (Figure 1). Ten patients (48%) were residents of a LTCF at the time of admission. Isolates were classified as hospital-acquired in 8 patients (38%) and only 6 isolates (29%) could be strictly considered community-acquired. All patients met criteria for symptomatic UTI; 12 patients (57%) had complicated cystitis and 9 patients (43%) had complicated pyelonephritis. All but one patient had a concordant urinalysis with pyuria (95%). Patients who had a urinary catheter in place at the time of bacteriuria had those catheters removed or replaced in 9 of 15 cases (60%). Three patients (14%) developed bacteremia with a KPC-producing isolate having the same susceptibility pattern as their urinary isolate; none of these patients died. Among patients receiving at least one Gram-negative active antibiotic during hospitalization and prior to isolation of their KPC-producing isolate (16 of 21 patients – 76%), the most common classes administered were fluoroquinolones and fourth-generation cephalosporins (eight patients each).
Figure 1.
Dendrogram generated from 19 blaKPC-positive K. pneumoniae isolates using RW3A primers from seven patients. All isolates were found to be clonally-related (similarity index > 90%).
Active antibiotics were ultimately utilized in 14 of the 21 cases (67%). In patients receiving active antibiotic therapy, significant delays (median 72.5 hours, range 4–312 hours) in implementing these therapies were noted. Infectious Diseases consultation was obtained for seven patients (33%), of which six were given active antibiotics. Gentamicin was the most common active drug used for treatment in our cohort (7 of 14 patients), with no other single agent or class of agents used in more than 30% of cases. In vitro testing of an extended antibiotic panel revealed high rates of susceptibility for tigecycline (28 of 29 – 97%), minocycline (22 of 29 – 76%), and fosfomycin (25 of 29 – 86%) against these isolates (Table 2).
Table 2.
In vitro antibiotic susceptibilities for KPC-producing Enterobacteriaceae
| Antibiotic | Disk Diffusion Susceptibility % S / % I / % R |
E-test MICs | |
|---|---|---|---|
| MIC50 | MIC90 | ||
| Amikacin | 100% / 0% / 0% | ||
| Ampicillin | 0% / 0% / 100% | ||
| Ampicillin/sulbactam | 0% / 0% / 100% | ||
| Aztreonam | 0% / 0% / 100% | ||
| Cefazolin | 0% / 0% / 100% | ||
| Cefepime | 0% / 40% / 60% | ||
| Cefoxitin | 0% / 0% / 100% | ||
| Chloramphenicol | 256 | 256 | |
| Ciprofloxacin | 5% / 33% / 62% | ||
| Colistin | 100% / 0% / 0% | ||
| Ceftriaxone | 0% / 0% / 100% | ||
| Doripenem | 32 | 32 | |
| Doxycycline | 100% / 0% / 0% | ||
| Ertapenem | 32 | 32 | |
| Fosfomycina | 16 | 205 | |
| Gentamicin | 81% / 19% / 0% | ||
| Imipenem | 32 | 32 | |
| Meropenem | 32 | 32 | |
| Minocycline | 4 | 22 | |
| Nitrofurantoin | 19% / 24% / 57% | ||
| Piperacillin/tazobactam | 0% / 0% / 100% | ||
| Tetracycline | 6 | 18 | |
| Ticarcillin/clavulanate | 0% / 0% / 100% | ||
| Tigecyclineb | 1 | 2 | |
| Tobramycin | 54% / 31% / 15% | ||
| Trimethoprim/sulfamethoxazole | 0% / 0% / 100% | ||
Abbreviation: S, sensitive; I, intermediate; R, resistant
Clinical and Laboratory Standards Institute susceptibility breakpoints for fosfomycin: susceptible – ≤64 mg/L; intermediate – 128 mg/L; resistant – ≥256 mg/L
US Food and Drug Administration susceptibility breakpoints for tigecycline: susceptible – ≤2 mg/L; intermediate – 4 mg/L; resistant – ≥8 mg/L
Positive clinical response was observed in 16 patients (76%), including all seven patients receiving aminoglycoside therapy. Positive microbiologic response was observed in six patients (29%), and presumed microbiologic response in an additional ten patients (48%). All-cause mortality during hospitalization was 19% (four patients). Of the four patients who died, only one had neither a positive clinical nor microbiologic response.
Discussion
This is one of the first reports of clinical and microbiological outcomes for a cohort of patients with UTI caused by KPC-producing Enterobacteriaceae. Multiple prior reports have focused on epidemiological investigations of outbreaks that included but were not limited to UTIs due to KPC-producing pathogens; however, defined criteria for identifying infection and clinical outcomes of treatment were not reported separately for UTIs [17,18]. A recent report described microbiologic clearance rates for KPC-producing UTI isolates using different therapeutic regimens, however, clinical treatment response and clonal relatedness were not investigated [7].
Other investigators have described that clonally-related KPC-producing isolates have rapidly spread to encompass a large proportion of isolates between institutions across a geographically vast area [19]. As depicted in the dendrogram (Figure 1), all 19 KPC-producing K. pneumoniae isolates available for testing were found to be clonally-related (similarity index > 90%), although there were three unique clusters within the dendrogram demonstrating a higher similarity index. This finding suggests the likely endemicity of this pathogen in our area, possibly linked to local healthcare institutions, as patients were found to be epidemiologically heterogeneous. This postulate is further supported by our finding that a majority of patients had some form of readily-identifiable healthcare exposure, with only 29% (6/21) having community-acquired infections sensu stricto. The evolving endemic nature of KPC-producing organisms argues for improved identification and infection prevention measures within local healthcare institutions to prevent the further spread of these infections.
Our cohort was heavily antibiotic treatment-experienced, with a large proportion receiving at least one broad-spectrum Gram-negative active antibiotic prior to isolation of their KPC-producing isolate. Previous investigations have identified prior use of fluoroquinolones and broad-spectrum β-lactam antibiotics as risk factors for isolation of KPC-producing Enterobacteriaceae [10,20–23]. Consistent with these data, fluoroquinolones and cefepime were also the most common prior antibiotics utilized in our cohort, further suggesting that stewardship activities targeted towards these classes may yield benefit in reducing resistance development and complications of these infections.
Many patients experienced delays to appropriate antibiotic treatment, and some never received active antibiotics. In fact, all patients identified as having either negative or uncertain clinical response failed to receive active antibiotic therapy. In addition, a significant number of patients with urinary catheters at the time of KPC isolate identification did not have them removed during the treatment period, which is an important intervention for improving outcomes. One study reported that for carbapenem-resistant systemic infections, having the probable source of infection removed was an independent predictor for survival, whereas concurrent administration of active antibiotics was not [20]. Also, current UTI treatment guidelines favor catheter replacement prior to antibiotic treatment [24]. Regardless of occasional suboptimal management within our cohort, clinical outcomes were generally encouraging. Of those patients who died, all but one displayed either positive clinical or microbiologic response and none had concurrent KPC bacteremia, suggesting that mortality may have been due largely to factors other than UTI. Still, KPC UTIs are a potential precursor to KPC-associated systemic infections, which have consistently been reported to cause excess morbidity and mortality [20–22,25].
Gentamicin was the most common antibiotic administered in our cohort, and aminoglycosides generally displayed high rates of activity against tested isolates (Table 2). Aminoglycoside monotherapy was described in a meta-analysis to be equally efficacious to comparator antibiotics in the treatment of UTIs [26]. In patients with KPC-producing Enterobacteriaceae, aminoglycosides are often one of the few remaining therapeutic options, despite their potential nephrotoxicity and requirement for parenteral administration [27]. Of our patients that received gentamicin as a component of their treatment regimen, all had positive clinical response, and either or presumed microbiologic response. A recent investigation has also described favorable responses with aminoglycosides in the treatment of KPC-producing UTI [7]. These findings suggest that aminoglycosides are a viable option for treatment of UTIs caused by susceptible KPC-producing strains.
One patient in our study received tigecycline as a part of their treatment regimen, with clinical but without microbiologic response. High rates of in vitro susceptibility to this agent against KPC-producing isolates have been described in this and other studies [28]. It is generally thought that since tigecycline has limited excretion into the lower urinary system, caution should be exercised with its use for UTIs. However, recent investigations challenge this assumption [29]. The structurally-related minocycline was also found to have high rates of activity in our study, which suggests that either this agent or doxycycline may be potential oral options. We did not routinely determine doxycycline in vitro susceptibilities in our cohort, however, in three cases activity was presumed based on minocycline susceptibility and these patients received doxycycline as a component of therapy, with uniformly positive clinical outcomes. Although not clinically administered in our study, fosfomycin was also found to have in vitro activity against the isolates tested. A number of recent reports have reported high rates of activity of fosfomycin against KPC-producing organisms [30–32]. Pending additional data on clinical use, fosfomycin may be a viable option for UTIs that are confined to the lower urinary tract and caused by fosfomycin-susceptible strains of KPC-producing bacteria.
Limitations of this study include its retrospective design, small cohort size, availability of molecular epidemiological data for only a subset of our patients, and the possibility that blaKPC-positive strains without phenotypic carbapenem resistance may have been missed in our screening. Outpatient and long-term treatment outcomes, as well as potential adverse effects, could not be adequately evaluated with this study design. Although we describe in vitro MIC results for our urinary isolates which have been strictly validated for predicting drug efficacy at concentrations achieved in the bloodstream, drug concentrations above the MIC can be achieved in the treatment of UTI as a result of renal drug elimination.
Conclusion
The majority of patients were reported as having positive clinical responses to treatment of KPC-producing UTI, despite delay or lack of administration of active antibiotic therapy and/or failure to remove indwelling urinary catheters. Aminoglycoside therapy was most commonly used for treatment in our study, and the data suggest that it was effective in this limited group of patients; tetracycline derivatives also appeared to be promising options. Although not used clinically in our study, fosfomycin was highly active in vitro against the studied KPC-producing strains.
Acknowledgments
This study was carried out as part of our routine work, received no funding, and was completed with department resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors indicate that they have no conflicts of interest regarding the content of this article.
References
- 1.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande LM, Rhomberg PR, Sader HS, Jones RN. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States Medical Centers: report from the MYSTIC Program (1999–2005) Diagn Microbiol Infect Dis. 2006;56:367–372. doi: 10.1016/j.diagmicrobio.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Moland ES, Hong SG, Thomson KS, Larone DH, Hanson ND. Klebsiella pneumoniae isolate producing at least eight different beta-lactamases, including AmpC and KPC beta-lactamases. Antimicrob Agents Chemother. 2007;51:800–801. doi: 10.1128/AAC.01143-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicrob Agents Chemother. 2008;52:2680–2682. doi: 10.1128/AAC.00158-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maltezou HC, Giakkoupi P, Maragos A, et al. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece) J Infect. 2009;58:213–219. doi: 10.1016/j.jinf.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymixin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother. 2011;55:5893–5899. doi: 10.1128/AAC.00387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. M7-A7, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. M100-S16, Performance standards for antimicrobial susceptibility testing, 16th informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 10.Bratu S, Tolaney P, Karumudi U, et al. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother. 2005;56:128–132. doi: 10.1093/jac/dki175. [DOI] [PubMed] [Google Scholar]
- 11.Marschall J, Tibbetts RJ, Dunne WM, Jr, Frye JG, Fraser VJ, Warren DK. Presence of the KPC carbapenemase gene in Enterobacteriaceae causing bacteremia and its correlation with in vitro carbapenem susceptibility. J Clin Microbiol. 2009;47:239–241. doi: 10.1128/JCM.02123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Vecchio VG, Petroziello JM, Gress MJ, et al. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J Clin Microbiol. 1995;33:2141–2144. doi: 10.1128/jcm.33.8.2141-2144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao RS, Storch GA, Buller RS, et al. Blinded comparison of repetitive-sequence PCR and multilocus sequence typing for genotyping methicillin-resistant Staphylococcus aureus isolates from a children’s hospital in St. Louis, Missouri. J Clin Microbiol. 2006;44:2254–2257. doi: 10.1128/JCM.00690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren JW, Abrutyn E, Hebel JR, Johnson JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29:745–758. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 15.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32:1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 16.Neal DE., Jr Complicated urinary tract infections. Urol Clin N Am. 2008;35:13–22. doi: 10.1016/j.ucl.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Poulou A, Spanakis N, Pournaras S, et al. Recurrent healthcare-associated community-onset infections due to Klebsiella pneumoniae producing VIM-1 metallo-beta-lactamase. J Antimicrob Chemother. 2010;65:2538–2542. doi: 10.1093/jac/dkq363. [DOI] [PubMed] [Google Scholar]
- 18.Tsakris A, Poulou A, Markou F, et al. Dissemination of clinical isolates of Klebsiella oxytoca harboring CMY-31, VIM-1, and a new OXY-2 type variant in the community. Antimicrob Agents Chemother. 2011;55:3164–3168. doi: 10.1128/AAC.00102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother. 2009;63:427–437. doi: 10.1093/jac/dkn547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 21.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falagas ME, Rafailidis PI, Kofteridis D, et al. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case control study. J Antimicrob Chemother. 2007;60:1124–1130. doi: 10.1093/jac/dkm356. [DOI] [PubMed] [Google Scholar]
- 24.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 international clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 25.Mouloudi E, Protonotariou E, Zagorianou A, et al. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol. 2010;31:1250–1256. doi: 10.1086/657135. [DOI] [PubMed] [Google Scholar]
- 26.Vidal L, Gafter-Gvili A, Borok S, Fraser A, Leibovici L, Paul M. Efficacy and safety of aminoglycoside monotherapy: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2007;60:247–257. doi: 10.1093/jac/dkm193. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 28.Castanheira M, Sader HS, Jones RN. Antimicrobial susceptibility patterns of KPC-producing or CTX-M-producing Enterobacteriaceae. Microb Drug Resist. 2010;16:61–65. doi: 10.1089/mdr.2009.0031. [DOI] [PubMed] [Google Scholar]
- 29.Nix DE, Matthias KR. Should tigecycline be considered for urinary tract infections? A pharmacokinetic re-evaluation. J Antimicrob Chemother. 2010;65:1311–1312. doi: 10.1093/jac/dkq116. [DOI] [PubMed] [Google Scholar]
- 30.Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int J Antimicrob Agents. 2011;37:415–419. doi: 10.1016/j.ijantimicag.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother. 2010;54:526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falagas ME, Maraki S, Karageorgopoulos DE, Kastoris AC, Mavromanolakis E, Samonis G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int J Antimicrob Agents. 2010;35:240–243. doi: 10.1016/j.ijantimicag.2009.10.019. [DOI] [PubMed] [Google Scholar]