Abstract
It has been widely reported that T cells are capable of influencing osteoclast formation and bone remodelling, yet relatively little is known of the reciprocal effects of osteoclasts for affecting T cell function and/or activity. In this study we investigated the effects of human osteoclasts on the function of γδ T cells, a subset of non-CD4+ T cells implicated in a variety of inflammatory disease states. γδ T cells and CD4+ T cells were isolated from peripheral blood of healthy volunteers and were co-cultured with autologous mature osteoclasts (generated by treatment with M-CSF and RANKL) before phenotypical and functional changes in the T cell populations were assessed. Macrophages, osteoclasts, and conditioned medium derived from macrophages or osteoclasts induced activation of γδ T cells, as determined by the expression of the early activation marker CD69. TNFα was a major mediator of this stimulatory effect on γδ T cells. Consistent with this stimulatory effect, osteoclasts augmented proliferation of IL-2-stimulated γδ T cells and also supported the survival of unstimulated γδ and CD4+ T cells, although these effects required co-culture with osteoclasts. Co-culture with osteoclasts also increased the proportion of γδ T cells producing IFNγ, but did not modulate IFNγ or IL-17 production by CD4+ T cells. We provide new insights into the in vitro interactions between human γδ T cells and osteoclasts/macrophages, and demonstrate that osteoclasts or their precursors are capable of influencing γδ T function both via the release of soluble factors and also through direct cell–cell interactions.
Abbreviations: IFNγ, interferon gamma; TNFα, tumour necrosis factor alpha; αMEM, alpha minimal essential medium; M-CSF, macrophage colony-stimulating factor; RANKL, receptor activator of nuclear factor kappa-B ligand; FBS, foetal bovine serum; TCR, T cell receptor; MCP-1, monocyte chemotactic protein-1; RANTES, regulated on activation, normal T cell expressed and secreted
Keywords: Osteoclast, γδ T cell, Chemotaxis, CD69, TNFα, IFNγ
Highlights
-
•
Interactions of human macrophages and osteoclasts with γδ T cells were investigated.
-
•
Osteoclasts produce T cell-active chemokines and stimulate γδ T cell migration.
-
•
Osteoclasts induced activation of autologous γδ T cells via TNFα.
-
•
Osteoclasts promoted γδ T cell survival and augmented γδ T cell proliferation.
-
•
Macrophages and osteoclasts stimulate IFNγ production in γδ T cells.
Introduction
Interactions between the immune system and bone have been a subject of intensive research interest in recent years, with a focus on how activated immune cells, such as T cells, affect the formation and activity of bone-resorbing osteoclasts both in vitro and in vivo [1–6]. Such interactions are thought to play a crucial role in the enhanced bone and joint destruction observed in chronic autoimmune diseases such as rheumatoid arthritis, where pro-inflammatory cytokines especially TNFα, derived from CD4+ T cells present in the inflamed synovium [7], result in the increased formation of osteoclasts. Other important CD4+ T cell-derived stimulatory mediators of osteoclast formation include the critical osteoclast differentiation factor, RANKL [5,8], and the pro-inflammatory cytokine IL-17 [9], which indirectly increases the expression of RANKL on osteoblasts and stromal cells in the local bone microenvironment. The enhanced osteoclast activity in inflamed joints drives the destruction of subchondral bone in the joint, resulting in the deterioration in joint microarchitecture and function, a characteristic feature of rheumatoid arthritis. However, while the role of soluble mediators has been extensively investigated in this process, the co-localisation of T cells with osteoclasts at the endosteal bone surface suggests that cell–cell contact may also play an important role in the functional outcome of interactions between osteoclasts and T cells in vivo [10].
Given the extensive evidence of a role of T cells for affecting osteoclast formation and activity, the reciprocal interactions of osteoclasts on T cell function, particularly in vivo, are ill-defined. It is now apparent that osteoclasts themselves share properties typically associated with specialised antigen-presenting cells, since they are capable of antigen uptake, processing and presentation to CD4+ and CD8+ T cells [11], and express T cell co-stimulatory molecules such as CD40 and CD80 [11,12]. Osteoclasts have also been observed to secrete a variety of T cell-active chemokines, and have been shown to retain and recruit T cells in vitro [12] with such interactions resulting in the modulation of phenotype and responsiveness of CD4+ and CD8+ T cells [11–13]. Despite these well-characterised effects of osteoclasts on CD4+ and CD8+ T cells, it is as yet unclear what effect osteoclasts have on γδ T cells or other non-conventional T cell subsets.
In murine models of human rheumatoid arthritis, γδ T cells have been reported to be pathogenic through marked production of IL-17 [14]. However, the contribution of dysregulated γδ T cell responses to bone loss in chronic human inflammatory diseases is currently debated, with recent studies suggesting that IL-17 production by γδ T cells does not play a pathophysiological role in rheumatoid arthritis [10,15], despite an elevation in their numbers in the synovial fluid and the inflamed synovium in rheumatoid arthritis patients [16–19]. Interestingly, a recent study has reported the increased prevalence of IL-17-producing γδ T cells in ankylosing spondylitis patients, providing the first evidence for a potentially pathogenic role of γδ T cells in human autoimmune disease [20].
In this study we investigated the effects of osteoclasts (and their monocyte/macrophage-lineage precursors) on γδ T cell function and reveal a novel immunostimulatory effect of macrophages/osteoclasts on γδ T cells. Osteoclast- and macrophage-derived soluble factors, particularly TNFα, were capable of inducing activation of γδ T cells, with further stimulatory effects of osteoclasts on γδ T cell survival, proliferation and cytokine production mediated during co-culture of these cells. This study therefore suggests a new immunostimulatory effect of macrophages and osteoclasts on γδ T cells and reveals an intriguing role for macrophages and osteoclasts in modulating the behaviour of innate immune cells, such as γδ T cells.
Materials & methods
All chemicals were from Sigma Chemical Co. (UK) unless otherwise stated.
Isolation of osteoclast precursors and generation of mature osteoclasts
All work with human subjects was approved by the North of Scotland Research Ethics Committee prior to commencement of these studies. Osteoclasts were generated as previously described [21]. Briefly, PBMCs were isolated using density-gradient separation and monocyte/macrophage-lineage cells were culture-expanded and differentiated to macrophages for 5–7 days in complete αMEM (containing 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 10% (v/v) FBS) supplemented with 20 ng/ml recombinant human M-CSF (R&D Systems). Mature osteoclasts were generated from precursors by treatment with 2 ng/ml recombinant murine RANKL (R&D Systems) and 20 ng/ml M-CSF, for 5–6 days. In some experiments macrophages were expanded in parallel cultures, supplemented with M-CSF only. Cells were supplied with fresh cytokines every 48 h.
T cell isolation and activation
γδ T cells and CD4+ T cells were isolated from peripheral blood mononuclear cells (PBMCs) of healthy donors using magnetic bead separation, as previously described [21]. The purity of the isolated T cell subsets was routinely ≥ 90% for γδ T cells and ≥ 98% for CD4+ T cells. In some experiments γδ and CD4+ T cells were activated with anti-CD3/anti-CD28-coated T-Activator Dynabeads® (Invitrogen) at a bead-to-cell ratio 1:1 for 24 h, or alternatively with 100 U/ml IL-2, prior to incubation with autologous macrophages or osteoclasts.
Determination of cytokine/chemokine production by osteoclasts
Osteoclasts were differentiated as described, then cultured for 48 h to generate conditioned medium. Cells and debris were then removed by sequential centrifugation at 300 g and 13,000 g, prior to determination of chemokine profiles using a Proteome Profiler Human Cytokine Array Kit, Panel A (R&D Systems), according to the manufacturer's instructions. Briefly, conditioned medium from macrophage or osteoclast cultures were incubated with a cocktail of supplied biotinylated detection antibodies prior to incubation with the array. The presence of cytokine/antibody complexes is then determined through binding to an immobilised capture antibody present on the array and subsequent streptavidin–horseradish peroxidase and chemiluminescent detection. Levels of MCP-1/CCL2, RANTES/CCL5 and IL-8/CXCL8 were quantified using a Milliplex MAP Human Cytokine/Chemokine array (Millipore), according to the manufacturer's instructions.
Chemotaxis assay
Differentiated osteoclasts were generated and then cultured for 48 h in serum-free medium supplemented with 20 ng/ml M-CSF and 2 ng/ml RANKL. Conditioned medium was harvested, centrifuged to remove cells and debris, and 600 μl/well was added to 24-well plates. Serum-free medium and medium containing 10% FBS, were supplemented with M-CSF and RANKL, and used as negative and positive controls, respectively. Prior to the chemotaxis assay, γδ T cells were activated for 12 h with 100 U/ml rhIL-2. γδ T cells were then re-suspended in serum-free medium at 106 cells/ml and 80 μl of cell suspension was added into Transwell inserts (8 μm pore size). γδ T cells were incubated for 4 h at 37 °C to allow migration through the Transwell membrane. Cells that had migrated into the bottom chamber were harvested and quantified using flow cytometric analysis on an LSRII flow cytometer (BD Biosciences) by counting an equivalent volume of cell suspension for each sample. γδ T cell migration was expressed as the fold-change of migrated γδ T cells relative to FBS-induced migration.
T cell activation assay
M-CSF-expanded macrophages, or differentiated osteoclasts, were cultured in 96-well plates at a density of 104 cells/well and allowed to adhere for 4 h, in the presence of M-CSF alone, or with M-CSF plus RANKL, respectively. In some experiments, mature osteoclasts were treated for 24 h with 5 ng/ml TNFα (Peprotech) and 20 ng/ml IFNγ (R&D Systems), followed by a 24 h wash-out period (hereafter referred to as treated osteoclasts), prior to culture in 96-well plates. Autologous γδ T cells or CD4+ T cells (both 5 × 104) were added to cultures of macrophages or osteoclasts for 72 h. As a positive control, γδ T cells were cultured in the presence of 100 U/ml IL-2, and CD4+ T cells were activated with anti-CD3/CD28-coated T-Activator Dynabeads at a bead-to-cell ratio of 1:1.
In some experiments, γδ T cells and CD4+ T cells were cultured with conditioned medium from macrophage, osteoclast or treated osteoclast cultures. To generate conditioned medium, macrophages, osteoclasts, and osteoclasts pre-treated with TNFα and IFNγ for 24 h, were supplemented with fresh medium and further cultured for 48 h. Cells and debris were then removed by sequential centrifugation at 300 g and 13,000 g, prior to addition to T cell cultures. The following neutralising antibodies were used: monoclonal mouse anti-human TNFα antibody, or mouse IgG1, κ isotype control (10 μg/ml — both Biolegend). Antibodies were pre-incubated with conditioned medium for 30 min prior to addition of T cells.
Following culture, γδ T cells and CD4+ T cells were harvested and labelled with eFluor780 fixable viability dye (eBioscience), then stained with anti-human γδ-TCR-FITC or anti-human CD4-FITC (both Beckman Coulter), respectively, in combination with anti-human CD69-PE antibody (BD Biosciences). CD69 expression by viable γδ T cells or CD4+ T cells was then assessed using flow cytometric analysis with an LSR II flow cytometer, and data were analysed with FlowJo software (Tree Star Inc.).
T cell proliferation and survival assays
Mature osteoclasts were seeded in 96-well plates as per the T cell activation assay. Autologous γδ and CD4+ T cells were labelled with 1 μM CellTrace™ CFSE (Molecular Probes) according to the manufacturer's instructions, and 5 × 104 T cells (plus 100 U/ml IL-2) were cultured alone, or in the presence of osteoclasts, for 5 days. Cultures were supplemented with fresh M-CSF and RANKL every 48 h to maintain osteoclast viability. In selected experiments, γδ T cells and CD4+ T cells were cultured with osteoclast conditioned medium for 5 days. γδ T cells and CD4+ T cells were then harvested and proliferation was assessed by quantifying CFSE fluorescence using an LSRII flow cytometer. Data were analysed with FlowJo software.
To assess T cell survival in the absence or presence of osteoclasts, autologous γδ T cells and CD4+ T cells were co-cultured with osteoclasts for 5 days, at a T cell:osteoclast ratio of 5:1. In some experiments a monoclonal mouse anti-human TNFα neutralising antibody (or respective mouse IgG1, κ isotype control — both 10 μg/ml) was used to determine the contribution of TNFα to the survival effects of osteoclasts on γδ T cells. Antibodies were pre-incubated with osteoclasts for 30 min prior to addition of γδ T cells. γδ T cells and CD4+ T cells were then harvested and stained with Annexin V-Pacific Blue and 7-AAD (both eBioscience). T cell apoptosis/necrosis was assessed using flow cytometric analysis performed on an LSRII flow cytometer. Data were analysed with FlowJo software.
Intracellular cytokine staining
Following co-culture of γδ and CD4+ T cells with autologous macrophages or osteoclasts for 3 days, T cells were harvested and stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml ionomycin in the presence of Golgistop reagent (BD Biosciences) for a further 6 h. γδ T cells and CD4+ T cells were then harvested and stained with anti-human γδ-TCR-FITC or anti-human CD4-FITC, respectively, prior to fixation and permeabilisation with a Cytofix/Cytoperm kit (BD Biosciences). T cells were then stained using a monoclonal mouse anti-human IFNγ-V450 antibody or mouse IgG1, κ-V450 isotype control (both BD Biosciences), and monoclonal mouse anti-human-IL-17-PE or mouse IgG1-PE isotype control (both eBiosciences). IFNγ- and IL-17-producing T cells were then assessed using flow cytometric analysis with an LSR II flow cytometer, and data were analysed with FlowJo software.
Statistical analysis
Data were analysed using the Kruskal–Wallis one-way analysis of variance on ranks (SigmaPlot®11.0), with inter-group comparisons analysed using the Wilcoxon matched-pairs rank test. p values ≤ 0.05 were considered statistically significant.
Results
Chemokine and cytokine production by osteoclasts
In order to determine whether osteoclasts produce chemokines, and are therefore potentially capable of recruiting γδ T cells, we determined chemokine production by osteoclasts using a Proteome Profiler Array. Conditioned medium from cultures of unstimulated mature osteoclasts contained a variety of chemokines, including MCP-1/CCL2, GROα/CXCL1 and IL-8/CXCL8 (Fig. 1A), indicating that osteoclasts had the capacity to recruit immune cells, including T cells and NK cells (via MCP-1/CCL2), and granulocytes (via GROα/CXCL1 and IL-8/CXCL8). Other factors produced by unstimulated osteoclasts detected on the array included IL-1RA, soluble ICAM-1 (sICAM-1) and Serpin E1. We also quantified production of a variety of chemokines and detected marked levels of MCP-1/CCL2 (753.02 ± 170.17 pg/ml), IL-8/CXCL8 (606.43 ± 44.95 pg/ml) and RANTES/CCL5 (331.81 ± 18.42 pg/ml) in osteoclast conditioned medium, thereby further supporting the idea that osteoclasts are capable of influencing the recruitment of a variety of immune cells.
Fig. 1.
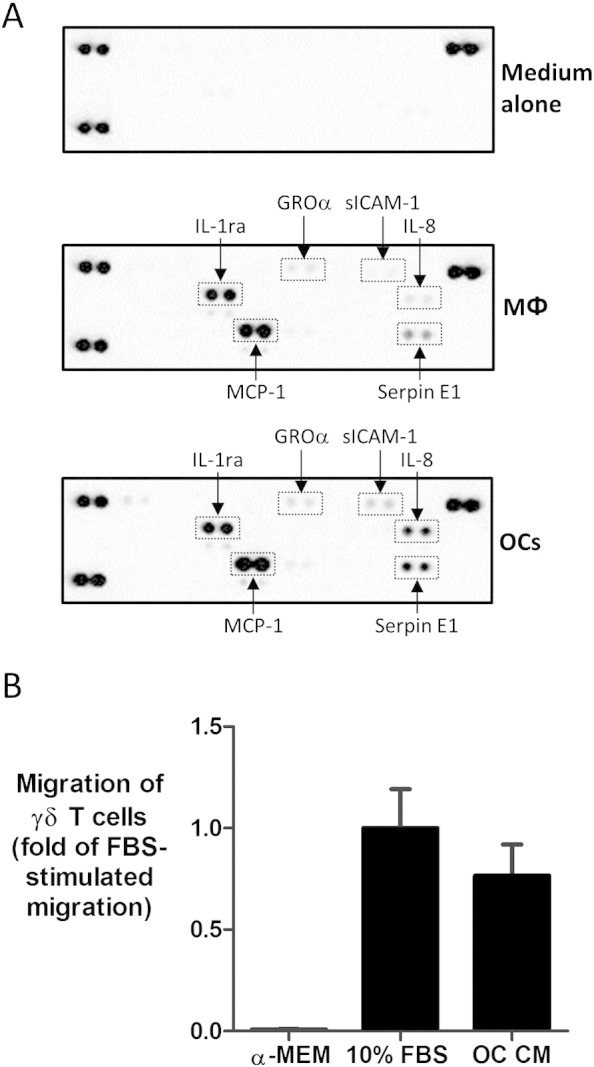
Osteoclasts produce T cell-active chemokines capable of inducing γδ T cell chemotaxis. A.) Conditioned medium was harvested from 48 h cultures of macrophages (MΦ) or mature osteoclasts (OC) and cytokine/chemokine profiles were determined using a Proteome Profiler Human Cytokine Array Kit (R&D Systems), according to the manufacturer's instructions. Proteins of interest are highlighted (GROα = CXCL1; IL-8 = CXCL8; MCP-1 = CCL2). Data shown are representative of two independent experiments using conditioned medium from macrophages and osteoclasts derived from two different donors. B.) A Transwell chemotaxis assay was conducted using serum-free medium, medium containing 10% FBS, or serum-free osteoclast conditioned medium (OC CM) as chemoattractants. Purified γδ T cells (pre-activated with 100 U/ml IL-2 for 12 h) were added into the Transwell inserts (8 μm pore size) and the cells were incubated for 4 h at 37 °C. Migrated cells were harvested and quantified using flow cytometric analysis. Migration of γδ T cells is normalised to fold-change of FBS-stimulated migration. Data shown are mean + S.E.M. using γδ T cells from four independent donors.
Osteoclasts release soluble factors capable of recruiting γδ T cells
We then sought to determine if soluble mediators released by osteoclasts could induce the migration of γδ T cells. Due to the potential confounding effects of FBS present in conditioned medium for stimulating T cell migration directly, we generated conditioned medium from osteoclasts cultured for 48 h in the absence of serum but supplemented with M-CSF and RANKL; conditions which did not adversely affect osteoclast viability as assessed by cellular morphology (data not shown). γδ T cells were pre-activated with 100 U/ml IL-2 for 12 h prior to addition, since unstimulated γδ T cells had limited motility in response to FBS-induced migration (data not shown), consistent with a previous study of T cell chemotaxis [22]. While activated γδ T cells did not migrate towards serum-free medium (Fig. 1B), FBS induced marked γδ T cell migration (~ 15–20% of input cells — data not shown). Interestingly, serum-free osteoclast conditioned medium also induced marked migration of γδ T cells across the Transwell membrane, comparable to that observed with FBS, indicating that osteoclasts release soluble factors capable of inducing the migration of γδ T cells.
Osteoclasts induce activation of γδ T cells and CD4+ T cells under co-culture conditions
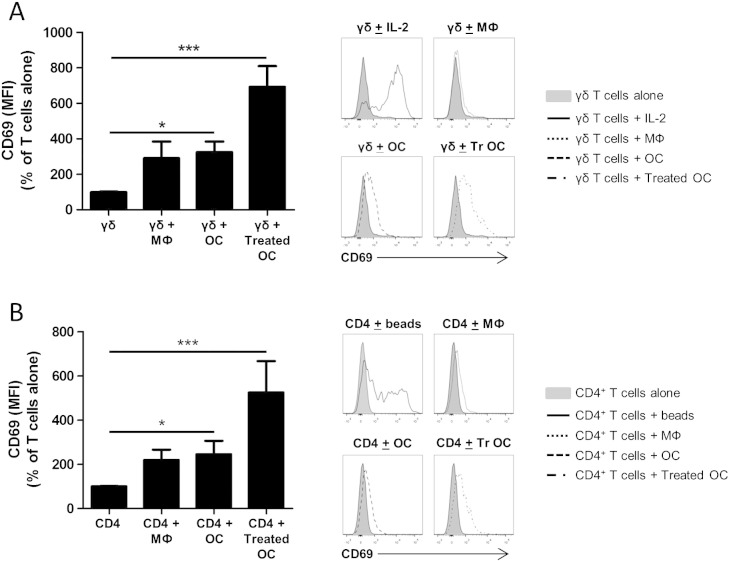
We next assessed whether osteoclasts could induce activation of T cells, using the early activation marker CD69. When γδ T cells or CD4+ T cells were co-cultured with osteoclasts for 3 days a significant increase in CD69 expression was observed in both the γδ T cell (Fig. 2A) and CD4+ T cell populations (Fig. 2B). A non-significant trend for macrophages to induce CD69 expression on both γδ T cells (Fig. 2A) and CD4+ T cells (Fig. 2B) comparable to that observed with osteoclasts was also demonstrated. Following co-culture with treated osteoclasts (i.e. osteoclasts pre-treated with TNFα and IFNγ for 24 h), CD69 expression was further increased on γδ T cells, although this was not statistically different from untreated osteoclasts. A similar further upregulation of CD69 expression on CD4+ T cells was also observed following co-culture with treated osteoclasts.
Fig. 2.
Osteoclasts induce CD69 expression by γδ T cells and CD4+ T cells. Quantification of CD69 expression on γδ T cells (A — left panel) and CD4+ T cells (B — left panel) following a 3 day culture with autologous macrophages (MΦ) or osteoclasts (OC), at a T-cell:osteoclast ratio of 5:1. In some experiments osteoclasts were pre-treated with 5 ng/ml TNFα and 20 ng/ml IFNγ for 24 h (Treated OC), prior to addition of T cells. Following the incubation period, both γδ and CD4+ T cells were harvested and CD69 expression determined as detailed in Section 2. Representative histograms showing γδ T cell (A — right panel) and CD4+ T cell (B — right panel) CD69 expression alone, or in the presence of macrophages, osteoclasts, or treated osteoclasts. Data shown are the mean + SEM of four independent experiments, performed in duplicate, from different donors. (n = 4, *p < 0.05, ***p < 0.001 compared to T cells alone).
Soluble factors produced by osteoclasts are sufficient to activate γδ T cells
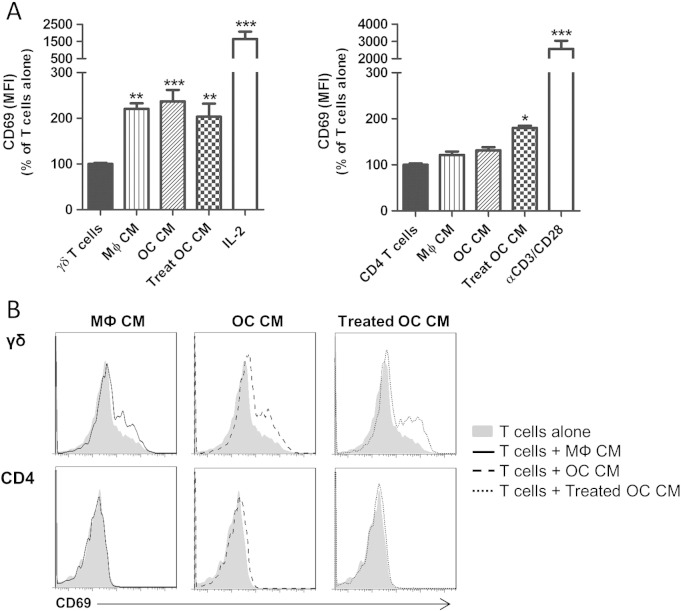
To determine if cell–cell contact was necessary for these stimulatory effects on CD69 expression we cultured γδ T cells and CD4+ T cells with conditioned medium from macrophages, osteoclasts or treated osteoclasts. Conditioned medium from macrophages, osteoclasts and treated osteoclasts all significantly increased CD69 expression on γδ T cells to a similar extent (Fig. 3). This was in contrast to our findings with CD4+ T cells, since conditioned medium from macrophages or untreated osteoclasts consistently failed to induce upregulation of CD69 on CD4+ T cells. However, conditioned medium from treated osteoclasts did induce a significant increase in CD69 expression on CD4+ T cells. Taken together, these results indicate that γδ T cell activation by macrophages or osteoclasts is mediated by soluble factors and does not fundamentally require cell–cell contact. However, the stimulatory effect of osteoclasts on CD4+ T cells requires co-culture conditions, suggesting that cell–cell interactions play an important role in this process.
Fig. 3.
Osteoclasts induce γδ T cell activation via the release of soluble factors. (A) CD69 expression on γδ T cells and CD4+ T cells was assessed in the presence of 100% (v/v) conditioned medium (CM) from macrophages (MФ), osteoclasts (OC) or treated osteoclasts (Treat OC). As positive controls, γδ T cells were activated with 100 U/ml IL-2 and CD4+ T cells with anti-CD3/CD28 Dynabeads® at a bead-to-cell ratio of 1:1. After 3 days in culture γδ T cells and CD4+ T cells were harvested and CD69 expression on viable γδ or CD4+ T cells determined as detailed in Section 2. Data shown are the mean + SEM of three independent experiments, performed in duplicate, from individual donors (n = 3, **p < 0.01, ***p < 0.001 compared to T cells alone). (B) Representative histograms showing CD69 expression on viable γδ T cells (upper row) and CD4+ T cells (bottom row) cultured alone or in the presence of CM.
TNFα is a major mediator of the stimulatory effect of osteoclasts on γδ T cells
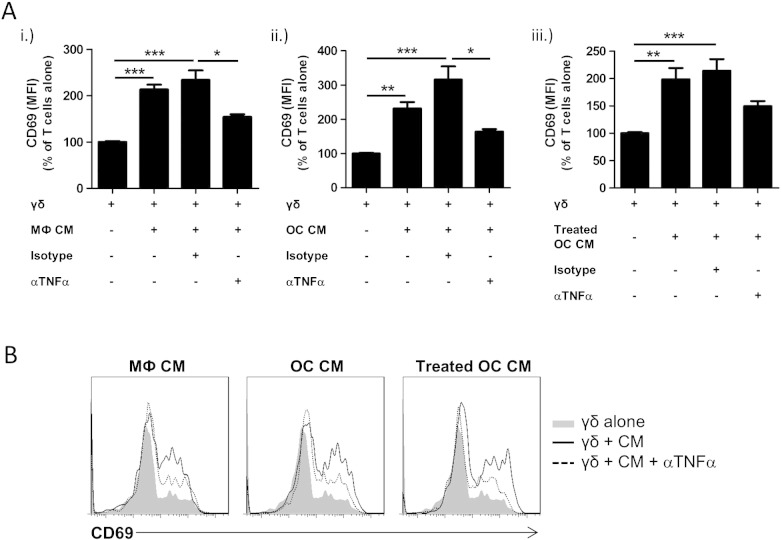
TNFα is a potent stimulator of T cell activation and is capable of co-stimulatory effects on T cell survival [23,24]. We therefore investigated whether macrophages and osteoclasts were triggering γδ T cell activation via production of TNFα. Using a neutralising anti-TNFα antibody we observed that the stimulatory effect of macrophage- and osteoclast-derived conditioned medium on CD69 expression by γδ T cells was significantly reduced versus the isotype control (Fig. 4). There was also a trend for TNFα neutralisation to diminish the stimulatory effects of treated osteoclast-derived conditioned medium but this was not statistically significant versus the isotype control. While the stimulatory effect of conditioned medium on γδ T cell activation was attenuated by anti-TNFα treatment, it was not abolished entirely, indicating that other stimulatory factors are present in osteoclast-derived conditioned medium that trigger γδ T cell activation.
Fig. 4.
TNFα is a mediator of the stimulatory effect of macrophage/osteoclast conditioned medium on γδ T cells. (A) γδ T cells were cultured with 100% (v/v) conditioned medium (CM) from (i.) macrophages (MФ), (ii.) untreated osteoclasts (OC), or (iii.) treated osteoclasts, in the absence or presence of anti-human TNFα antibody or isotype control (both 10 μg/ml). After 3 days in culture γδ T cells and CD4+ T cells were harvested and CD69 expression on viable γδ T cells determined as detailed in Section 2. Data shown are the mean + SEM of five independent experiments, performed in duplicate, from different donors (n = 5, *p < 0.05, **p < 0.01, ***p < 0.001). (B) Representative histograms showing the effect of TNFα neutralisation on CD69 expression induced by CM in γδ T cells.
Osteoclasts augment T cell proliferation and survival
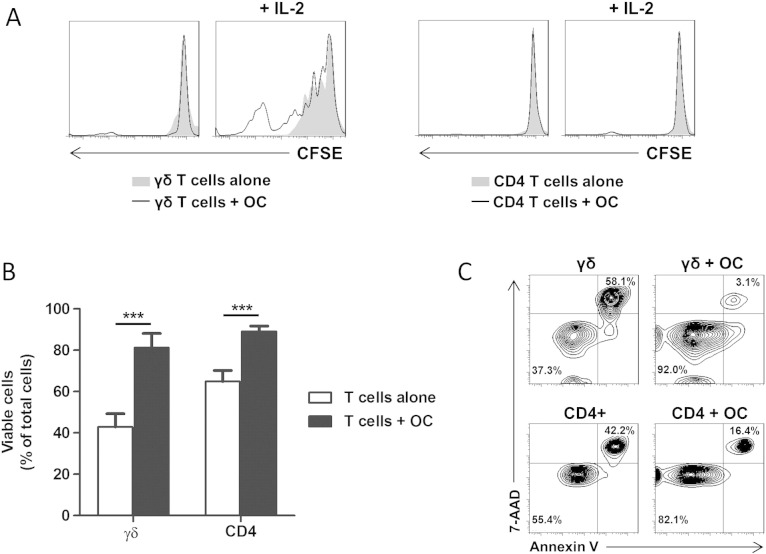
Following our observation that osteoclasts induce γδ T cell activation we then sought to determine whether these stimulatory effects of osteoclasts could trigger proliferative responses in γδ T cells. Using CFSE-labelled γδ and CD4+ T cells in co-cultures with autologous osteoclasts, we observed no proliferative effects of autologous osteoclasts on unstimulated γδ T cells or CD4+ T cells (Fig. 5A). However, activation of γδ T cells with IL-2 (which induced marked upregulation of CD69 on γδ T cells — Fig. 3A) resulted in extensive proliferation of γδ T cells, and this proliferative effect was further enhanced by co-culture with osteoclasts (Fig. 5A). In contrast to this, CD4+ T cells did not exhibit any proliferative responses to IL-2 alone or in co-culture with osteoclasts. This suggests that unstimulated osteoclasts provide co-stimulatory signals that augment IL-2-induced γδ T cell proliferation, but such co-stimulatory signals do not confer responsiveness of CD4+ T cells to IL-2 stimulation.
Fig. 5.
Osteoclasts augment the proliferation of activated γδ T cells and support T cell survival. (A) Representative histograms showing the effects of osteoclasts (OC) on γδ T cell proliferation in the absence or presence of IL-2 (left panels); CD4+ T cell proliferation in the absence or presence of IL-2 (right panels). γδ and CD4+ T cells were labelled with 1 μM CFSE dye prior to their incubation with autologous osteoclasts for 5 days. γδ T cells and CD4+ T cells were harvested and their proliferation was assessed based on dilution of CFSE fluorescence using flow cytometric analysis. Data shown are from one experiment and are representative of three independent experiments, performed in duplicate, from different donors. (B) γδ and CD4+ T cells were cultured alone or co-cultured with autologous osteoclasts (at a T cell:OC ratio of 5:1) for 5 days. Following this period, γδ T cells and CD4+ T cells were harvested and cell viability assessed as detailed in Section 2. Data shown are the mean + SEM from three independent experiments, performed in duplicate, from different donors (n = 3; ***p < 0.001). (C) Representative contour plots showing viability of T cells alone (left column) or in co-culture with osteoclasts (right column).
We next determined if osteoclasts could influence T cell survival by culturing γδ T cells or CD4+ T cells with autologous osteoclasts and consequently assessing T cell viability. After 5 days, purified cultures of unstimulated γδ T cells contained 42.9 ± 6.5% viable cells, which were significantly increased in the presence of osteoclasts to 81.2 ± 7.1% (Figs. 5B,C). Similarly, the viability of purified CD4+ T cells was significantly increased by the presence of osteoclasts, from 64.8 ± 5.5% to 89.1 ± 2.7%. This observed increase in cell viability conferred by osteoclasts was not simply due to engulfment of apoptotic T cells (and a consequent increase in the apparent viability of T cells in these co-cultures), since the recovered cell numbers from these co-cultures did not markedly differ from the purified T cell cultures alone (data not shown). This pro-survival effect of osteoclasts on T cells was dependent on co-culture conditions, since conditioned medium from osteoclast cultures had no protective effect on T cell survival (data not shown), thereby suggesting that osteoclast-derived soluble factors are themselves insufficient to maintain T cell viability.
Due to our previously observed stimulatory effects of TNFα on CD69 expression by γδ T cells and CD4+ T cells (Fig. 4A), we next investigated if osteoclast-derived TNFα was responsible for these pro-T cell survival effects using co-cultures of osteoclasts and γδ T cells. Following neutralisation of TNFα we observed no decrease in the osteoclast-induced survival of γδ T cells (Supplemental Fig. 1), thereby suggesting that TNFα is not a mediator of the protective effects of osteoclasts on T cell viability.
Due to our previously observed stimulatory effects of TNFα on CD69 expression by γδ T cells and CD4+ T cells (Fig. 4A), we next investigated if osteoclast-derived TNFα was responsible for these pro-T cell survival effects using co-cultures of osteoclasts and γδ T cells. Following neutralisation of TNFα we observed no decrease in the osteoclast-induced survival of γδ T cells (Supplemental Fig. 1), thereby suggesting that TNFα is not a mediator of the protective effects of osteoclasts on T cell viability.
Osteoclasts modulate cytokine production by γδ T cells but not CD4+ T cells
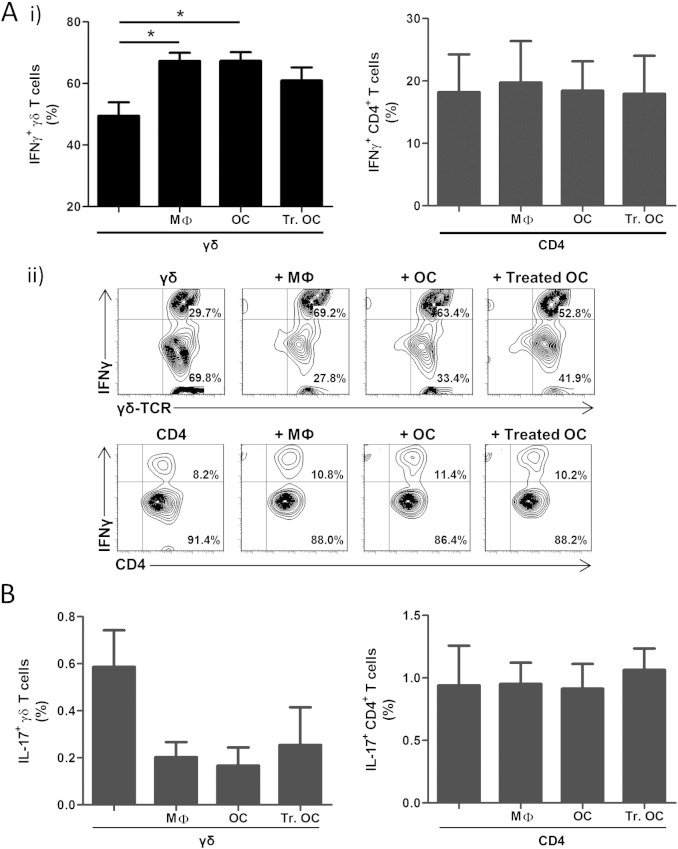
We have previously shown that anti-CD3/CD28-induced activation of purified human γδ T cells results in marked production of IFNγ, with little or no production of IL-17 [21]. We therefore determined if co-culture with macrophages or osteoclasts influenced the production of IFNγ or IL-17 by γδ T cells or CD4+ T cells.
Following co-culture with macrophages, osteoclasts or IFNγ/TNFα-treated osteoclasts, T cells were non-specifically activated with PMA and ionomycin, to stimulate intracellular cytokine production. Co-culture with macrophages or osteoclasts significantly increased the proportion of IFNγ+ γδ T cells, from 49.5 ± 11.5% in purified γδ T cell cultures to 67.3 ± 6.9%, or 67.4 ± 7.4%, with macrophages or osteoclasts, respectively (Fig. 6A). A similar, although non-significant, trend was also observed for treated osteoclasts to increase the proportion of IFNγ+ γδ T cells (61.0 ± 11.3%). The increase in IFNγ+ γδ T cells was consistently associated with a decreased proportion of IL-17+ γδ T cells, from ~ 0.6% in purified γδ T cell cultures to ~ 0.2% in co-cultures with macrophages or osteoclasts (Fig. 6B). Interestingly, there was no enhanced production of IFNγ following co-culture of γδ T cells with treated osteoclasts, suggesting that exposure of osteoclasts to pro-inflammatory cytokines (such as TNFα and IFNγ) does not enhance this stimulatory effect on IFNγ production by γδ T cells.
Fig. 6.
Macrophages and osteoclasts increase the proportion of IFNγ-producing γδ T cells. γδ T cells or CD4+ T cells were cultured alone, or in co-culture with autologous macrophages (MФ), osteoclasts (OC) or IFNγ/TNFα-treated osteoclasts (Treated OC) at a T-cell:OC ratio of 5:1, for 3 days. γδ T cells and CD4+ T cells were then harvested, activated with 50 ng/ml PMA and 1 μg/ml ionomycin in the presence of GolgiStop reagent, and assessed for intracellular IFNγ and IL-17 production using flow cytometric analysis, as detailed in Section 2. Gates for IFNγ+ and IL-17+ T cells were set using fluorescence minus one (FMO) and isotype controls. Data shown are the mean + SEM values for IFNγ-producing (A.i) or IL-17-producing (B) γδ T cells (left panels) or CD4+ T cells (right panels) from seven independent experiments from different donors for γδ T cells (n = 7; *p < 0.05) and four independent experiments from different donors for CD4+ T cells (n = 4). A.ii) Representative contour plots of IFNγ+ γδ T cells (top panel) and CD4+ T cells (bottom panel).
The modulatory effect of macrophages or osteoclasts on γδ T cell-derived cytokine production was not observed with CD4+ T cells, since the presence of macrophages and osteoclasts did not affect the proportion of CD4+ T cells producing IFNγ (~ 18 ± 6% IFNγ+), or IL-17 (~ 0.9% IL-17+) (Figs. 6A,B). Taken together, this data suggests that osteoclasts are capable of modulating γδ T cell phenotype by enhancing their Th1-like (IFNγ-producing) bias, but have little/no effect on CD4+ T cell phenotype.
Discussion
To date, numerous studies have focussed on the effects of immune cells for affecting osteoclastogenesis (for review see [25]), while the reciprocal effects of osteoclasts for affecting immune cells, particularly the function of various T cell subsets, awaits more thorough investigation.
In this study we investigated the effects of mature human osteoclasts or macrophages on the function of γδ T cells, a subset of T cells previously implicated in the pathogenesis of a variety of chronic inflammatory diseases [14,20,26,27]. Unstimulated osteoclasts were found to produce a range of chemokines capable of influencing the recruitment of a range of immune cells, and soluble factors produced by osteoclasts stimulated the chemotaxis of purified γδ T cells, thereby suggesting that osteoclasts may be capable of orchestrating immune responses in vivo. Of particular note, and consistent with a previous study [12], osteoclasts produced marked quantities of MCP-1/CCL2, which has recently been reported to be a crucial mediator of the migration of cytotoxic γδ T cells to tumour beds in a murine model of melanoma [28]. The potential recruitment of γδ T cells may also involve osteoclast-derived RANTES/CCL5, since γδ T cells express CCR5 (a receptor for RANTES), as well as CCR2 [29], which governs responsiveness to MCP-1/CCL2. Furthermore, this study reveals that osteoclasts may also influence the migration of neutrophils to sites of excessive osteoclast activity such as that observed in rheumatoid arthritis, since osteoclasts produced IL-8/CXCL8 and GROα/CXCL1, which mediate neutrophil chemotaxis and are elevated in synovial fluid of rheumatoid arthritis patients [30–32]. Taken together, these studies suggest that osteoclasts play a vital role in orchestrating immune cell migration into inflamed joints in chronic inflammatory conditions, and would contribute to the recruitment of γδ T cells into the inflamed synovium and synovial fluid of rheumatoid arthritis patients [16–19].
The exact role of γδ T cells in the synovial microenvironment of rheumatoid arthritis patients is currently debated, with murine models suggesting potentially pathogenic or protective roles for infiltrating γδ T cells, depending on the model system used and timing of antibody-mediated γδ T cell depletion [10,14,15,33]. Our findings that the co-culturing of γδ T cells or CD4+ T cells with osteoclasts can induce T cell activation suggests that the interactions of T cells with osteoclasts in vivo could induce T cell activation. However, while close proximity of CD4+ T cells with osteoclasts has been demonstrated in rheumatoid arthritis patients [10], the same study failed to identify γδ T cells associated with osteoclasts, with γδ T cells localised mainly to soft tissue structures such as synovium and tendon. Therefore, the induction of CD4+ T cell activation through interaction with osteoclasts, particularly osteoclasts exposed to a pro-inflammatory environment, may be of functional relevance in vivo, but evidence for direct interactions of γδ T cells with osteoclasts in vivo is currently lacking. Despite this, our findings suggest that osteoclasts can still influence γδ T cell function in the absence of direct cell–cell contact via the production of stimulatory mediators (such as TNFα, which is abundant in the inflamed synovium of rheumatoid arthritis patients [7,34]) in the joint microenvironment.
We also report here that osteoclasts support both γδ and CD4+ T cell survival, in accordance with a recent study [12]. This survival effect appears to rely on cell–cell contact and, although the specific mechanism remains to be elucidated, previous studies have suggested that LFA-1:ICAM-1 and CD28:CD80 interactions are important mediators of the survival effects of dendritic cells on CD4+ T cell survival [35]. In support of a role for CD28 co-stimulation in mediating the survival and proliferative effects on γδ T cells, a recent study reported that murine γδ T cells co-cultured with antigen-presenting cells showed an increased proliferation in the presence of CD28 agonists, and antibody-mediated blockade of CD28-signalling prevented γδ T cell proliferation [36]. Since CD80 and CD86 (the ligands of CD28) are expressed on osteoclasts [11], we suggest that co-stimulation of CD28 on γδ T cells and on CD4+ T cells may be the cell-contact-dependent mechanism responsible for the osteoclast-mediated support of γδ and CD4+ T cell survival and IL-2-induced γδ T cell proliferation.
Our study also reveals that co-culture with macrophages or osteoclasts induces an enhanced Th1-like bias in γδ T cells as assessed by IFNγ production, demonstrating that the observed macrophage/osteoclast-induced increase in CD69 expression has a functional outcome for γδ T cells in vitro. While the relevance of this finding requires formal verification in vivo, for example using animal model systems of erosive bone diseases or human samples, our study highlights a potentially intriguing capacity of macrophages and osteoclasts to influence γδ T cell function. This may be of particular relevance in the context of aminobisphosphonates (N-BPs), widely-used drugs to treat diseases of excessive osteoclast activity [37], since the major subset of γδ T cells in human peripheral blood, Vγ9Vδ2+ T cells, are potently activated by N-BPs [38–41]. The relatively selective internalisation of N-BPs, released from bone mineral by osteoclasts during the process of bone-resorption, and perhaps also by bone-associated macrophages [42] in near-proximity to resorbing osteoclasts, would therefore bestow osteoclasts and macrophages with an enhanced ability to activate Vγ9Vδ2+ T cells, resulting in IFNγ production and potentially Vγ9Vδ2+ T cell-mediated cytotoxic effects on osteoclasts themselves, as has recently been demonstrated in vitro [43]. While the formal demonstration of interactions between Vγ9Vδ2+ T cells and osteoclasts has yet to be demonstrated in N-BP-treated patients in vivo, such immunostimulatory effects of macrophages/osteoclasts on Vγ9Vδ2+ T cells could potentially contribute to the increased disease-free survival of early-stage breast cancer patients treated with the N-BP zoledronic acid and adjuvant endocrine therapy [44–46].
Conclusion
Our work provides further evidence for a role of osteoclasts as immunomodulatory cells, capable of affecting γδ T cell function and behaviour. This supports the notion that osteoclasts may play important roles in both the recruitment and retention of immune cells, particularly in chronic inflammatory diseases such as rheumatoid arthritis, through complex mechanisms involving the release of soluble factors and cell–cell interactions.
The following are the supplementary data related to this article.
TNFα is not a mediator of the enhanced γδ T cell survival induced by osteoclasts. γδ was cultured alone or co-cultured with autologous osteoclasts (at a T cell:OC ratio of 5:1) for 5 days, in the absence or presence of anti-human TNFα antibody or isotype control (both 10 μg/ml). Following this period, γδ T cells were harvested and cell viability assessed as detailed in Section 2. Data shown are the mean + SEM from four independent experiments from different donors (n = 4; *p< 0.05).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bone.2014.10.019.
Acknowledgments
The authors would like to acknowledge the Oliver Bird Foundation (RHE/00092/S1 24105) (A.P.) and Arthritis Research UK (18439) (K.T.) for funding this work, and to thank Dr Heather M. Wilson for the helpful comments on the manuscript.
References
- 1.Gao Y., Grassi F., Ryan M.R., Terauchi M., Page K., Yang X. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J.Y., Tawfeek H., Bedi B., Yang X., Adams J., Gao K.Y. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2011;108:768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbie-Ryan M., Pacifici R., Weitzmann M.N. IL-7 drives T cell-mediated bone loss following ovariectomy. Ann N Y Acad Sci. 2006;1068:348–351. doi: 10.1196/annals.1346.051. [DOI] [PubMed] [Google Scholar]
- 4.Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 5.Horwood N.J., Kartsogiannis V., Quinn J.M., Romas E., Martin T.J., Gillespie M.T. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 6.Horwood N.J., Udagawa N., Elliott J., Grail D., Okamura H., Kurimoto M. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest. 1998;101:595–603. doi: 10.1172/JCI1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romas E., Gillespie M.T., Martin T.J. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 8.Kong Y.Y., Feige U., Sarosi I., Bolon B., Tafuri A., Morony S. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 9.Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollinger B., Junt T., Metzler B., Walker U.A., Tyndall A., Allard C. Th17 cells, not IL-17 + gammadelta T cells, drive arthritic bone destruction in mice and humans. J Immunol. 2011;186:2602–2612. doi: 10.4049/jimmunol.1003370. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Hong S., Qian J., Zheng Y., Yang J., Yi Q. Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4 + and CD8 + T cells. Blood. 2010;116:210–217. doi: 10.1182/blood-2009-11-255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassi F., Manferdini C., Cattini L., Piacentini A., Gabusi E., Facchini A. T cell suppression by osteoclasts in vitro. J Cell Physiol. 2011;226:982–990. doi: 10.1002/jcp.22411. [DOI] [PubMed] [Google Scholar]
- 13.Kiesel J.R., Buchwald Z.S., Aurora R. Cross-presentation by osteoclasts induces FoxP3 in CD8 + T cells. J Immunol. 2009;182:5477–5487. doi: 10.4049/jimmunol.0803897. [DOI] [PubMed] [Google Scholar]
- 14.Roark C.L., French J.D., Taylor M.A., Bendele A.M., Born W.K., O'Brien R.L. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito Y., Usui T., Kobayashi S., Iguchi-Hashimoto M., Ito H., Yoshitomi H. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 16.Meliconi R., Pitzalis C., Kingsley G.H., Panayi G.S. Gamma/delta T cells and their subpopulations in blood and synovial fluid from rheumatoid arthritis and spondyloarthritis. Clin Immunol Immunopathol. 1991;59:165–172. doi: 10.1016/0090-1229(91)90090-w. [DOI] [PubMed] [Google Scholar]
- 17.Mitogawa T., Nishiya K., Ota Z. Frequency of gamma delta T cells in peripheral blood, synovial fluid, synovial membrane and lungs from patients with rheumatoid arthritis. Acta Med Okayama. 1992;46:371–379. doi: 10.18926/AMO/32664. [DOI] [PubMed] [Google Scholar]
- 18.Keystone E.C., Rittershaus C., Wood N., Snow K.M., Flatow J., Purvis J.C. Elevation of a gamma delta T cell subset in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 1991;84:78–82. [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan F.M., Londei M., Jackson A.M., Hercend T., Brenner M.B., Maini R.N. T cells expressing gamma delta chain receptors in rheumatoid arthritis. J Autoimmun. 1988;1:319–326. doi: 10.1016/0896-8411(88)90002-9. [DOI] [PubMed] [Google Scholar]
- 20.Kenna T.J., Davidson S.I., Duan R., Bradbury L.A., McFarlane J., Smith M. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive gamma/delta T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012;64:1420–1429. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 21.Pappalardo A., Thompson K. Activated gammadelta T cells inhibit osteoclast differentiation and resorptive activity in vitro. Clin Exp Immunol. 2013;174:281–291. doi: 10.1111/cei.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taub D.D., Key M.L., Clark D., Turcovski-Corrales S.M. Chemotaxis of T lymphocytes on extracellular matrix proteins. Analysis of the in vitro method to quantitate chemotaxis of human T cells. J Immunol Methods. 1995;184:187–198. doi: 10.1016/0022-1759(95)00087-q. [DOI] [PubMed] [Google Scholar]
- 23.Fotin-Mleczek M., Henkler F., Hausser A., Glauner H., Samel D., Graness A. Tumor necrosis factor receptor-associated factor (TRAF) 1 regulates CD40-induced TRAF2-mediated NF-kappaB activation. J Biol Chem. 2004;279:677–685. doi: 10.1074/jbc.M310969200. [DOI] [PubMed] [Google Scholar]
- 24.Yokota S., Geppert T.D., Lipsky P.E. Enhancement of antigen- and mitogen-induced human T lymphocyte proliferation by tumor necrosis factor-alpha. J Immunol. 1988;140:531–536. [PubMed] [Google Scholar]
- 25.Danks L., Takayanagi H. Immunology and bone. J Biochem. 2013;154:29–39. doi: 10.1093/jb/mvt049. [DOI] [PubMed] [Google Scholar]
- 26.Blink S.E., Miller S.D. The contribution of gammadelta T cells to the pathogenesis of EAE and MS. Curr Mol Med. 2009;9:15–22. doi: 10.2174/156652409787314516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton C.E., Mielke L.A., Mills K.H. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 28.Lanca T., Costa M.F., Goncalves-Sousa N., Rei M., Grosso A.R., Penido C. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic gammadelta T lymphocytes to tumor beds. J Immunol. 2013;190:6673–6680. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 29.Brandes M., Willimann K., Lang A.B., Nam K.H., Jin C., Brenner M.B. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102:3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- 30.Koch A.E., Kunkel S.L., Shah M.R., Hosaka S., Halloran M.M., Haines G.K. Growth-related gene product alpha. A chemotactic cytokine for neutrophils in rheumatoid arthritis. J Immunol. 1995;155:3660–3666. [PubMed] [Google Scholar]
- 31.Valcamonica E., Chighizola C.B., Comi D., De Lucia O., Pisoni L., Murgo A. Levels of chemerin and interleukin 8 in the synovial fluid of patients with inflammatory arthritides and osteoarthritis. Clin Exp Rheumatol. 2014;32:243–250. [PubMed] [Google Scholar]
- 32.Wright H.L., Bucknall R.C., Moots R.J., Edwards S.W. Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatology (Oxford) 2012;51:451–459. doi: 10.1093/rheumatology/ker338. [DOI] [PubMed] [Google Scholar]
- 33.Peterman G.M., Spencer C., Sperling A.I., Bluestone J.A. Role of gamma delta T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–6558. [PubMed] [Google Scholar]
- 34.Buchan G., Barrett K., Turner M., Chantry D., Maini R.N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988;73:449–455. [PMC free article] [PubMed] [Google Scholar]
- 35.Feuillet V., Lucas B., Di Santo J.P., Bismuth G., Trautmann A. Multiple survival signals are delivered by dendritic cells to naive CD4 + T cells. Eur J Immunol. 2005;35:2563–2572. doi: 10.1002/eji.200526127. [DOI] [PubMed] [Google Scholar]
- 36.Ribot J.C., Debarros A., Mancio-Silva L., Pamplona A., Silva-Santos B. B7-CD28 costimulatory signals control the survival and proliferation of murine and human gammadelta T cells via IL-2 production. J Immunol. 2012;189:1202–1208. doi: 10.4049/jimmunol.1200268. [DOI] [PubMed] [Google Scholar]
- 37.Roelofs A.J., Thompson K., Gordon S., Rogers M.J. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 38.Thompson K., Rogers M.J. Statins prevent bisphosphonate-induced gamma, delta-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- 39.Roelofs A.J., Jauhiainen M., Monkkonen H., Rogers M.J., Monkkonen J., Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunzmann V., Bauer E., Feurle J., Weissinger F., Tony H.P., Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 41.Miyagawa F., Tanaka Y., Yamashita S., Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–5514. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 42.Chang M.K., Raggatt L.J., Alexander K.A., Kuliwaba J.S., Fazzalari N.L., Schroder K. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 43.Cui Q., Shibata H., Oda A., Amou H., Nakano A., Yata K. Targeting myeloma-osteoclast interaction with Vgamma9Vdelta2 T cells. Int J Hematol. 2011;94:63–70. doi: 10.1007/s12185-011-0885-9. [DOI] [PubMed] [Google Scholar]
- 44.Coleman R., de Boer R., Eidtmann H., Llombart A., Davidson N., Neven P. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol. 2013;24:398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 45.Eidtmann H., de Boer R., Bundred N., Llombart-Cussac A., Davidson N., Neven P. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST study. Ann Oncol. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 46.Gnant M., Mlineritsch B., Schippinger W., Luschin-Ebengreuth G., Postlberger S., Menzel C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TNFα is not a mediator of the enhanced γδ T cell survival induced by osteoclasts. γδ was cultured alone or co-cultured with autologous osteoclasts (at a T cell:OC ratio of 5:1) for 5 days, in the absence or presence of anti-human TNFα antibody or isotype control (both 10 μg/ml). Following this period, γδ T cells were harvested and cell viability assessed as detailed in Section 2. Data shown are the mean + SEM from four independent experiments from different donors (n = 4; *p< 0.05).