Abstract
Tea flavonoids and polyphenols are well known for their extraordinary antioxidant activity which is considered important for anti-aging processes in animals. This study evaluated the anti-wrinkle effects of three different kinds of tea (Camellia sinensis) water extracts (CSWEs) including green, white, and black teas using a photoaged hairless mouse model. Data showed that the CSWE-treatment greatly improved skin conditions of mice suffering from UVB-induced photoaging, based on the parameters including the skin erythema index, moisture capacity, and transepidermal water loss. In addition, the wrinkle measurement and image analysis of skin replicas indicated that CSWEs remarkably inhibited wrinkle formation. In histological examination, the CSWE-treated mice exhibited diminished epidermal thickness and increased collagen and elastic fiber content, key signatures for skin restoration. Furthermore, the reduced expression of MMP-3, a collagen-degradative enzyme, was observed in the skin of CSWE-treated animals. Interestingly, comparative data between green, white, and black tea indicated that the anti-wrinkle activity of white tea and black tea is equally greater than that of green tea. Taken together, these data clearly demonstrated that CSWEs could be used as an effective anti-wrinkle agent in photoaged animal skin, implying their extended uses in therapeutics.
Keywords: Aging, Antioxidant, Camellia sinensis, MMP-3, Polyphenols
INTRODUCTION
Ultraviolet (UV) irradiation results in diverse clinical skin changes such as wrinkling, sunburn, immune-suppression, cancer, and premature skin aging (photoaging) (1). UV exposure to skin induces extensive generation of reactive oxygen species (ROS). In vivo, ROS partly play a positive role such as energy production, phagocytosis, regulation of cell growth, and intracellular signaling. On the other hand, ROS can react with DNA, proteins, fatty acids, and saccharides causing oxidative damage. Such injuries result in a number of harmful effects: disturbed cell metabolism, morphological and ultrastructural changes, attack on the regulation pathways and alterations in the differentiation, proliferation and apoptosis of skin cells (2). Photodamaged skin is characterized by epidermal hyperplasia and altered biomechanical properties of the dermis, ultimately leading to wrinkle formation. Qualitative and/or quantitative alterations of the dermal extracellular matrix components involved in the photoagaing process are represented by accumulation of elastotic material and immunohistochemical changes in collagen, the main macro-molecular component of the skin (3). Acute exposure of human skin to UV irradiation in vivo up-regulates synthesis of several matrix metalloproteinases (MMPs) including MMP-1 (interstitial collagenase), MMP-3 (stromelysin-1), and MMP-9 (92-kd gelatinase B), all of which are involved in degradation of skin collagen (4).
Retinoids are known to induce the proliferation of keratinocytes and fibroblasts, promote collagen synthesis, and decrease the MMPs expression level in the dermis (5). Plant extracts have been widely used as topical applications for wound-healing, anti-aging, and disease treatments (6). In particular, the antioxidant activity of herbal phenolics, namely phenolic acids and flavonoids, has been given much attention. Tea leaves (Camelia sinensis L.) are a rich source for polyphenols, natural compounds consisting of flavonoids, mainly catechins [(−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin-3-gallte (ECG), (−)-epigallocatechin (EGC), (−)-epicatechin (EC)], which undergo oxidation to form theaflavins and thearubigens in the manufacturing of black tea (7,8). The epicatechin derivatives, which are commonly called polyphenols, are the active ingredients in green tea and possess antioxidant, anti-inflammatory and anti-carcinogenic properties (9). EGCG has been shown to suppress skin cancer caused by ultraviolet irradiation or carcinogens (10) and reduce the expression of extracellular matrix degradation (11). Polyphenol in green tea has a skin anti-aging effect through stimulating the proliferation of keratinocyte (12). White tea manufactured by a minimal process is also known to have a skin photo-protective effect (13). Black tea reduces the number of sunburn cells caused by ultraviolet irradiation (14), and shows skin cancer suppression, anti-inflammatory (15), anti-oxidation and antimicrobial activity (16). Despite the well-established health benefits, the effects of white and black teas on skin aging have not been extensively investigated so far.
In this study, the anti-wrinkle effects of three different tea (Camellia sinensis) water extracts (CSWEs) including green, white, and black teas were evaluated based on physiological and histological observation and MMP expression analysis using a photoaged hairless mouse model.
MATERIALS AND METHODS
Preparation of tea extracts. Green, white, and black teas were obtained from an oriental medicinal herb market, Daegu, Korea. Six-hundred gram of green tea, white tea and black tea each suspended in 6 L distilled water was boiled for 2 hr in a heating extractor (COSMOS-660, Kyungseo Machine Co., Korea) and concentrated. The aqueous extracts were then lyophilized into powder. These specimens were dissolved to 2% concentrations in the vehicle [propylene glycol : ethanol : water (5 : 3 : 2)] for the downstream experiments.
Experimental animal. Seven-weeks-old female SKH-1 hairless mice were purchased from Charles River (Japan). The animals were acclimatized for 1 wk before use and maintained throughout at standard conditions: 22 ± 1℃, 50 ± 5% relative humidity, and 12/12 hr light/dark cycle. Animals were allowed free access to water and diet. The mice were divided into six groups seven mice each, which consisted of no UV irradiation group as the normal group (N), UV irradiation group as the control group (C), UV irradiation and 0.01% retinoic acid treatment group as the positive control group (RA), and UV irradiation and 2% green tea (GT), 2% white tea (WT), or 2% black tea (BT) treatment group. The skin was extracted, a part of the skin was fixed with 10% neutral buffered formalin solution for histological analysis, and the rest of the skin was stored in deep freezer (−80℃) for MMP-3 analysis. Both animal care and the protocol for this study were in accordance with IACUC (Institutional Animal Care and Use Committee) and OECD guidelines.
UV irradiation. The mice were irradiated dorsally using an UVB radiation (302 nm sunlamp) for 12 wks, three times a week. The irradiation dose was one MED (minimal erythemal dose: 60 mJ/cm2) in the 1st wk, two MED (120 mJ/cm2) in the 2nd wk, three MED (180 mJ/cm2) in the 3rd wk, and four MED (240 mJ/cm2) between the 4th and 12th wk. Four MED irradiation was applied once a week during the period of sample application.
Application of the test compounds. After wrinkle induction using an UVB irradiation for 12 wks, saline and CSWEs were applied 200 μl each time, for four weeks, five times a wk (2%: 0.26 g/kg BW/day). RA was diluted to 0.01% with polyethylene glycol.
Measurement of skin conditions. Skin moisture capacity, erythema index, and transepidermal water loss (TEWL) were measured using Corneometer® CM825, Mexameter® MX18, and Tewameter® TM300 (Courage & Khazaka, Köln, Germany), respectively.
Measurement of skin wrinkle. At the end of the experiment, skin replicas were made from the back skin of the hairless mice using a SILFLO impression material (FLEXICO, England). The Visioline® VL650 (Courage & Khazaka, Koln, Germany) was used to assess the skin surface. Total wrinkle area (mm2) was calculated.
Histological observation. After fixation with 10% neutral buffered formalin solution for 24 hr at room temperature, the extracted skin tissue was subjected to the processes of washing, dehydration, clearing, and infiltration, and was embedded with paraffin to be sliced into a section of 4 μm in thickness. After staining the section with H&E, Masson’s trichrome, Verhoeff's and Toluidine, the patterns of changes in skin tissue were observed under the optical microscope.
Reverse transcription-polymerase chain reaction (RTPCR). The total RNA was extracted from the dorsal skin samples by homogenization with 400 μl of lysis/binding buffer per 50-mg samples using a high pure RNA tissue kit (Roche, Germany) per the manufacturer instructions. The purity was checked by measuring the optical density (OD) at 260 nm and 280 nm with a spectrometer to confirm the A260/A280 ratio was between 1.8 and 2.0. The total RNA (1 μg/μl) was used to synthesize cDNA utilizing a Cycle Script RT PreMix (dT20) kit with the PCR-cycling parameters of 95℃ for 5 min followed by 12 cycles of 30℃ for 1 min, and 50℃ for 4 min. MMP-3 was amplified using an AccupowerTM PCR PreMix kit with 2 μl template, 15.2 μl sterile water, and 1.4 μl of 100 pmol/μl each of gene-specific primers (mouse MMP-3 forward 5'-TAGCAGGTTATCCTAAAAGCA-3', reverse 5'-CCAGCTATTGCTCTTCAAT- 3'; GAPDH forward 5'-CCCACTAACATCAAATGGGG- 3', reverse 5'-ACACATTGGGGGTAGGAACA-3') with the following PCR conditions: 35 cycles of 94℃ for 45 s, 60℃ for 45 s, and 72℃ for 60 s. The amplification products were run on a 1.5% agarose gel and visualized with ethidium bromide staining. The DNA band densities were evaluated with the KODAK Gel Logic 100 image analysis system.
Statistical analysis. Differences between the groups were evaluated statistically using one-way analysis of variance (ANOVA) followed by the Duncan's multiple range test as a post hoc comparison using SPSS WIN (v20.0). Statistical significance was set at p < 0.05, p < 0.01 and p < 0.001.
RESULTS
Skin conditions. The skin moisture capacity of all the treatment groups (i.e., RA, GT, WT, and BT) was significantly higher compared to the control group (C) throughout the experiment period (Table 1). At wk 4, the moisture capacity of RA, GT, WT, and BT groups was 207%, 196%, 233%, and 219%, respectively, significantly (p < 0.001) higher than that of C group. While the skin erythema index increases over time in C group which didn’t receive any treatment, it gradually decreased in the 4 treatment groups. At wk 4, the skin erythema index of RA, GT, WT, and BT groups was 32%, 41%, 46%, and 42%, respectively, significantly (p < 0.001) lower than that of C group. Overall, the skin TEWL of all the treatment groups was at the basal levels similar to N group in contrast to C group that displayed a significant increase of TEWL over time. At wk 4, the skin TEWL of RA, GT, WT, and BT groups was 58%, 82%, 87%, and 86%, respectively, significantly (p < 0.001) lower than that of C group.
Table 1. Changes in moisture capacity, erythema index and transepidermal water loss (TEWL) of SKH-1 hairless mice.
| Index | Week | N | C | RA 0.01% | GT 2% | WT 2% | BT 2% |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Moisture capacity | 1 | 60.48 ± 9.53b | 39.86 ± 1.59a | 57.99 ± 7.31b | 57.04 ± 10.01b | 64.37 ± 2.25b | 64.78 ± 2.39b |
| 2 | 51.46 ± 9.66b | 31.02 ± 1.75a | 65.70 ± 5.75c | 69.09 ± 9.84c | 76.16 ± 2.66c | 75.82 ± 1.31c | |
| 3 | 36.31 ± 7.25b | 18.52 ± 3.09a | 71.42 ± 5.82cd | 69.61 ± 4.22c | 78.79 ± 1.71de | 80.90 ± 0.81e | |
| 4 | 46.06 ± 5.95b | 28.90 ± 2.10a | 88.59 ± 4.87cd | 85.67 ± 5038c | 95.89 ± 2.42de | 98.55 ± 5.98e | |
| Erythema index | 1 | 240.67 ± 30.67a | 346.9 ± 14.48d | 314.38 ± 17.33c | 279.76 ± 11.72b | 274.17 ± 5.87b | 275.86 ± 5.64b |
| 2 | 246.33 ± 23.30a | 357.52 ± 14.46c | 301.10 ± 16.60b | 267.81 ± 12.00a | 255.24 ± 8.46a | 274.10 ± 11.51ab | |
| 3 | 261.71 ± 21.60a | 377.43 ± 13.36c | 294.43 ± 13.14b | 260.93 ± 11.09a | 236.75 ± 12.71a | 261.31 ± 9.58a | |
| 4 | 285.50 ± 34.20b | 413.43 ± 12.21c | 282.64 ± 15.50b | 245.50 ± 12.07a | 223.64 ± 4.24a | 238.86 ± 8.52a | |
| TEWL | 1 | 8.29 ± 2.07a | 19.16 ± 2.92bc | 24.16 ± 4.49c** | 14.67 ± 3.01b** | 14.21 ± 2.03b** | 13.81 ± 1.68b** |
| 2 | 11.08 ± 2.37a | 22.86 ± 2.71b | 18.33 ± 4.85b** | 11.66 ± 2.24a | 10.79 ± 5.47a | 9.84 ± 1.53a | |
| 3 | 11.90 ± 1.26b | 24.79 ± 1.51d | 18.76 ± 3.66c | 9.86 ± 1.83ab | 8.76 ± 1.45ab | 7.74 ± 1.20a | |
| 4 | 15.77 ± 3.06b | 40.27 ± 2.24c | 16.90 ± 3.59b | 7.39 ± 0.85a | 5.34 ± 1.50a | 5.53 ± 0.66a | |
Unit: Moisture capacity and erythema index (arbitrary unit), TEWL (g/m2/h). N: saline-treated normal group, C: UVB-irradiated control group, RA 0.01%: 0.01% retinoic acid-treated group, GT 2%: 2% green tea-treated group, WT 2%: 2% white tea-treated group, BT 2%: 2% black teatreated group. Values represent the mean ± SD of 7 mice. a,b,c,d,eValues with different superscripts in the same raw are significantly different (p< 0.001) by ANOVA and Duncan’s multiple range test. **p < 0.01 significantly different from the control group.
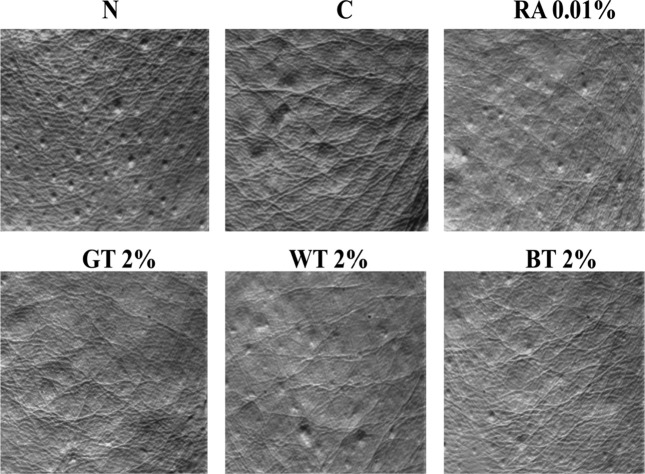
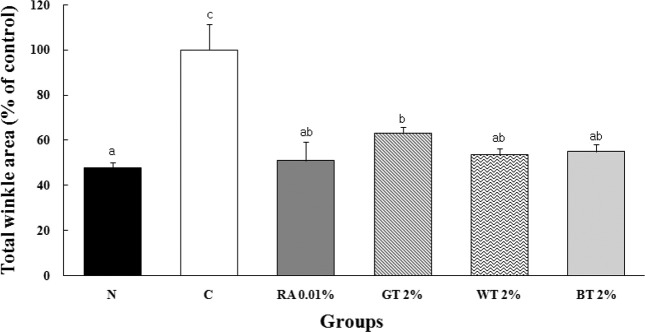
Replica image analysis. There was no wrinkle formation in N group, whereas varying degrees of fine wrinkles were observed on the replica images of the control and treatment groups (Fig. 1). In UV-irradiated animals, there was a tendency toward decrease of wrinkle formation in the treatment groups over time, with the least amount of visible wrinkle at wk 4. As compared with C group, RA and CSWE groups (GT, WT, and BT) greatly improved wrinkle conditions (i.e., shallow furrows and thin and narrow crests). The C group showed significantly (p < 0.001) wider wrinkle area than N group by 110%, and RA, GT, WT, and BT groups showed significantly (p < 0.001) narrower area of wrinkle than C group by 49%, 37%, 46%, and 45%, respectively (Fig. 2).
Fig. 1. Comparison in replica images of SKH-1 hairless mice skin applied with test compounds for 4 wks. N: saline-treated normal group, C: UVB-irradiated control group, RA 0.01%: 0.01% retinoic acid-treated group, GT 2%: 2% green tea-treated group, WT 2%: 2% white tea-treated group, BT 2%: 2% black teatreated group. As compared with C group, RA and CSWE groups reduced wrinkle formation in a pattern of shallow furrows and thin and narrow crests.
Fig. 2. Comparison of wrinkle area of SKH-1 hairless mice skin applied with test compounds for 4 wks. Values represent the mean ± SD of 7 mice. a,b,cValues with different superscripts are significantly different (p < 0.001) by ANOVA and Duncan’s multiple range test. A significant decrease in wrinkle area was observed in RA and CSWE groups.
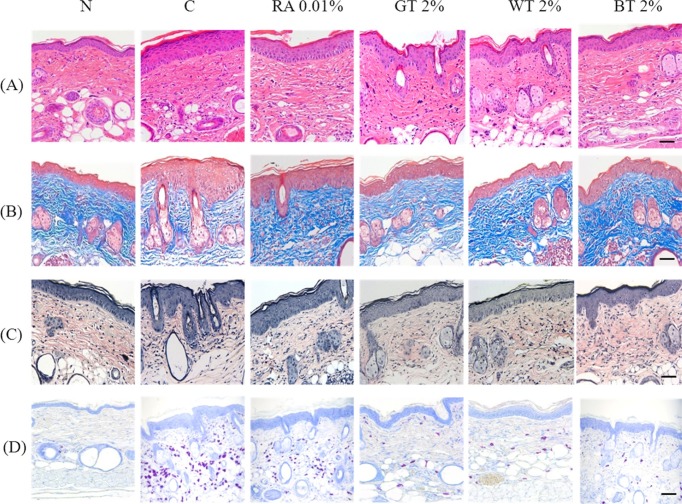
Histological observation. Microscopic examination was performed in the skin. The epidermis and dermis of C group were remarkably thickened by UV exposure and a moderate number of inflammatory cells including lymphocytes, neutrophils, and macrophages were infiltrated in the dermis. Inflammatory cells were rarely found in the dermis of RA and CSWE groups. The thick epidermal layer found in the C group is shown in Fig. 3A. Collagen fibers in N group displayed highly dense regular arrangement while they were severely broken and reduced in the structural density in the dermis of C group. As expected, collagen fibers in the dermis of RA and CSWE groups were almost intact with a regular arrangement (Fig. 3B). Similarly, elas-tic fibers in N group had a regular arrangement but an elastosis with denatured and tangled elastic fibers was observed in C group. Both the RA and CSWE groups featured less denaturalization of elastic fibers was observed compared to the C group (Fig. 3C). A large number of mast cells and prominent degranulation were found in the C group. In contrast, relatively less number of mast cells were found in the RA and CSWE groups and the degree of degranulation was negligible (Fig. 3D).
Fig. 3. Histological observation on SKH-1 hairless mice skin applied with test compounds for 4 wks. (A) H&E stain, ×200, (B) Masson’s trichrome stain, ×200, (C) Verhoeff’s stain, ×200, (D) Toluidine blue stain, ×200 magnification. N: saline-treated normal group, C: UVBirradiated control group, RA 0.01%: 0.01% retinoic acid-treated group, GT 2%: 2% green tea-treated group, WT 2%: 2% white teatreated group, BT 2%: 2% black tea-treated group. Scale bar 100 μm. The epidermis and dermis of control group were remarkably thickened in comparison with normal group. A moderate large numbers of neutrophil and lymphocyte were infiltrated in the dermis of control group (A), and collagen fibers (B) and elastic fibers (C) in the dermis were arranged irregularly in control groups. A large number of mast cells were found in the dermis and hypodermis of control group and their degranulation was severe, whereas a few numbers of mast cells were observed in the dermis of RA and CSWE groups (D).
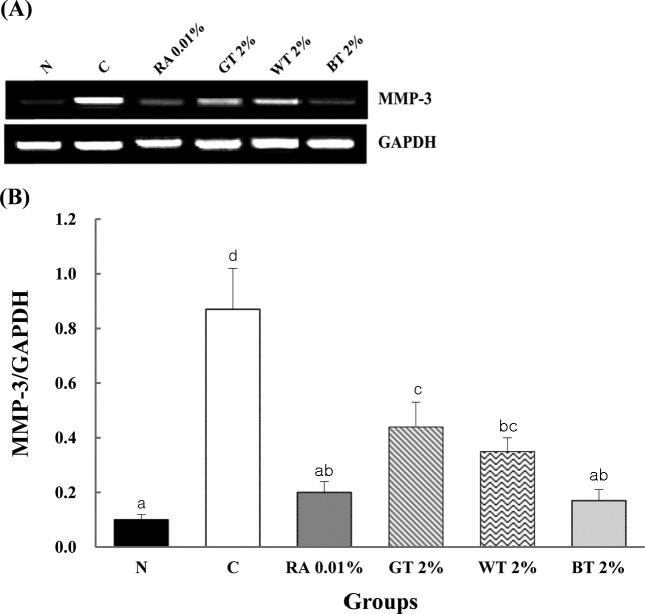
Reverse transcription polymerase chain reaction (RTPCR). An overall decrease in the expression of MMP-3 mRNA was observed in both RA and CSWE groups compared to the C group. At wk 4, MMP-3 mRNA expression was significantly (p < 0.001) decreased by 77%, 49%, 60%, and 80%, respectively in RA, GT, WT, and BT groups compared to the C group (Fig. 4).
Fig. 4. Comparison in MMP-3 mRNA expression of SKH-1 hairless mice applied with test compounds for 4 wks. (A) MMP-3 transcript levels decreased by treatment with RA and CSWEs compared to UVB-irradiated control group, as determined by RT-PCR. Expression was normalized to GAPDH levels. (B) Quantification of MMP-3 transcript expression in mice treated with saline (N), UVB radiation (C), 0.01% retinoic acid (RA 0.01%), 2% green tea (GT 2%), 2% white tea (WT 2%), or 2% black tea (BT 2%). Values represent the mean ± SD of 5 mice. a,b,c,dValues with different superscripts are significantly different (p < 0.001) by ANOVA and Duncan’s multiple range test. A significant decrease in MMP-3 mRNA expression was observed in RA and CSWE groups compared to UVB-irradiated control group.
DISCUSSION
In this study, we found that CSWEs can effectively reduce skin damage and promote anti-wrinkling processes in UVirradiated hairless mouse model, which is associated with increased moisture capacity, decrease of erythema index, decreased TEWL, replica image analysis, histological examination, and MMP-3 expression. The health benefits of tea have been mainly attributed to the relatively high levels of flavonoids, including catechins and other polyphenols (17). Tea polyphenols act as antioxidants in vitro by scavenging reactive oxygen and nitrogen species and chelating redox-active transition metal ions (18). Given the high antioxidant activity of tea, we sought to investigate the antiwrinkle effect of CSWEs through rigorous tests using an animal model. In an observation of skin wrinkle, RA and CSWE groups reduced wrinkle formation in a pattern of shallow furrows and thin and narrow crests compared with C group (Fig. 1). The RA and CSWE groups showed significantly (p < 0.001) lower wrinkle areas compared with C group, and the reduction rate was in the order of RA >WT > BT > GT (Fig. 2). In particular, BT and WT showed similar values to the RA group, indicating that the CSWE has the efficacy of reducing wrinkle formation on the photoaged skin. Kim et al. (19) reported that treatment of EGCG, a major polyphenolic constituent of green tea, had shown to reduce UVA-induced skin damage (roughness and sagginess) and prevent the decrease of dermal collagen in hairless mouse skin, and also block the UV-induced increase of collagen degradation and collagenase mRNA level in fibroblast culture.
According to the histological observation with Masson’s trichrome and Verhoeff's stain, collagen fibers in the dermis were reduced and arranged irregularly in the C group, while they were almost intact with a regular arrangement both in the RA and CSWE groups. In addition, an elastosis was observed moderately in the C group. However, it was almost disappeared both in the RA and CSWE groups (Fig. 3). Thus, these data support that the damage on the skin tissue reduced by treatment with CSWE. Kligman (20) reported an observation of reduction in collagen and modified elastosis by UV irradiation to mouse skin. Imokawa (21) also observed the improvement in wrinkles and elasticity by RA in mouse skin.
Photoaging is characterized by degradation of collagen and accumulation of abnormal elastin in the superficial dermis; several MMPs have been implicated in this process (22-24). Latent MMP-1, -7, and -9 derived from keratinocytes or dermal fibroblasts could be maximally activated by MMP-3, and ultimately initiate degradation of collagen types I and III. Simultaneous expression of MMP-2, -3, -7, and -9 could lead to degradation of non-collagenous extracellular matrix, including the basement membrane and proteoglycans (25). In this study, at wk 4, compared to the C group, the MMP-3 mRNA expression was significantly (p < 0.001) reduced in the order of BT > RA >WT > GT (Fig. 4). In particular, BT group showed similar value to the RA group. The RA and CSWE groups also showed remarkably lower MMP-2 and MMP-9 activities than that of the C group (data not shown). Vayalil et al. (22) reported that topical application of EGCG had been shown to reduce UVinduced production of MMP-2, -3, -7, and -9, which are known to degrade collagen and lead to photodamage.
In this study, the degree of erythema was measured to examine the inflammatory reaction. The skin erythema indices of all the groups were significantly (p < 0.001) lower compared to the C group during the whole period of experiment. At wk 4, compared to the C group, the skin erythema indices were significantly (p < 0.001) reduced in the order of WT > BT > GT > RA (Table 1). Elamets et al. (26) reported that the EGCG and ECG polyphenolic fractions were most efficient at inhibiting erythema, whereas EGC and EC had little effect. In an observation of H&E stain, a moderate number of inflammatory cells including macrophages, neutrophils, and lymphocytes were infiltrated in the thick dermis of the C group, whereas only a few inflammatory cells were found in the dermis of RA and CSWE groups. Dona et al. (27) and Katiyar et al. (28) reported that the topical application of EGCG before UVB exposure reduced the number of CD11b+ monocytes/macrophages and neutrophils that infiltrated in the exposed skin. In an observation of toluidine blue stain, the number and degree of degranulation of mast cells were reduced in the RA and CSWE groups, compared with C group. Thus, it is affirmed that the inflammation on the skin tissue reduced by treatment with CSWEs.
Human skin acts as a barrier between the internal and the external environment, protecting the body from mechanical damage, noxious substances, penetration by pathogens and radiation. The skin also plays a vital role in regulating body homeostasis by reducing TEWL to a minimum via the stratum corneum (SC). The TEWL is used for assessing epidermal barrier function. Therefore, in the current study, TEWL was used as one of the parameters for evaluating the antiaging properties. The skin moisture capacity of all the groups was significantly (p < 0.001) higher compared to the C group throughout the experiment period. At wk 4, compared to the C group, the skin moisture capacity was significantly (p < 0.001) increased in the order of BT >WT > RA > GT (Table 1). At wk 4, compared to the C group, the skin TEWLs were significantly (p < 0.001) reduced in the order of WT > BT > GT > RA. It was found that the damage to the skin barrier in the CSWE groups decreased, because the skin moisture capacity was significantly higher and the TEWL was significantly lower, compared to the C group. Green tea polyphenols have been reported to have many effects on cellular and molecular responses in the epidermis, but more important effect of this general class of ingredients is on TEWL. For instance, polyphenols, such as catechins when ingested in beverages, have been shown to improve SC barrier function (29), although at much higher concentrations compared with the current skin topical application study. Puch et al. (30) demonstrated enhancement of the skin barrier function in human volunteers after consumption of green tea containing milk.
All together, the findings of this study indicated that CSWEs derived from green, white and black teas were effective in the wrinkle improvement by reducing dermal extracellular matrix damage, and alleviating inflammation and skin barrier damage in an in vivo hairless mouse photoaged model. These anti-wrinkle activities are likely attributable to the high levels of EGCG and polyphenols in green tea and white tea, and to theaflavins and thearubigins formed during the oxidation of black tea. In particular, the water extracts from white and black teas had a better effect than green tea on the wrinkle improvement. Interestingly, despite differences in the processing between white tea and black tea, they did not significantly differ each other in the ability to improve photoaged skin. In conclusion, this study suggests a high possibility of the practical use of white and black teas as anti-wrinkle agents.
References
- 1.Fisher G.J., Wang Z.Q., Datta S.C., Varani J., Kang S., Voorhees J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. (1997);337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 2.Zhaorigetu S., Yanaka N., Sasaki M., Watanabe H., Kato N. Inhibitory effects of silk protein, sericin on UVB-induced acute damage and tumor promotion by reducing oxidative stress in the skin of hairless mouse. J. Photochem. Photobiol. B. (2003);71:11–17. doi: 10.1016/S1011-1344(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 3.Chaquour B., Seité S., Coutant K., Fourtanier A., Borel J.P., Bellon G. Chronic UVB and all trans retinoic acid induced qualitative and quantitative changes in hairless mouse skin. J. Photochem. Photobiol B. (1995);28:125–135. doi: 10.1016/1011-1344(94)07080-8. [DOI] [PubMed] [Google Scholar]
- 4.Honda A., Abe R., Makino T., Norisugi O., Fujita Y., Watanabe H., Nishihira J., Iwakura Y., Yamagishi S., Shimizu H., Shimizu T. Interleukin-1beta and macrophage migration inhibitory factor (MIF) in dermal fibroblasts mediate UVA-induced matrix metalloproteinase-1 expression. J. Dermatol. Sci. (2008);49:63–72. doi: 10.1016/j.jdermsci.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Varani J., Gendimenico G.J., Shah B., Gibbs D., Capetola R.J., Mezick J.A., Voorhees J.J. A direct comparison of pharmacologic effects of retinoids on skin cells in vitro and in vivo. Skin Pharmacol. Physiol. (1991);4:254–261. doi: 10.1159/000210959. [DOI] [PubMed] [Google Scholar]
- 6.Hsu S. Green tea and the skin. J. Am. Acad. Dermatol. (2005);52:1049–1059. doi: 10.1016/j.jaad.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Yen G.C., Chen H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. (1995);43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- 8.Ho C.T., Chen Q., Shi H., Zhang K.Q., Rosen R.T. Antioxidative effect of polyphenol extract prepared from various Chinese teas. Prev. Med. (1992);21:520–525. doi: 10.1016/0091-7435(92)90059-Q. [DOI] [PubMed] [Google Scholar]
- 9.Katiyar S.K., Afaq F., Perez A., Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. (2001);22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z.Y., Agarwal R., Bickers D.R., Mukhtar H. Protection against ultraviolet B radiation-induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis. (1991 );12:1527–1530. doi: 10.1093/carcin/12.8.1527. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.H., Chung J.H., Cho K.H. The effects of epigallocatechin-3-gallate on extracellular matrix metabolism. J. Dermatol. Sci. (2005);40:195–204. doi: 10.1016/j.jdermsci.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Hsu S., Bollag W.B., Lewis J., Huang Q., Singh B., Sharawy M., Yamamoto T., Schuster G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J. Pharmacol. Exp.Ther. (2003);306:29–34. doi: 10.1124/jpet.103.049734. [DOI] [PubMed] [Google Scholar]
- 13.Camouse M.M., Domingo D.S., Swain F.R., Conrad E.P., Matsui M.S., Maes D., Declercq L., Cooper K.D., Stevens S.R., Baron E.D. Topical application of green and white tea extracts provides protection from solar-simulated ultraviolet light in human skin. Exp. Dermatol. (2009);18:522–526. doi: 10.1111/j.1600-0625.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 14.Record I.R., Dreosti I.E. Protection by black tea and green tea against UVB and UVA + B induced skin cancer in hairless mice. Mutat. Res. Fundam. Mol. Mech. Mutagen. (1998);422:191–199. doi: 10.1016/S0027-5107(98)00192-4. [DOI] [PubMed] [Google Scholar]
- 15.Ratnasooriya W.D., Fernancho T.S.P. Anti-inflammatory activity of Sri Lankan black tea (Camellia sinensis L.) in rats. Pharmacogn. Res. (2009);1:11–20. [Google Scholar]
- 16.Bancirova M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. (2010);43:1379–1382. doi: 10.1016/j.foodres.2010.04.020. [DOI] [Google Scholar]
- 17.Shahidi F. Antioxidants in food and food antioxidants. Nahrung. (2000);44:158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Frei B., Higdon J.V. Antioxidant activity of tea polyphenols in vivo: evidence from animal. J. Nutr. (2003);133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 19.Kim J., Hwang J.S., Cho Y.K., Han Y., Jeon Y.J., Yang K.H. Protective effects of (−)-epigallocatechin-3-gallate on UVA- and UVB-induced skin damage. Skin Pharmacol. Appl. Skin Physiol. (2001);14:11–19. doi: 10.1159/000056329. [DOI] [PubMed] [Google Scholar]
- 20.Kligman L.H. The hairless mouse model for photoaging. Clin. Dermatol. (1996);14:183–195. doi: 10.1016/0738-081X(95)00154-8. [DOI] [PubMed] [Google Scholar]
- 21.Imokawa G. Recent advances in characterizing biological mechanisms underlying UV-induced wrinkles: a pivotal role of fibrobrast-derived elastase. Arch. Dermatol. Res. (2008);300(Suppl 1):S7–S20. doi: 10.1007/s00403-007-0798-x. [DOI] [PubMed] [Google Scholar]
- 22.Vayalil P.K., Mittal A., Hara Y., Elmets C.A., Katiyar S.K. Green tea polyphenols prevent ultraviolet lightinduced oxidative damage and matrix metalloproteinases expression in mouse skin. J. Invest. Dermatol. (2004);122:1480–1487. doi: 10.1111/j.0022-202X.2004.22622.x. [DOI] [PubMed] [Google Scholar]
- 23.Inomata S., Matsunaga Y., Amano S., Takada K., Kobayashi K., Tsunenaga M., Nishiyama T., Kohno Y., Fukuda M. Possible involvement of gelastinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Invest. Dermatol. (2003);120:128–134. doi: 10.1046/j.1523-1747.2003.12021.x. [DOI] [PubMed] [Google Scholar]
- 24.Fisher G.J., Kang S., Varani J., Bata-Csorgo Z., Wan Y., Datta S., Voorhees J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. (2002);138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 25.Fisher G.J., Voorhees J.J. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J. Invest. Dermatol. Symp. Proc. (1998);3:61–68. [PubMed] [Google Scholar]
- 26.Elamets C.A., Singh D., Tubesing K., Matsui M., Katiyar S., Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. (2001);44:425–432. doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- 27.Donà M., Dell’Aica I., Calabrese F., Benelli R., Morini M., Albini A., Garbisa S. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. (2003);170:4335–4341. doi: 10.4049/jimmunol.170.8.4335. [DOI] [PubMed] [Google Scholar]
- 28.Katiyar S.K., Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukocyte Biol. (2001);69:719–726. [PubMed] [Google Scholar]
- 29.Heinrich U., Neukam K., Tronnier H., Sies H., Stahl W. Long-term ingestion of high flavanol cocoa provides photoprotection against UV-induced erythema and improves skin condition in women. J. Nutr. (2006);136:1565–1569. doi: 10.1093/jn/136.6.1565. [DOI] [PubMed] [Google Scholar]
- 30.Puch F., Samson-Villeger S., Guyonnet D., Blachon J.L., Rawlings A.V., Lassel T. Consumption of functional fermented milk containing borage oil, green tea and vitamin E enhances skin barrier function. Exp. Dermatol. (2008);17:668–674. doi: 10.1111/j.1600-0625.2007.00688.x. [DOI] [PubMed] [Google Scholar]