Abstract
Peppermint (Mentha piperita) is a plant native to Europe and has been widely used as a carminative and gastric stimulant worldwide. This plant also has been used in cosmetic formulations as a fragrance component and skin conditioning agent. This study investigated the effect of peppermint oil on hair growth in C57BL/6 mice. The animals were randomized into 4 groups based on different topical applications: saline (SA), jojoba oil (JO), 3% minoxidil (MXD), and 3% peppermint oil (PEO). The hair growth effects of the 4-week topical applications were evaluated in terms of hair growth, histological analysis, enzymatic activity of alkaline phosphatase (ALP), and gene expression of insulin-like growth factor-1 (IGF-1), known bio-markers for the enhanced hair growth. Of the 4 experimental groups, PEO group showed the most prominent hair growth effects; a significant increase in dermal thickness, follicle number, and follicle depth. ALP activity and IGF-1 expression also significantly increased in PEO group. Body weight gain and food efficiency were not significantly different between groups. These results suggest that PEO induces a rapid anagen stage and could be used for a practical agent for hair growth without change of body weight gain and food efficiency.
Keywords: Alkaline phosphatase, Hair growth, Hair follicle, Insulin-like growth factor-1, Peppermint oil
INTRODUCTION
Hair loss is a distressing condition that is associated with a multitude of natural, medical, or nutritional conditions. For example, androgenetic alopecia in men, or male pattern baldness, is increasingly recognized as a physically and psychologically serious medical condition that often requires a professional care by generalist clinicians (1).
The only products sanctioned by the US FDA for hair loss treatment are oral finasteride (Proscar®) and topical minoxidil (Rogaine®). Minoxidil was originally created as a hypertension medication by Upjohn Pharmaceuticals (2). Upjohn itself has warned of possible negative side effects of the medication including increased heart rate, difficulty breathing, rapid weight gain, edema, seborrhoeic dermatitis, scalp itching, and scaling (3-5).
Traditional plant remedies have been used for centuries in the treatment for hair loss, but only a few have been scientifically evaluated (5). Peppermint (Mentha piperita) extracted from peppermint leaves is generally regarded as an excellent carminative and gastric stimulant, and also has been used in cosmetic formulations as a fragrance component and a general skin conditioning agent. The principal ingredient of peppermint oil, menthol, is primarily responsible for its beneficial effects (6). In vitro, peppermint has been reported to show anti-inflammatory, antimicrobial, and antifungal activities as well as strong antioxidant activity, and antiallergenic and antitumor actions (7,8). Several clinical trials examining the effects of peppermint oil (PEO) on irritable bowel syndrome have been reported (9). However, experimental trial of PEO in its hair growth activity has not been fully reported. The aim of this study was to address the therapeutic potential of PEO for hair loss via the comparative analysis between PEO and minoxidil.
MATERIALS AND METHODS
Materials. This study used peppermint oil (Sanoflore®, France) certified as 100% pure and natural essential oil by an organic product certification organization (ECOCERT-F- 32600) and jojoba oil (Desert Whale, USA). The chemical compositions of peppermint oil and jojoba oil used are listed in Table 1. The 3% minoxidil was obtained from Hyundai Pharmacia (Korea).
Table 1. Composition of peppermint oil and jojoba oil.
| Peppermint oil | % | Jojoba oil | % |
|---|---|---|---|
|
| |||
| Menthol | 42.34 | Docosenyl elcosenoate | 41.53 |
| Menthone | 19.39 | Elcosenyl elcosenoate | 27.65 |
| Menthyl acetate | 5.24 | Elcosenyl docosenoate | 10.33 |
| 1, 8-Cineole | 4.60 | Tetracosanyl elcosanate | 6.27 |
| Menthofuranne | 3.92 | Elcosenyl oleate | 5.38 |
| Neomenthol | 3.03 | Docosenyl stearate | 3.21 |
| Isomenthone | 2.98 | Other esters | 5.63 |
| β-Caryophyllene | 2.08 | ||
| Germacrene D | 1.94 | ||
| Limonene | 1.38 | ||
| β-Pinene | 1.18 | ||
| Terpinene-4-ol | 0.94 | ||
| α-Pinene | 0.77 | ||
| The others | 10.21 | ||
| Total | 100.00 | Total | 100.00 |
Experimental animal. Five-week-old male C57BL/6 mice (Daehan Biolink Co., Korea) were allowed to adapt to their new environment for one week, with food and water provided ad libitum under 22 ± 1℃ room temperature, 50 ± 5% relative humidity and 12 hrs of a light/dark cycle before the experiment was begun. The dorsal area (2 cm × 4 cm) of the 6-week-old C57BL/6 mice was shaved with an animal clipper. Upon shaving the mice all of the hair follicles were synchronized in the telogen stage, showing pink color. All animals were randomized into 4 groups based on different topical applications: saline (SA), jojoba oil (JO), 3% minoxidil (MXD), and 3% peppermint oil (PEO, diluted in jojoba oil). Each compound (100 μl) was topically applied to the shaved dorsal area once a day, 6 days a week, for 4 weeks. Both animal care and the protocol for this study were in accordance with IACUC (Institutional Animal Care and Use Committee) and OECD guidelines.
Hair growth observation. To assess the hair growth in each group, photographs of the animals were taken at week 1, 2, 3, and 4 after topical application was begun. The hair growth effect was scored as follows, 0: no hair growth; 1: less than 20% growth; 2: 20% to less than 40% growth; 3: 40% to less than 60% growth; 4: 60% to less than 80% growth; and 5: 80% to 100% growth.
Histological analysis. The mice were euthanized with diethyl ether and extracted skin tissue. Number of mice sacrificed at week 1, 2, and 4 was and 3, 3, and 5, respectively, and their dermal skin samples were fixed in 10% buffered formalin for 24 hrs, followed by paraffin wax embedding using standard techniques. General histology was visualized by hematoxylin-eosin (H&E) staining, and we subsequently observed the number, elongation and depth of hair follicles by fluorescent microscopy (Axio imager, Carl Zeiss, Germany). The dermal thickness and follicle depth were also measured by using the scale bar tool of the fluorescent microscope.
Detection of alkaline phosphatase activity in dermal skin. The extracted dorsal skin was minced and homogenized with a homogenizer (T25 basic, IKA, Malaysia) by adding 4 times phosphate buffered saline (PBS) to give a 20% homogenate. The homogenate was centrifuged at 12,000 rpm, 4℃, for 20 min (AVANTI, Beckman Coulter Inc., USA). The supernatants were kept in a deep freezer at −80℃ and used for the assay. The activity of alkaline phosphatase (ALP) was analyzed with an auto biochemistry analyzer (Konelab 20XT, Thermo, Finland).
Isolation of total RNA and cDNA synthesis. Total RNA was isolated from the extracted dorsal skin using the High Pure RNA Isolation Kit (Roche Applied Science, Penzberg, Germany) following the manufacturer's protocol. The quantity and quality of the isolated total RNA were determined by the UV/Vis spectrophotometer (Mecasys Co., Korea). Only samples with 2.0 > OD 260/280 > 1.8 were further analyzed. cDNA was synthesized from 1 μg of total RNA, using AccuPower CycleScript RT PreMix Kit (Bioneer, Korea) in a final volume of 205 μl.
Reverse transcription polymerase chain reaction. First, cDNA was diluted 1:10 with sterile deionized water, and 2 μl of the diluted cDNA was added to AccupowerTM PCR PreMix (Bioneer, Korea) and 10 pmol/L specific primer. This reaction mixture was filled up to a final volume of 20 μl with water. PCR was carried out in a PCR cycler (MycyclerTM thermal cycler, BioRad, USA). Cycling protocol for insulin-like growth factor-1 (IGF-1) was as follows: 1 cycle 94℃ for 5 min, followed by 35 cycles, 94℃ for 30 s, 60℃ for 30 s, 72℃ for 30 s, and a final extension at 72℃ for 5 min. Cycling protocol for GAPDH was as follows: 1 cycle 94℃ for 5 min, followed by 35 cycles, 94℃ for 30 s, 58℃ for 30 s, 72℃ for 30 s, and a final extension at 72℃ for 5 min. Reaction products were electrophoresed in 1.5% agarose gels and visualized with using ethidium bromide (EtBr). Each band was densitometrically quantified by image analyzer (Kodak 1D v3.6 image Analysis system, USA) and normalized with GAPDH intensity. The primer sequences used were as follows: IGF-1 forward 5'- AGAGACCCTTTGCGGGGCTGA-3', reverse 5'-CTTCTGAGTCTTGGGCATGT- 3'; GAPDH forward 5'-AACGGATTTGGTCGTATTGG- 3', reverse 5'-AGCCTTCTCCATGGTGGTGAAGAC- 3'.
Water and food intakes, food efficiency ratio and body weight change. The water and food intakes of experimental animals were measured once a week, and the weight was measured immediately before the experiment started and at 09:00~10:00 a.m. once a week during the experimental period.
Statistical analysis. The data were statistically analyzed by Student’s t-test for the comparison among groups using SPSS WIN (v21.0). The results were considered statistically significant if the p-values were less than 0.05.
RESULTS
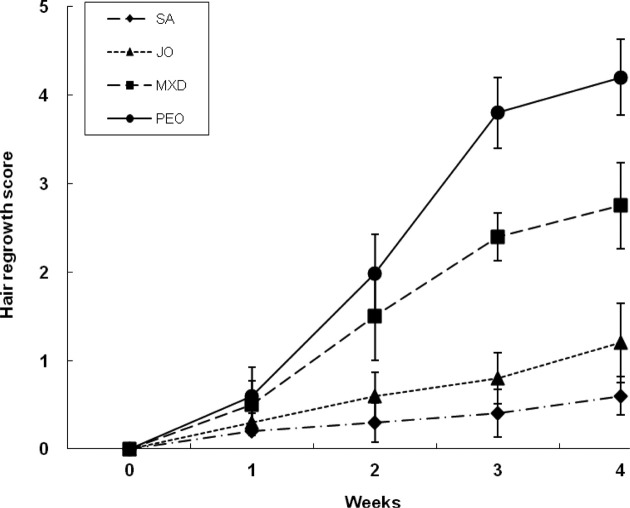
Hair growth promotion. From week 2, PEO grew hair more rapidly than SA and JO. At week 3, PEO remarkably promoted hair growth than SA and JO, even greater than MXD. At week 4, PEO showed hair growth about 92%, whereas MXD about 55% (Fig. 1).
Fig. 1. Comparison of the hair growth effect in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. The hair growth effect was calculated using scoring index: 0~19% (1), 20~39% (2), 40~59% (3), 60~79% (4), and 80~100% (5). Each point represents the mean ± SD of 5 mice. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
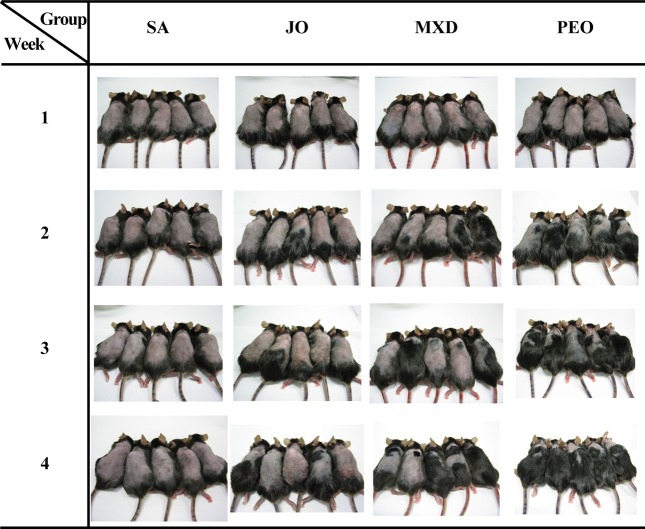
Hair growth promotion was evaluated by observing the darkening of the skin color, which indicated telogen to anagen conversion, bright pink in telogen and grey/black in anagen. At week 1, PEO changed the dorsal skin color from pink to grey/black and from week 2, it showed a considerably rapid increase in hair growth (Fig. 2). These results clearly demonstrate that the topical application of PEO induces rapid anagen hair growth in telogen mouse skin.
Fig. 2. Gross observation of back skins in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
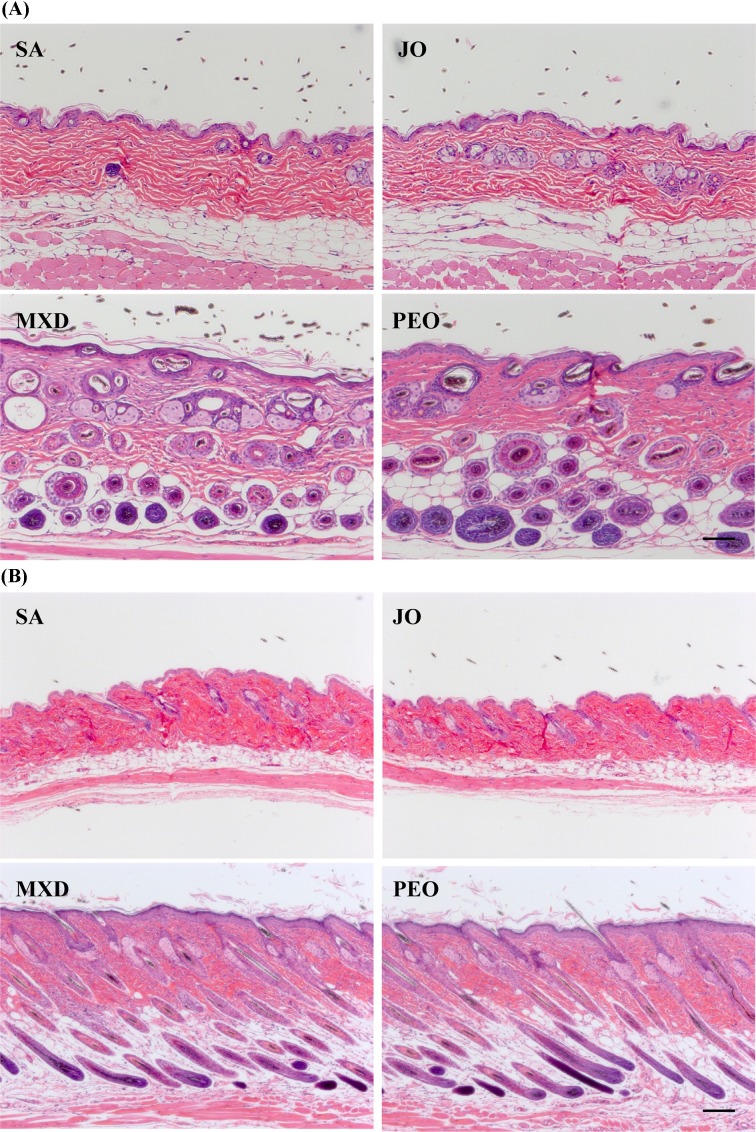
Increase of dermal thickness, hair follicle number and hair follicle depth in histological analysis. Histological analysis showed that 4-wk topical application of PEO and MXD induced very thick and long hair growth and promoted the elongation of hair follicles from dermis to subcutis (Fig. 3). These results indicate that the hair follicles of PEO and MXD groups at week 4 were in the anagen stage. We also observed a slight increase of epidermal thickness in PEO group.
Fig. 3. Histological observation of hair follicles in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. H&E stain, ×100. Scale bar 200 μm. (A) transverse view of hair follicles, (B) vertical view of hair follicles. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
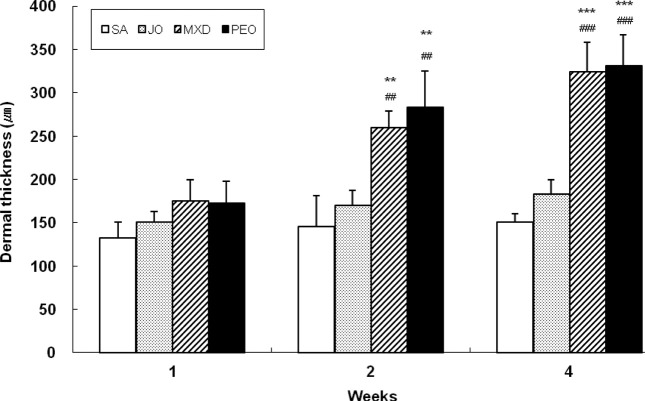
At week 2, PEO showed dermal thickness to 95% and 66% greater than SA and JO, respectively (p < 0.01). At week 4, PEO showed it to 120% and 81% greater than SA and JO, respectively (p < 0.01), comparable to MXD (Fig. 4).
Fig. 4. Change of dermal thickness in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. The dermal thickness was measured by the average of two different points in the field with an eyepiece of ×100 magnification. Values are the mean ± SD of 3, 3, and 5 mice at week 1, 2, and 4, respectively. The value is significantly different fromSA group (**; p < 0.01, ***; p< 0.001) and JO group (##; p< 0.01, ###; p< 0.001) by Student’s t-test. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
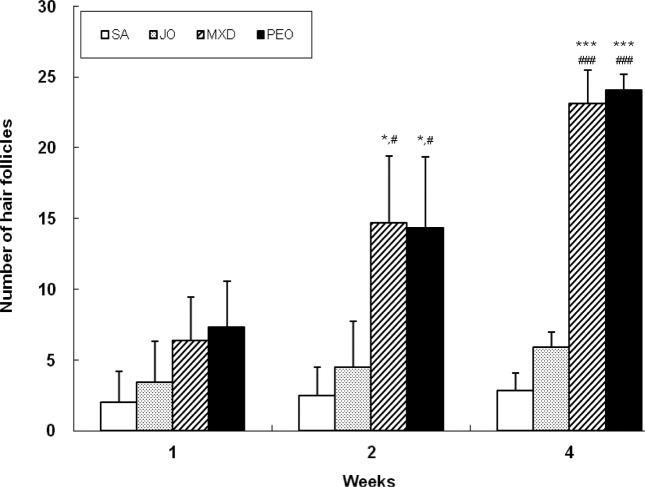
Fig. 5 shows the growth promoting activity of hair follicle number. At week 2, the hair follicle number of PEO group was 473% and 218% greater than SA and JO groups, respectively (p < 0.05). At week 4, PEO group had 740% and 307% more hair follicles than SA and JO groups, respectively (p < 0.001), comparable to MXD group. We also found that the number of hair follicles increased as hair regrew.
Fig. 5. Change of follicle number in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. The follicle number was determined by the average of two different points in the field with an eyepiece of ×100 magnification. Values are the mean ± SD of 3, 3, and 5 mice at week 1, 2, and 4, respectively. The value is significantly different from SA group (*; p<0.05; ***; p< 0.001) and JO group (#; p < 0.05, ###; p< 0.001) by Student’s t-test. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
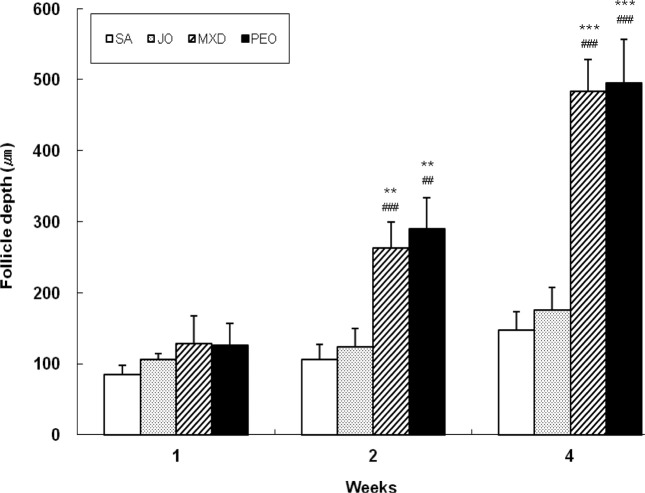
Fig. 6 shows the growth promoting activity of hair follicle depth. At week 2, the depth of hair follicles of PEO group was 172% and 133% greater than SA and JO groups, respectively (p < 0.01). At week 4, the depth of hair follicles of PEO group was 236% and 182% greater than SA and JO groups, respectively (p < 0.001), comparable to MXD. Histological studies revealed that PEO markedly stimulated the skin and thickened it. The depth, size, and number of hair follicles were also markedly increased in PEO treated skin. These results clearly demonstrate that topical application of PEO markedly stimulated hair growth and induced rapid anagen hair growth in telogen mouse skin.
Fig. 6. Change of follicle depth in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. The follicle depth was measured by the average of two different points in the field with an eyepiece of ×100 magnification. Values are the mean ± SD of 3, 3, and 5 mice at week 1, 2, and 4, respectively. The value is significantly different from SA group (**; p < 0.01; ***; p<0.001) and JO group (##; p< 0.01; ###; p< 0.001) by Student’s t-test. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
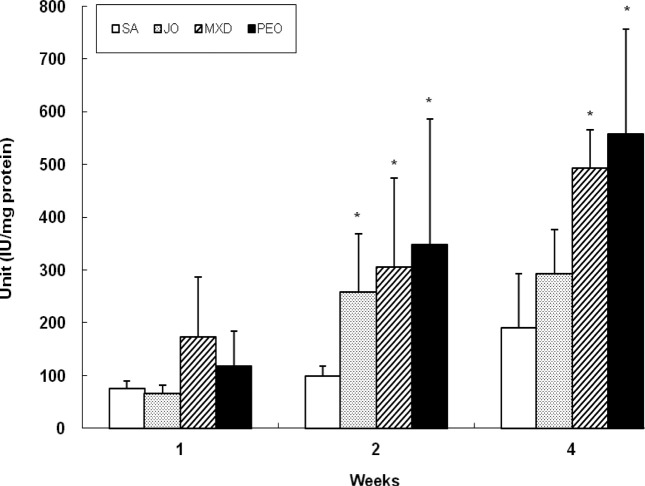
Change of ALP enzyme activity with hair cycle. At week 2, PEO showed 253% (p < 0.05), 35%, and 13% greater ALP activity compared to SA, JO, and MXD, respectively (Fig. 7). At week 4, PEO showed 192% (p < 0.05), 90%, and 13% greater ALP activity compared to SA, JO, and MXD, respectively. After topical application on the backs of C57BL/6 mice for 4 wks, PEO induced the earliest telogen-to-anagen conversion, with MXD, JO and SA following in order. The increase in ALP activity of PEO group was fast compared to MXD group, with remarkably significant compared to SA and JO groups.
Fig. 7. Change of skin ALP activity in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. Values are the mean ± SD of 3, 3, and 5 mice at week 1, 2, and 4, respectively. The value is significantly different from SA group (*; p< 0.05) by Student’s t-test. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
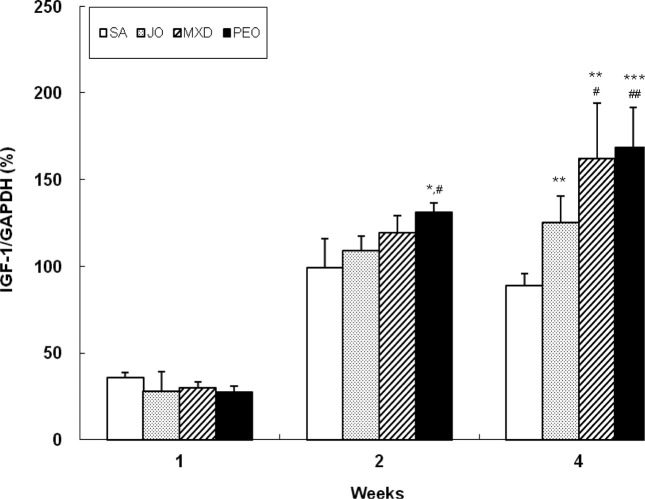
Comparison of IGF-1 mRNA expression. At week 2, PEO showed 33% and 21% greater IGF-1 mRNA expression compared to SA and JO, respectively (p < 0.05) (Fig. 8). At week 4, PEO showed 89% (p < 0.001) and 34% (p < 0.01) greater IGF-1 mRNA expression compared to SA and JO, respectively, comparable to MXD. PEO showed remarkably increased IGF-1 mRNA expression, even better than MXD.
Fig. 8. Change of IGF-1 expression in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. Values are the mean ± SD of 3, 3, and 5 mice at week 1, 2, and 4, respectively. The value is significantly different from SA group (*; p< 0.05; **; p< 0.01, ***; p< 0.001) and JO group (#; p< 0.05; ##; p< 0.01) by Student’s t-test. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
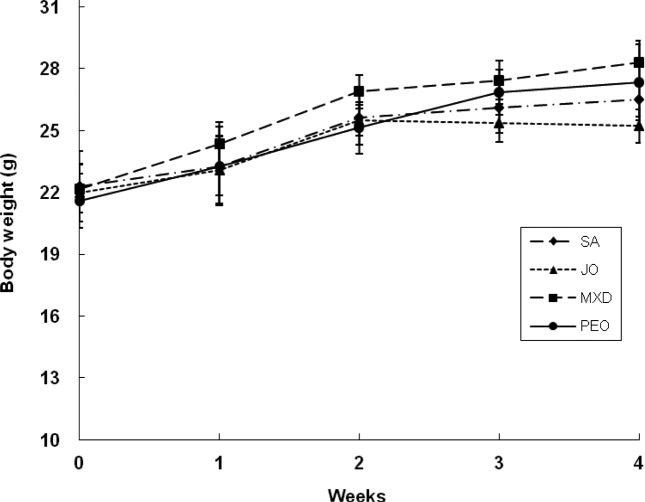
Change of water and food intakes, food efficiency ratio, and body weight. Body weight gain, food efficiency, and weight of MXD group were higher than the other groups but did not show significant difference (Table 2 and Fig. 9).
Table 2. Daily water intake, food intake, body weight gain, and food efficiency ratio in C57BL/6 mice applied with test compounds for 4 wks.
| Items Groups | SA | JO | MXD | PEO |
|---|---|---|---|---|
|
| ||||
| Water intake (ml/day) | 6.96 ± 0.12 | 7.12 ± 0.19 | 7.16 ± 0.12 | 7.14 ± 0.15 |
| Food intake (g/day) | 4.61 ± 0.28 | 4.51 ± 0.27 | 4.78 ± 0.28 | 4.64 ± 0.20 |
| Body weight gain (g/day) | 0.20 ± 0.02 | 0.18 ± 0.04 | 0.25 ± 0.03 | 0.20 ± 0.04 |
| Food efficiency ratio (%)1) | 4.38 ± 0.47 | 3.93 ± 0.75 | 5.22 ± 0.69 | 4.36 ± 0.67 |
Values are the mean ± SD of 11 mice. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
1)Food efficiency ratio (%): (Body weight gain/Food intake) × 100.
Fig. 9. Change of body weight in C57BL/6 mice. The back skins of the mice were shaved and test compounds were topically applied for 4 wks. Values are the mean ± SD of 11 mice. SA; saline, JO; jojoba oil, MXD; 3% minoxidil, PEO; 3% peppermint oil.
DISCUSSION
MXD has been widely used to treat androgenetic alopecia, but little is known about its pharmacological activity or about the identity of its target cells in hair follicles (10). Topically applied MXD was believed to stimulate hair growth by indirect drug action, i.e. by inducing vasodilatation and increasing blood flow to the follicular dermal papilla cells, or by creating a local irritation (11). The follicular dermal papilla cells are the most likely target site for the action of MXD (12). Mori and Uno (13) reported that topical application of MXD specifically stimulates the secondary germ of the telogen follicles, resulting in their rapid progression to anagen follicles. Thus, hair follicle is useful marker that is associated with hair cycle (14). Anagen I-VI development is characterized by increasing length of the hair follicle and catagen I-VII by decreasing length. During telogen, the hair follicle reaches its minimal length. Synchronized hair follicle cycling in mice is also associated with stage-dependent changes in dermal thickness. A hair follicle in telogen, anagen I or anagen II has not yet reached the subcutis. During the anagen III stage, the follicles move from the dermis down to the subcutis.
We found that PEO remarkably promoted hair growth compared to SA and JO, even faster than MXD without significant change of body weight gain and food efficiency. Chen et al. (15) reported that MXD took only about 10 days for the hair of mice to fully regrew after the topical application of MXD, indicating the enhancing effect of MXD in the proliferative rate of hair growth. In our study, histological analysis showed that MXD promoted hair growth in terms of hair follicle number, follicle depth, and dermal thickness at week 2.
Menthol is a major constituent of peppermint oil, which is a cyclic alcohol. Menthol has been widely used as a component of food and cosmetics. It has been reported that menthol increases the sensitivity of cutaneous cold receptors by modulating Ca2+ currents of neuronal membranes (16). Menthol is the most effective penetration enhancer that, along with limonene, can be considered the prototype for the use of terpenes as penetration enhancers (17). For years terpenes (e.g., menthol, β-pinene, terpinene-4-ol, α- pinene, 1,8-cineole) have been used alone or as constituents of essential oils in medicine, cosmetics and household products. In the experimental dermopharmacy and technology of transdermal drug forms, terpenes have also been intensively explored as penetration enhancers (18). When skin is treated with terpenes, the existing network of hydrogen bonds between ceramides may loosen because of competitive hydrogen bonding (19). The high accumulation of most of the terpenes in the skin layers proves that these compounds easily permeate the stratum corneum and that they may easily penetrate into blood circulation in vivo (20).
In our study, we found that PEO induced very thick and long hair after 4-week topical application and promoted the elongation of hair follicles from the epidermis down to the subcutis in a vertical section (Fig. 3), showing in the stage of anagen III. Application of MXD caused similar results. We observed that this increase in hair follicle length was not associated with any loss of hair follicle architecture and that the increase in hair follicle length was associated with an increase in the length of the keratinized hair shaft.
The drugs for alopecia treatment have been developed to maintain or induce the anagen stage of hair cycle. ALP activity was particularly detected in the dermal papilla. ALP activity in the dermal papilla was moderate in very early anagen, reached a maximal level in early anagen, and was kept at a low level during catagen (21). The bulbar dermal sheath showed intense ALP activity only in early anagen (22). Although results from clinical trials vary, the majority of the evidence indicates that there is a direct correlation between the hair follicle depth and the level of ALP activity. In our study, PEO induced significantly high ALP activity at week 2, even greater than MXD. This study demonstrates that PEO stimulates both dermal papilla and ALP activity, which promotes blood circulation by relaxing vascular smooth muscle (8).
To better understand the influence of the endocrine system in hair growth, we analyzed the mRNA expression of IGF-1 gene. It is a potent mitogen supporting cell growth and survival (23) and also plays a role to increase hair thickness (24). In our study, PEO showed remarkably increased IGF-1 mRNA expression at week 2, whereas MXD at week 4.
In conclusion, our experimental data suggest that 3% PEO facilitates hair growth by promoting the conservation of vascularization of hair dermal papilla, which may contribute to the induction of early anagen stage. In addition, PEO effectively stimulated hair growth in an animal model via several mechanisms and thus could be used as a therapeutic or preventive alternative medicine for hair loss in humans.
References
- 1.Stough D., Stenn K., Haber R., Parsley W.M., Vogel J.E., Whiting D.A., Washenik K. Psychological effect, pathophysiology, and management of androgenetic alopecia in men. Mayo Clin. Proc. (2005);80:1316–1322. doi: 10.4065/80.10.1316. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro J., Price V.H. Hair regrowth therapeutic agents. Dermatol. Clin. (1998);16:341–356. doi: 10.1016/S0733-8635(05)70017-6. [DOI] [PubMed] [Google Scholar]
- 3.Buhl A.E., Waldon D.J., Baker C.A., Johnson G.A. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J. Invest. Dermatol. (1990);95:553–557. doi: 10.1111/1523-1747.ep12504905. [DOI] [PubMed] [Google Scholar]
- 4.Trüeb R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. (2002);37:981–990. doi: 10.1016/S0531-5565(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 5.Messenger A.G., Rundegren J. Minoxidil: mechanisms of action on hair growth. Br. J. Dermatol. (2004);150:186–194. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 6.Hills J.M., Aaronson P.I. The mechanism of action of peppermint oil on gastrointestinal smooth muscle: an analysis using patch clamp electrophysiology and isolated tissue pharmacology in rabbit and guinea pig. Gastroenterology. (1991);101:55–65. doi: 10.1016/0016-5085(91)90459-x. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T., Sugimoto Y., Masuda H., Kamei C. Effect of peppermint (Mentha piperita L.) extracts on experimental allergic rhinitis in rats. Biol. Pharm. Bull. (2001);24:92–95. doi: 10.1248/bpb.24.92. [DOI] [PubMed] [Google Scholar]
- 8.Comar K.M., Kirby D.F. Herbal remedies in gastroenterology. J. Clin. Gastroenterol. (2005);39:457–468. doi: 10.1097/01.mcg.0000165650.09500.3a. [DOI] [PubMed] [Google Scholar]
- 9.Schelz Z., Molnar J., Hohmann J. Antimicrobial and antiplasmid activities of essential oils. Fitoerapia. (2006);77:279–285. doi: 10.1016/j.fitote.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Han J.H., Kwon O.S., Chung J.H., Cho K.H., Eun H.C., Kim K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol Sci. (2004);34:91–98. doi: 10.1016/j.jdermsci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Wester R.C., Maibach H.I., Guy R.H., Novak E. Minoxidil stimulates cutaneous blood flow in human balding scalps: pharmacodynamics measured by laser Doppler velocimetry and photopulse plethysmography. J. Invest Dermatol. (1984);82:515–517. doi: 10.1111/1523-1747.ep12261084. [DOI] [PubMed] [Google Scholar]
- 12.Headington J.T. Hair follicle biology and topical minoxidil: possible mechanisms of action. Dermatologica. (1987);175(Supple 2):19–22. doi: 10.1159/000248894. [DOI] [PubMed] [Google Scholar]
- 13.Mori O., Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J. Dermatol. (1990);17:276–281. doi: 10.1111/j.1346-8138.1990.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 14.Müller-Röver S., Handjiski B., van der Veen C., Eichmüller S., Foitzik K., McKay I.A., Stenn K.S., Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. (2001);117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen C.H., Sheu M.T., Wu A.B., Lin K.P., Ho H.O. Simultaneous effects of tocopheryl polyethylene glycol succinate (TPGS) on local hair growth promotion and systemic absorption of topically applied minoxidil in a mouse model. Int. J. Pharm. (2005);306:91–98. doi: 10.1016/j.ijpharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Chiyotani A., Tamaoki J., Takeuchi S., Kondo M., Isono K., Konno K. Stimulation by menthol of Cl secretion via a Ca2+-dependent mechanism in canine airway epithelium. Br. J. Pharmacol. (1994);112:571–575. doi: 10.1111/j.1476-5381.1994.tb13112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aqil M., Ahad A., Sultana Y., Ali A. Status of terpenes as skin penetration enhancers. Drug Discovery Today. (2007);12:1061–1067. doi: 10.1016/j.drudis.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Cal K., Kupiec K., Sznitowska M. Effect of physicochemical properties of cyclic terpenes on their ex vivo skin absorption and elimination kinetics. J. Dermatol. Sci. (2006);41:137–142. doi: 10.1016/j.jdermsci.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Jain A.K., Thomas N.S., Panchagnula R. Transdermal drug delivery of imipramine hydrochloride. J. Controlled Release. (2002);79:93–101. doi: 10.1016/S0168-3659(01)00524-7. [DOI] [PubMed] [Google Scholar]
- 20.Cal K., Sopala M. Ex vivo skin absorption of terpenes from Vicks VapoRub® ointment. Med. Sci. Monit. (2008);14:Pl19–123. [PubMed] [Google Scholar]
- 21.Iida M., Ihara S., Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev. Growth Differ. (2007);49:185–195. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamada K., Suzuki K. Evaluation of biochemical indices as a hair cycle marker in C3H mice. Exp. Anim. (1996);45:251–256. doi: 10.1538/expanim.45.251. [DOI] [PubMed] [Google Scholar]
- 23.Stewart C.E., Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol. Rev. (1996);76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 24.Weger N., Schlake T. IGF-1 signalling controls the hair growth cycle and the differentiation of hair shafts. J. Invest. Dermatol. (2005);125:873–882. doi: 10.1111/j.0022-202X.2005.23946.x. [DOI] [PubMed] [Google Scholar]