Abstract
Polyunsaturated fatty acids (PUFAs) display immunomodulatory properties in the brain, n-3 PUFAs being able to reduce inflammation whereas n-6 PUFAs are more pro-inflammatory. It has been extensively demonstrated that exposure to a peripheral immune challenge leads to the production and release of inflammatory mediators in the brain in association with cognitive deficits. The question arises whether n-3 PUFA supplementation could downregulate the brain inflammatory response and subsequent cognitive alterations. In this study, we used a genetically modified mouse line carrying the fat-1 gene from the roundworm Caenorhabditis elegans, encoding an n-3 PUFA desaturase that catalyzes conversion of n-6 into n-3 PUFA. Consequently, these mice display endogenously elevated n-3 PUFA tissue contents. Fat-1 mice or wild-type (WT) littermates were injected peripherally with lipopolysaccharide (LPS), a bacterial endotoxin, to induce an inflammatory episode. Our results showed that LPS altered differently the phenotype of microglia and the expression of cytokines and chemokines in Fat-1 and WT mice. In Fat-1 mice, pro-inflammatory factors synthesis was lowered compared with WT mice, whereas anti-inflammatory mechanisms were favored 24 h after LPS treatment. Moreover, LPS injection impaired spatial memory in WT mice, whereas interestingly, the Fat-1 mice showed normal cognitive performances. All together, these data suggest that the central n-3 PUFA increase observed in Fat-1 mice modulated the brain innate immune system activity, leading to the protection of animals against LPS-induced pro-inflammatory cytokine production and subsequent spatial memory alteration.
INTRODUCTION
Increasing attention has been paid to the role of n-3 and n-6 polyunsaturated fatty acids (PUFAs) in the brain and an increasing database attests of their powerful immunomodulatory effects (Calder, 2001; Laye, 2010; Labrousse et al, 2012; Orr et al, 2013). n-3 PUFAs are precursors of lipid derivatives (neuroprotectins and resolvins) with anti-inflammatory properties, whereas n-6 PUFAs are mostly the precursors of the pro-inflammatory prostaglandins, and stimulate the production and activity of inflammatory cytokines. The dramatic reduction in the dietary supply of n-3 PUFAs in Western societies and the corresponding increase in n-6 PUFAs leads to an imbalanced n-6/n-3 ratio currently estimated at 12–20 in developed countries instead of the recommended ratio of 5 (Simopoulos, 2001). By affecting brain PUFAs composition, this could therefore contribute to the sensitization of the brain to inflammatory cytokines, and thus to the development of neurodegenerative and/or behavioral disorders (Laye, 2010).
Microglia are the resident macrophages of the brain, and constitute the first line of immune defense of the brain (Ransohoff and Cardona, 2010). Once stimulated by an immune challenge, microglia are capable of acquiring diverse and complex phenotypes as well as performing several macrophage-like functions including inflammatory and anti-inflammatory cytokine production (Garden and Moller, 2006; Biber et al, 2007; Madore et al, 2013). OFF signals (eg CX3CL1) that are released by healthy neurons to keep microglia in a surveillance mode in normal conditions are also downregulated under inflammation (Biber et al, 2007). Moreover, exposure to pathogens results in altered memory performances (Yirmiya and Goshen, 2011), as part of the general sickness behavior syndrome (Dantzer et al, 2008). More specifically, it has been extensively demonstrated that microglia-derived inflammatory mediators are directly involved in the cognitive disturbances that accompany exposure to endotoxin (Yirmiya and Goshen, 2011). Central nervous system inflammation occurs in a large variety of pathologies including multiple sclerosis, Alzheimer's disease, and Parkinson's disease (Laye, 2010). Thus, identifying inflammatory modulators is a growing area of interest, because it may provide novel targets in disease prevention and treatment.
In this study, we used a genetically modified mouse line to study the impact of n-3 PUFA supplementation on brain inflammatory response and subsequent cognitive alterations. These mice carry the fat-1 gene from the roundworm nematode Caenorhabditis elegans, encoding an n-3 PUFA desaturase, absent in mammals, that catalyzes the conversion of n-6 into n-3 PUFA (Kang et al, 2004). Therefore, these mice have endogenously elevated n-3 PUFA tissue content and exhibit lower n-6/n-3 PUFA ratio compared with their wild-type (WT) littermates. Indeed, Fat-1 mouse displays brain docosahexaenoic acid (DHA) levels that are generally attainable through fish oil feeding (Orr et al, 2010) and present lower n-6 PUFA levels in the brain (Boudrault et al, 2010).
In the present study, Fat-1 mice or WT littermates were injected peripherally with lipopolysaccharide (LPS), a potent inducer of cerebral inflammation, and we assessed cognitive performances, cytokine production, and microglial phenotype 24 h later. Our results showed that Fat-1 mice were protected against systemic inflammation, in the form of lower production of pro-inflammatory mediators, together with an increased anti-inflammatory profile of microglia. Fat-1 mice also displayed protection against inflammation-induced cognitive impairment.
MATERIALS AND METHODS
Animals and Treatment
Heterozygous transgenic Fat-1 mice were generated as described previously (Kang et al, 2004) and backcrossed onto a C57BL/6J background. The presence of the fat-1 gene in each mouse was confirmed both by genotyping and brain tissue fatty acid analysis profile (see below). Transgenic and WT animals were kept under pathogen-free conditions in standard cages in temperature- and humidity-controlled conditions with a 12-h light/dark cycle. We used adult male Fat-1 transgenic mice and nontransgenic WT littermates (as controls) all along the study. Studies were carried out according to the Quality Reference System of INRA (http://www.international.inra.fr/content/download/947/11111/file/requirements) and approved by the local ethical committee for care and use of animals (approval ID: A33-063-920).
Of importance and contrary to previous studies on Fat-1 mice, all animals were fed ad libitum with a standard diet (A04, SAFE, Augy, France; Table 1), as we recently demonstrated that the long-term consumption of inadequate n-6/n-3 PUFAs ratio triggers spatial memory impairment and depressive and anxiety-like symptoms in mice (Lafourcade et al, 2011; Moranis et al, 2012). By giving a standard diet to the animals, we prevented any behavioral alterations in WT littermates from occurring. Behavioral experiments were conducted on adult mice (3–5-month old). To induce an inflammatory reaction, a dose of 125 μg/kg of LPS (Escherichia coli, 0127:B8, Sigma-Aldrich, Lyon, France), diluted in saline (NaCl 0.9%) was intraperitoneally injected between 9 and 11am. LPS is a component of the cell wall of Gram-negative bacteria known at this dose as a useful model for investigation of changes that accompany inflammation in brain such as spatial memory impairment (Laye et al, 1994; Mingam et al, 2008 ). Control mice received an injection of saline solution (0.9%). Food consumption (assessed by weighing the pellets) and body weight were monitored 2 and 24 h after LPS injection as a marker of sickness. For qPCR experiments and lipid measurements, mice were quickly anesthetized by isoflurane inhalation and euthanized by decapitation 2 or 24 h after treatment. Twenty-four hours after the treatment, mice have recovered from the sickness symptoms and present a normal locomotor activity, allowing us to investigate their working memory abilities (O'Connor et al, 2009).
Table 1. Fatty Acid Composition of the Dietary Lipids (% wt of Total Fatty Acids).
| Lipids | Standard diet (A04) |
|---|---|
| 16:0 | 20.2 |
| 18:0 | 2.2 |
| Other saturated FAs | 1.8 |
| Total saturated FAs | 24.2 |
| 18:1n-9 | 19.2 |
| 18:1n-7 | 1.5 |
| Other monounsaturated FAs | 3.9 |
| Total monounsaturated FAs | 24.6 |
| 18:2n-6 (LA) | 45.6 |
| Other n-6 PUFAs | 0.3 |
| Total n-6 PUFAs | 45.9 |
| 18:3n-3 (ALA) | 3.3 |
| 20:5 n-3 | 0.6 |
| 22:5 n-3 | 0.1 |
| 22:6 n-3 | 0.8 |
| Total n-3 PUFAs | 4.8 |
| Total PUFAs | 50.7 |
Abbreviations: AA, arachidonic acid; ALA, α-linolenic acid; FAs, fatty acids; PUFAs, polyunsaturated fatty acids.
Genotyping
DNA was extracted from approximately 2–3 mm of the mouse tail. Tissue was digested in 200 μl of NaOH 0.05 M for 20 min at 100 °C and then 50 μl of Tris HCl 1 M pH8.0 was added to the mix. After 10 min of centrifugation, supernatant containing DNA was removed and left at 4 °C. Two microliters of DNA extracts were used for genotyping by PCR. The primers used for the fat-1 gene were 5′-TGTTCATGCCTTCTTCTTCTTTTTCC-3′ and 5′-GCGACCATACCTCAAACTTGG-3′. The 20 μl PCR reaction mix also contained 5 μl of deoxyribonucleotide mix, 10 μl of PCR 2 × mix (Promega, WI, USA), and 0.2 μl of GoTaq DNA Polymerase (Promega). The thermocycler program consisted of a period of 5 min at 94 °C followed by 2 cycles of 1 min at 94 °C, 1 min at 60 °C, 1 min at 72 °C, then 30 cycles of 30 s at 94 °C, 30 s at 60 °C, 45 s at 72 °C. The run was completed with 5 min at 72 °C, and then cooled to 4 °C. Amplified PCR products were run through a 2% agarose gel and visualized using Ethidium Bromide on UV table with gel imager (Syngene, Saint Quentin en Yvelines, France).
Fatty Acids Analysis in Brain Phospholipids
We measured the levels of n-3 and n-6 PUFAs in the hippocampus of WT (n=4/saline; n=6/LPS) and Fat-1 mice (n=4/saline; n=4/LPS) as previously described (Lafourcade et al, 2011; Labrousse et al, 2012; Larrieu et al, 2012), as a control of lipid content in mice fed with a standard diet. Fatty acid composition is expressed as the percentage of total fatty acids.
Behavioral Measurement
Y-maze
The Y-maze paradigm was used to assess spatial working memory as previously described by our group (Labrousse et al, 2009; Moranis et al, 2012). Spatial working memory was assessed in Fat-1 (n=9–10/saline and n=8/LPS) and WT mice (n=9–12/saline and n=9–10/LPS). The apparatus was a Y-shaped maze made of gray plastic. Each arm was 34 cm long, 8 cm wide, and 14 cm high. The floor of the maze was covered with corncob litter, which was mixed between each trial to remove olfactory cues. Visual cues were placed in the testing room and kept constant during the whole test. In the first trial of the test, one arm of the Y-maze was closed with a guillotine door and mice were allowed to visit two arms of the Y-maze for 5 min. After 30-min inter-trial interval, mice were placed back in the start arm and allowed free access to the three arms for 5 min. Spontaneous spatial recognition was also measured using a 5 min inter-trial interval (ITI) between acquisition and retrieval. This ITI was employed to control for potential motivational deficits and to verity that all groups performed more visits to the novel arm when the mnemonic demand was minimal. Start and closed arms were randomly assigned for each mouse. We compared the time spent exploring the novel and the familiar arm during the 5 min of the second trial.
Quantitative Real-Time PCR
Total RNA was extracted from hippocampi using TRIzol (Invitrogen, Life Technologies) (WT: n=5/saline; n=5/LPS; Fat-1: n=5/saline; n=5/LPS). RNA purity and concentration were determined using a Nanodrop spectrophotometer (Nanodrop technologies, Wilmington, DE). One microgram of RNA was reverse transcribed to synthesize cDNA using Superscript III (Invitrogen, Life Technologies) and random hexamers according to the manufacturer's protocol (Labrousse et al, 2012).
Quantitative PCR was performed to measure cytokine expression using the Applied Biosystems (California, USA) assay-on demand gene expression protocol as previously described (Mingam et al, 2008). In brief, cDNA was amplified by real-time PCR where a target cDNA and a reference cDNA (β2-microglobulin) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7500-sequence detection system (Applied Biosystems). Data were analyzed using the comparative threshold cycle method, results are expressed as relative fold change (Mingam et al, 2008) to control target mRNA expression.
Isolation of Microglia
Microglial cells were isolated from whole brain homogenates as previously described (Henry et al, 2008; Henry et al, 2009; Wynne et al, 2010; Wohleb et al, 2012; Madore et al, 2013). Brains were homogenized in Hanks' Balanced Salt Solution, pH 7.4 passing through a 70 μm nylon cell strainer. Homogenates were centrifuged at 600 g for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE-Healthcare). A discontinuous Percoll density gradient was set up as follows: 70, 50, 35, and 0% isotonic Percoll. Gradients were centrifuged at 2000 g for 20 min. Microglia cells were collected at the interface between the 70 and 50% Percoll layers (Frank et al, 2006; Nair et al, 2007). Cells were washed and counted with a hemacytometer. For each brain extraction, approximately 3 × 105 cells were isolated. Microglial cells were re-suspended in phosphate buffer saline solution/0.1% bovine serum albumin to perform flow cytometric analysis.
Flow Cytometry
Microglial preparations were incubated with anti-CD16/CD32 antibody (eBiosciences) to block Fc receptors for 10 min on ice. Cells were washed and then incubated for 30 min with the appropriate conjugated antibodies: anti-CD11b-APC, anti-CD45-PerCP Cy5.5, anti-MHC-II-FITC (eBio-sciences), and anti-CD36-PE (Biolegend, Saint Quentin Yvelines, France). Cells were washed and then re-suspended in fluorescence-assisted cell sorting buffer (phosphate buffer saline solution/bovine serum albumin 0.1%) for analysis. Non-specific binding was assessed by using non-specific, isotype-matched antibodies. Antigen expression was determined using a Becton-Dickinson LSR Fortessa cytometer. Ten thousand events were recorded for each sample and isotype-matched conjugate. Data were analyzed using FlowJo software (OR, USA) and gating for each antibody was determined based on non-specific binding of appropriate negative isotype-stained controls (Madore et al, 2013).
Statistical Analyses
All data are expressed as mean±SEM. For the analysis of PUFA level, cytokine expression and flow cytometry data, a two-way ANOVA with genotype (WT vs Fat-1) and treatment (saline vs LPS) as factors was performed. When a significant interaction was reported, ANOVAs were followed by post hoc Fisher's LSD test comparisons. For Y-maze analyses, a three-way ANOVA with treatment and genotype as between-subjects factors and arm (novel vs familiar) as the within-subjects factor was performed. Specific comparisons between novel and familiar arms were assessed by paired Student's t-tests. Statistical significance was set at p<0.05.
RESULTS
N-6/n-3 PUFA Ratio Decreases in the Hippocampus of Fat-1 Mice
We first measured n-3 and n-6 PUFA contents in the hippocampus of Fat-1 mice or WT littermates fed with a standard diet (Table 2). Arachidonic acid (AA, 20:4 n-6) and n-6 docosatetraenoic acid (22:4 n-6) levels were significantly lower in the hippocampus of Fat-1 mice compared with WT littermates (F(1,13)=5.174, p<0.05 and F(1,13)=14.31, p<0.01, respectively). Conversely, DHA (22:6 n-3) level was unchanged whereas n-3 docosapentaenoic acid (n-3 DPA, 22:5 n-3) and eicosapentaenoic acid (EPA, 20:5 n-3) levels were significantly higher in Fat-1 mice compared with WT littermates (n-3 DPA: F(1,14)=12.28, p<0.01; EPA: F(1,14)=13.10, p<0.01). Overall, Fat-1 mice had a lower n-6/n-3 ratio than WT littermates (F(1,13)=13.59, p<0.01).
Table 2. Fatty Acid Composition of Total Lipids of Adult Hippocampus.
| Fatty acids mg/100 mg fatty acids |
Saline |
LPS |
Statistical effects |
||||
|---|---|---|---|---|---|---|---|
| WT N=4 | Fat-1 N=4 | WT N=6 | Fat-1 N=4 | LPS | Genotype | LPS × genotype | |
| 18:2 n-6 | 0.4±0.01 | 0.5±0.03 | 0.5±0.02 | 0.5±0.05 | NS | <0.01 | NS |
| 20:4 n-6 | 10.0±0.09 | 9.5±0.33 | 9.9±0.12 | 9.7±0.21 | NS | <0.05 | NS |
| 22:4 n-6 | 2.1.0±0.03 | 1.9±0.08 | 2.2±0.03 | 1.8±0.12 | N5 | <0.01 | NS |
| 22:5 n-6 | 0.2±0.02 | 0.2±0.05 | 0.2±0.02 | 0.2±0.05 | NS | NS | NS |
| 20:5 n-3 | 0.03±0.002 | 0.08±0.02 | 0.04±0.01 | 0.10±0.03 | NS | <0.01 | NS |
| 22:5 n-3 | 0.15±0.01 | 0.22±0.05 | 0.15±0.01 | 0.26±0.04 | NS | <0.01 | NS |
| 22:6 n-3 | 14.6±0.20 | 14.5±0.17 | 14.5±0.21 | 15.0±0.33 | NS | NS | NS |
| n-6/n-3 | 0.89±0.02 | 0.86±0.02 | 0.90±0.01 | 0.84±0.04 | NS | <0.01 | NS |
18:2 n-6: linoleic acid; 20:4 n-6: arachidonic acid (AA); 22:4 n-6: docosatetraenoic acid (DTA); 22:5 n-6: docosapentaenoic acid (DPA n-6); 20:5 n-3: eicosaperrtaenoicacid (EPA); 22:5 n-3: docosapentaenoic acid (DPA n-3); 22:6 n-3: docosahexaenoic acid (DHA). Data are mean±SEM.
We also measured peripherally for the tail fatty acid composition of Fat-1 mice and found more DHA (4.7% in Fat-1 vs 2.8% in WT), more EPA (1.6 vs 0.6%) and much less AA (0.8 vs 8.9%), showing a n-6/n-3 ratio of 1.2 in Fat-1 vs 6.0 in WT (data not shown).
LPS Impairs Spatial Memory in WT but not in Fat-1 Mice
LPS treatment has been previously reported to alter memory performance in rats and mice (Aubert et al, 1995; Pugh et al, 1998; Arai et al, 2001; Sparkman et al, 2005; Thomson and Sutherland, 2005; Tanaka et al, 2006). In the present study, memory performance was assessed 24 h after intraperitoneal LPS injection when most of symptoms of sickness has disappeared and especially motor dysfunctions that may interact with behavioral paradigms (Cunningham and Sanderson, 2008; O'Connor et al, 2009; Yirmiya and Goshen, 2011). We used a novelty discrimination task in a Y-shaped maze to assess spatial working memory more specifically (Labrousse et al, 2012; Moranis et al, 2012).
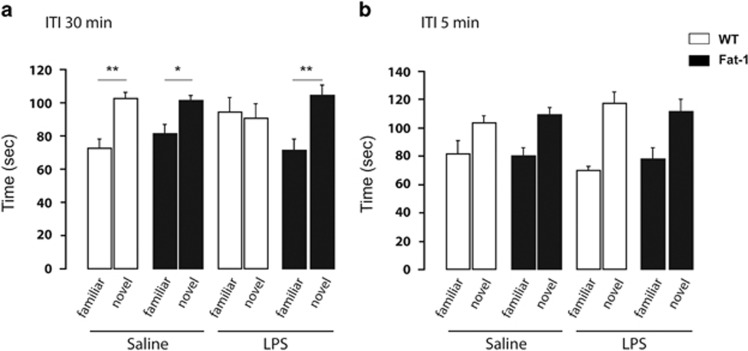
In a first set of experiments, we used an ITI of 30 min for all experimental groups. Spatial memory performance was significantly impaired only in WT mice injected with LPS (Figure 1a). A three-way ANOVA (Genotype × treatment × arm) revealed a significant effect of arm (F(1,29)=13.70, p<0.001) and a significant interaction between treatment, genotype, and arm (F(1,29)=4.30, p<0.05). Further analyses revealed that only WT mice injected with LPS exhibited a random exploration of the two arms (paired-t-test, t(7)=0.23, p=0.82), whereas the other groups significantly distinguished between the novel and the familiar arm (paired-t-test, WT/saline: t(8)=−4.08, p<0.01; Fat-1/saline: t(8)=−2.58, p<0.05; Fat-1/LPS: t(6)=−4.33, p<0.01).
Figure 1.
LPS impairs spatial memory in WT but not in Fat-1 mice. Spatial memory was assessed in the Y-maze paradigm for Fat-1 mice and WT littermates. (a) Time spent (in seconds) in the novel or the familiar arm after a 30-min ITI. Only WT mice injected with LPS exhibited a random exploration of the two arms. (b) Time spent (in sec) in the novel or the familiar arm after a 5-min ITI. All the mice spent more time in the novel arm. Data are expressed as mean±SEM. *p<0.05, **p<0.01.
To evaluate whether the impairment revealed in WT mice injected with LPS was a memory deficit or a performance deficit related to motivational alterations, animals underwent a spatial recognition test with minimal ITI (1 min) between acquisition and retrieval. In this condition, all mice recognized the novel arm (arm effect, F(1,27)=33.07, p<0.0001) showing a normal response to novelty as well as no abnormal fatigue, anxiety or lack of motivation (Figure 1b).
LPS Alters Differently the Expression of Cytokines and Chemokines in the Hippocampus of WT Compared with Fat-1 Mice
From the behavioral experiments, Fat-1 mice were protected from LPS effects on spatial memory impairment as measured in the Y-maze paradigm. Brain pro-inflammatory cytokines, especially interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6, are involved in the memory disturbances that accompany exposure to immune challenge (Yirmiya and Goshen, 2011). Our next objective was thus to quantify the expression level of cytokines mRNA in the hippocampus of Fat-1 mice and WT littermates following LPS injection.
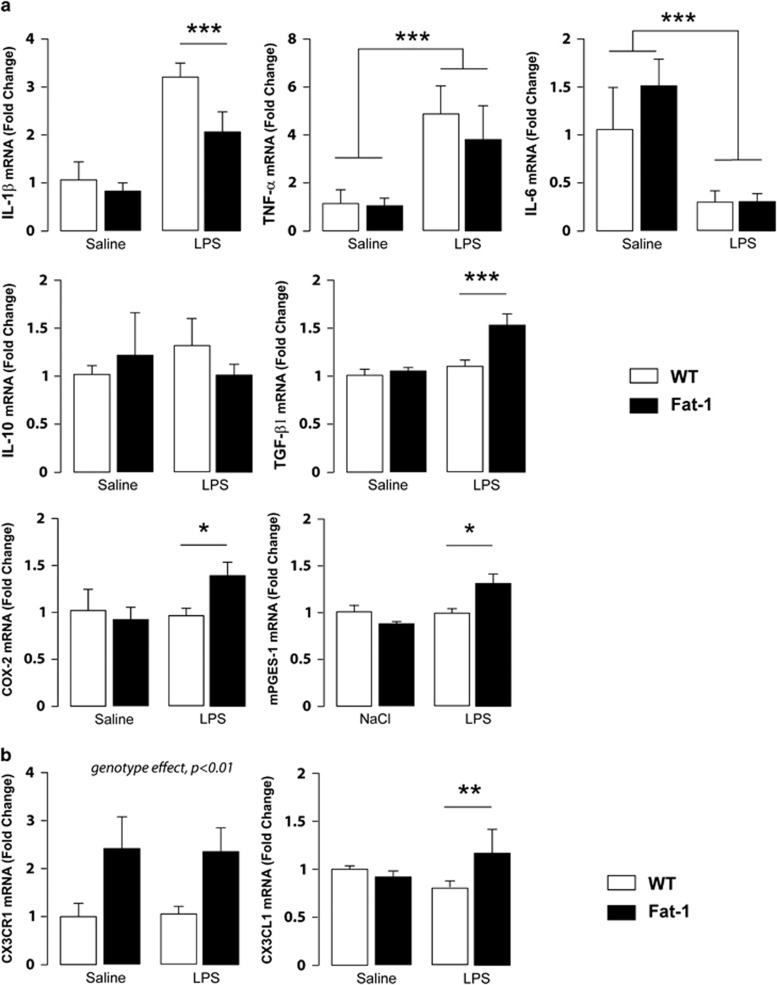
Pro-inflammatory cytokines mRNA expression was thus determined 24 h after LPS injection, the time point we used to conduct behavioral experiments (Figure 2a). Interestingly, although IL-1β mRNA expression was induced 24 h post LPS injection (treatment effect F(1,14)=124.3, p<0.0001), this increase was significantly smaller in Fat-1 mice than in WT littermates (interaction F(1,14)=9.045, p<0.01; Fisher's LSD post hoc test, p<0.001). Moreover, our results showed that TNF-α mRNA expression was significantly increased 24 h post LPS injection for both genotypes (treatment effect F(1,15)=47.55, p<0.0001) whereas IL-6 mRNA was significantly decreased after LPS treatment in all groups (treatment effect F(1,13)=65.39, p<0.0001). Regarding anti-inflammatory cytokines, no significant difference was found for IL-10 mRNA expression whatever the genotype or treatment of the animals. On the other hand, TGF-β1 was significantly increased in Fat-1 mice 24 h after LPS treatment compared with all other groups (interaction F(1,14)=5.124, p<0.05; Fat-1/LPS group different from the other groups, Fisher's LSD post hoc test, p<0.01).
Figure 2.
LPS alters differently the expression of cytokines and chemokines in the hippocampus of WT compared with Fat-1 mice. (a) Quantification of cytokines (IL-1β, TNF-α, IL-6, IL-10, TGF-β1), COX-2, and mPGES-1 mRNAs in the hippocampus of Fat-1 and WT littermates. (b) Quantification of the chemokine CX3CL1 (or fractalkine) and its receptor CX3CR1 mRNA expression in hippocampus of Fat-1 and WT littermates. Data are expressed as mean±SEM. ***p<0.001; **p<0.01; *p<0.05.
We also measured cyclooxygenase-2 (COX-2) and microsomal prostaglandin E synthase-1 (mPGES1), two enzymes necessary for prostaglandin synthesis. As for TGF-β1, COX-2 and mPGES1 mRNA expression was significantly increased in Fat-1 mice 24 h after LPS treatment compared with all other groups (interaction F(1,13)=10.15, p<0.01; Fat-1/LPS group different from the other groups, Fisher's LSD post hoc test, p<0.01).
The mechanisms leading to cytokine dysregulation within the brain and thus risk factors for cognitive disorders remain poorly defined. However, research on ON/OFF signals released by neurons has begun to provide valuable insight into the origin of neuronal factors to regulate the production of cytokines by microglia. CX3CL1, a chemokine produced by neurons, controls the overexpression of IL-1β, TNFα, and IL-6 through its effect on its receptor CX3CR1 (Biber et al, 2007). In addition, disruption of CX3CL1/CX3CR1 signaling in rodents leads to increased hippocampal IL-1β and cognitive function (Rogers et al, 2011). We thus analyzed CX3CR1 and CX3CL1 mRNA expression in the hippocampus of Fat-1 and WT mice 24 h after LPS treatment. Regarding cytokine mRNA expression, Fat-1 mice showed a striking reduced pro-inflammatory profile compared with WT littermates after LPS injection. CX3CR1 receptor mRNA expression was significantly increased in Fat-1 mice whatever the treatment (Figure 2b) (Genotype effect, F(1,16)=9.059, p<0.01). CX3CL1 mRNA expression was significantly increased in the hippocampus of Fat-1 but not WT mice after LPS treatment (Interaction F(1,15)=12.244, p<0.01; Fat-1/LPS group different from Fat-1/saline and WT/LPS groups, Fisher's LSD post hoc test, p<0.05).
LPS Modulates Microglia Phenotype in the Brain of Fat-1 but not of WT Mice
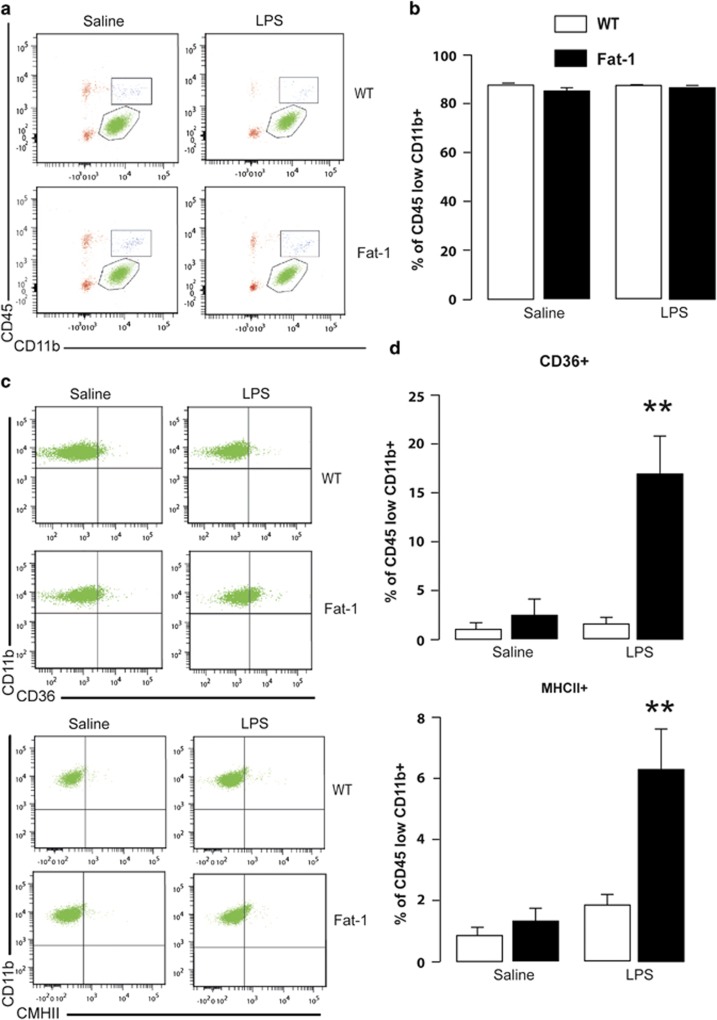
Once stimulated by an immune challenge, microglia acquire diverse and complex phenotypes that are likely to be the marker for different functions, including phagocytosis, inflammatory and anti-inflammatory cytokine production, or antigen presentation (Garden and Moller, 2006). We thus analyzed microglia phenotype by flow cytometry in the brain of Fat-1 and WT mice 24 h after LPS treatment.
Firstly, the representative bivariate dot plots showed that the majority of the viable cells were CD11b+/CD45low, likely to be microglia, in saline- and LPS-treated mice (Figure 3a). We first verified that the percentage of CD11b+/CD45low cells isolated from saline- and LPS-treated mice was not different (WT/saline: 87.62% WT/LPS: 87.56% Fat-1/saline: 85.2% and Fat-1/LPS: 86.53% on average) (Figure 3b).
Figure 3.
LPS modulates microglia phenotype in the brain of Fat-1 but not of WT mice. (a) Representative bivariate dot plots of Percoll-isolated cells gated on CD11b+/CD45low expression for microglia in saline or LPS-treated animals from both genotypes. (b) Average percentage of events that were CD11b+/CD45low in saline or LPS-treated animals from both genotypes. (c) Representative bivariate dot plots of Percoll-isolated cells gated on CD11b+/CD45low and CD36 or MHCII expression for microglia in saline or LPS-treated animals from both genotypes. (d) Average percentage of microglia that were CD36- or MHCII-positive. Data are expressed as mean±SEM (N=4 independent experiments; n=2 brains/N). **p<0.01.
Secondly, both TGF-β1 and COX-2 (significantly increased in LPS-treated Fat-1 mice) have previously been shown to favor anti-inflammatory/repair (M2) phenotype (Remington et al, 2007; Baitsch et al, 2011; Kawahara et al, 2012; Bhattacharjee et al, 2013; Chhor et al, 2013; Hirai et al, 2013). We thus determined the surface expression of MHCII and CD36 (two M2 phenotype markers) in the CD11b+/CD45low population (Figure 3c and d). We showed that Fat-1/LPS-treated animals had an increase in CD36 and MHCII surface expression 24 h post injection (CD36: interaction F(1,11)=8.91, p<0.05; Fat-1/LPS group different from the others, p<0.001; MHCII: interaction F(1,12)=7.21, p<0.05; Fat-1/LPS group different from the others, p<0.001).
LPS Decreases Food Intake and Body Weight in both WT and Fat-1 Mice
Overall, our data showed that in Fat-1 mice, pro-inflammatory factor synthesis was lowered compared with WT mice, whereas anti-inflammatory mechanisms were favored 24 h after LPS treatment. Thus, either Fat-1 mice display a lower LPS-induced inflammatory response compared with WT mice or it may be that the recovery from inflammation is faster in these mice. Both situations could explain the anti-inflammatory phenotype we observed 24 h post injection.
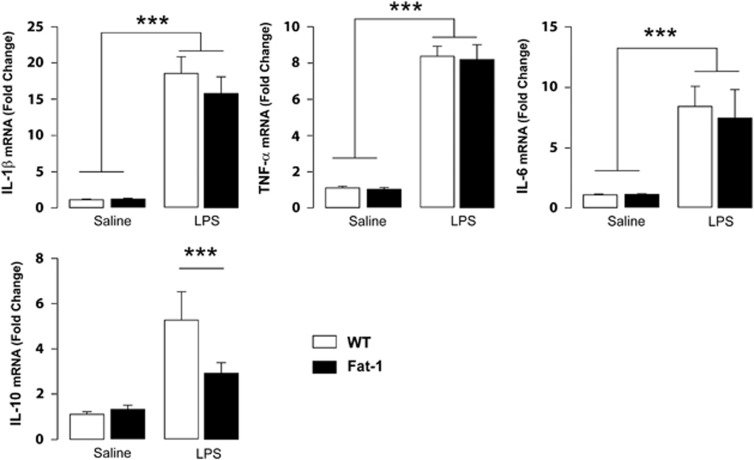
To address this question, we measured LPS-induced ‘sickness behavior' and cytokine expression in the first phase of inflammation (Dantzer et al, 2008). After LPS injection, activated microglia produce pro-inflammatory cytokines and secondary messengers that elicit a sickness behavior syndrome. Thus, WT and Fat-1 mice were injected with LPS or saline and cytokine expression was evaluated 2 h later in the hippocampus, ie, during the first wave of pro-inflammatory processes in the brain (Figure 4). IL-1β, IL-6, and TNF-α expression levels were increased 2 h post LPS injection at the same extent for both genotypes (IL-1β: treatment effect F(1,28)=100.0, p<0.0001; IL-6: treatment effect F(1,30)=23.62, p<0.0001; TNF-α: treatment effect F(1,30)=197.0, p<0.0001). Only IL-10 expression increase was significantly smaller 2 h post LPS injection in Fat-1 mice in comparison with WT littermates (treatment effect F(1,28)=20.97, p<0.0001; interaction F(1,28)=4.255, p<0.05; Fisher's LPSD post hoc test, p<0.05).
Figure 4.
LPS similarly alters the expression of cytokines in the hippocampus of WT and Fat-1 mice 2 h post injection. Quantification of cytokine mRNA expression 2 h after saline or LPS treatment in animals from both genotypes. Data are expressed as mean±SEM. ***p<0.01.
Regarding the sickness behavior, we sought to determine the effect of LPS on body weight and food consumption 2 and 24 h after the intraperitoneal injection in Fat-1 and WT mice (Table 3). Our results showed that LPS treatment induced a decrease in body weight in Fat-1 mice and WT littermates at 2 h (LPS effect F(1,32)=20.75, p<0.0001) and 24 h post-injection (LPS effect F(1,35)=82.761, p<0.001). Additionally, LPS injection also decreased food consumption in both groups at 2 h (LPS effect F(1,32)=18.78, p<0.0001) and 24 h post treatment (LPS effect F(1,35)=78.337, p<0.0001).
Table 3. Body Weight and Food Consumption of WT and Fat-1 Mice 2 and 24 h Post Treatment.
| Time after injection |
Saline |
LPS |
Statistical effects |
|||||
|---|---|---|---|---|---|---|---|---|
| WT N=10/12 | Fat-1 N=8/10 | WT N=9 | Fat-1 N=8/9 | LPS | Genotype | LP5 × genotype | ||
| Body weight loss | 2 h | +0.12±0.1 | +0.09±0.1 | +0.80±0.1 | +0.40±0.1 | <0.0001 | NS | NS |
| Food consumption | 2 h | 0.49.0±0.1 | 0.53±0.1 | 0.16±0.04 | 0.23±0.1 | <0.0001 | NS | NS |
| Body weight loss | 24 h | −0.18±0.2 | +0.07±0.4 | +1.42±0.1 | +1.67±0.2 | <0.0001 | NS | NS |
| Food consumption | 24 h | 5.18.0±0.2 | 4.75±0.2 | 3.22±0.2 | 2.68±0.3 | <0.0001 | <0.05 | NS |
Data are means±SEM.
DISCUSSION
This study is the first to report that Fat-1 mice that fed a standard diet had a higher level of n-3 PUFA content in the brain compared with WT animals. We also showed that LPS altered differently the phenotype of microglia and the expression of cytokines and chemokines in Fat-1 and WT mice. In Fat-1 mice, pro-inflammatory factor synthesis was lowered compared with WT mice, whereas anti-inflammatory mechanisms were favored 24 h after LPS treatment. It is likely that the recovery from inflammation was faster in Fat-1 mice explaining for such differences. Finally, we showed that LPS injection impaired spatial memory in WT mice, whereas interestingly, the Fat-1 mice showed normal cognitive performances. All together, these data suggested that the decrease of the central n-6/n-3 ratio observed in Fat-1 mice modulated the brain innate immune system activity, leading to the protection of animals against LPS-induced pro-inflammatory cytokine production and subsequent spatial memory alteration.
Dietary supplementation with n-3 PUFAs (mainly fish oil) is usually performed for enriching tissues with long-chain n-3 PUFAs such as EPA or DHA. This approach allows evaluating the effects of n-3 PUFAs in vivo. However, feeding animals with fish oil may induce confounding effects on the results because of other compounds provided in the fish oil such as vitamins. Thus, the use of the Fat-1 mice, a transgenic mouse line which expresses the C. elegans fat-1 gene and is capable of producing n-3 PUFAs from the n-6 type, is a model allowing the elimination of confounding factors provided by the diet. Moreover, we and others have previously demonstrated that a lifelong n-3 PUFA-deficient diet is related to increased depressive-like symptoms and spatial memory disturbance (Lafourcade et al, 2011; Moranis et al, 2012). Thus, both WT and Fat-1 mice were fed with a standard diet to avoid cognitive and emotional alterations in WT littermates. To verify that Fat-1 mice exposed to a standard diet indeed displayed a cerebral enrichment in n-3 PUFAs, we first measured hippocampal lipid fatty acid composition in both genotypes and showed that Fat-1 mice had a significant decrease of the n-6/n-3 ratio in the brain compared with WT littermates, even if exposed to a standard diet. However, contrary to what was demonstrated previously by Kang et al (2004), we could not find any effect of fat-1 gene on DHA levels in the hippocampus. This difference may be attributable to the lower levels of n-6 PUFA available in our standard chow compared with the deficient diet used by Kang et al (2004). It is thus likely that the fat-1-dependent n-6 to n-3 PUFA conversion is less effective in our experimental conditions, accounting for the lack of DHA increase. Of note, we found that tail fatty acid composition of Fat-1 mice contain more DHA (4.7% in Fat-1 vs 2.8% in WT), more EPA (1.6 vs 0.6%), and much less AA (0.8 vs 8.9%), showing a n-6/n-3 ratio of 1.2 in Fat-1 vs 6.0 in WT. Thus, Fat-1 mice displayed a classical and expected phenotype peripherally, ruling out a genetic drift of our colony. Consequently, the results that are presented in this manuscript are likely to be specific to the brain and/or are due to exposure of the mice to a standard diet.
However, Fat-1 mice interestingly displayed an increase of n-3 EPA and DPA levels in the hippocampus compared with WT littermates and a decrease in AA and docosatetraenoic acid levels, whereas amount of n-6 DPA did not change. These changes are in agreement with the effect of the fat-1 gene which can transform AA into EPA and docosatetraenoic acid into n-3 DPA (Kang, 2007). A previous study conducted on Fat-1 mice fed with an enriched n-6 PUFA diet could not find any difference in n-3 EPA and DPA levels measured after a whole brain lipid extraction (Orr et al, 2010). Here, we analyzed total fatty acid composition in the isolated hippocampus. It is thus likely that the slight but significant differences we found represented a hippocampus-specific effect.
An increasing number of publications attest of the powerful immunomodulatory effects of PUFAs in the brain (Calder, 2001; Laye, 2010; Orr et al, 2013) with n-3 PUFAs showing anti-inflammatory properties, whereas n-6 PUFAs stimulate the production and activity of inflammatory mediators. We thus hypothesized that LPS-induced cerebral immune system activation was limited in Fat-1 mice compared with WT littermates, accounting for a role of n-3 PUFAs on the basal activity of microglia. We thus quantified the expression levels of pro- and anti-inflammatory cytokines after LPS injection. Our data showed that IL-1β expression was much smaller in Fat-1 mice than in WT littermates 24 h after LPS injection. This confirms a previous report showing a significantly lower expression of IL-1β in the brain of Fat-1 mice, 24 h after an intracerebroventricular injection of LPS compared with WT littermates (Orr et al, 2013). Recently, Heerwagen et al (2013) also demonstrated that maternal expression of the fat-1 transgene protects mothers from increase in obesity-associated IL-1β overexpression in plasma. The reduced expression of IL-1β we observed in Fat-1 mice is of particular interest because this cytokine has been shown to specifically interfere with spatial learning which depends on normal hippocampal functioning (Gibertini et al, 1995; Song and Horrobin, 2004; Yirmiya and Goshen, 2011). Accordingly, using the Y-maze paradigm, we were able to show that 24 h after LPS injection, WT littermates spent the same amount of time in the novel and familiar arm whereas Fat-1 animals behaved like saline-injected animals, spending more time in the novel arm. This confirms our previous results demonstrating that during aging, a model of low grade inflammation, mice showed impaired spatial working memory when tested in the Y-maze, associated with an increase of IL-1β expression in the hippocampus (Labrousse et al, 2012). Short-term exposure to an EPA- and DHA-enriched diet was able to reduce IL-1β expression in the hippocampus and reverse spatial working memory deficits found in aged mice (Labrousse et al, 2012).Thus, reduced IL-1β expression may be one of the main mechanisms by which Fat-1 mice are protected against an immune challenge.
EPA and/or n-3 DPA may be good candidates to explain the modulation of IL-1β expression observed in Fat-1 mice. Interestingly, previous works have shown that specialized pro-resolving mediators derived from n-3 PUFAs, particularly from EPA, are anti-inflammatory and pro-resolving (Serhan et al, 2002; Serhan et al, 2008). Thus, a higher production of these specialized pro-resolving mediators may explain the anticipated recovery from inflammation we observed in Fat-1 mice. Indeed, we found almost no difference in the first wave of inflammatory factors induction (2 h post treatment), whereas Fat-1 mice displayed a faster recovery from inflammation (24 h post treatment). Moreover, it has been shown that EPA and/or EPA derivates are able to downregulate microglial activation (Kelly et al, 2011).
To further characterize the impact of the fat-1 gene on microglial physiology, we looked at microglial markers by flow cytometry. Notably, cell-sorting experiments revealed a higher proportion of CD36- and MHCII-positive microglial cells in Fat-1 mice treated with LPS, these two phenotypic markers being described as more anti-inflammatory and/or neuroprotective (Kawahara et al, 2012). All together, our results tend to show that the fat-1 gene conferred protection against an inflammatory challenge to the mice, characterized by a lower expression of pro-inflammatory markers and a switch of microglial phenotype towards a more anti-inflammatory/neuroprotective profile.
The increased expression of TGF-β1 that we observed in Fat-1 mice may explain, at least in part, the reduced inflammatory episode observed after an immune challenge as well as phenotypic modifications of microglia. Indeed, previous works conducted in vitro have shown that TGF-β1 decreases microglial activation over the course of an inflammatory challenge (Merrill and Zimmerman, 1991). Exposure to TGFβ is also known to induce a specific phenotype in macrophages, termed M2c. This phenotype is characterized by high levels of anti-inflammatory cytokines and low levels of pro-inflammatory cytokines production (Mantovani et al, 2005). Of note, we found that COX-2 and mPGES1 mRNA expression was significantly increased in Fat-1 mice 24 h after LPS treatment. Although these enzymes are known as markers of the M2c phenotype, they may also display a pro-inflammatory action in certain conditions (Matsumura et al, 1998; Quan et al, 1998; Nadjar et al, 2010). However, a recent in vivo study also suggested a role of PGE2, a well-described prostaglandin produced in the brain as a product of COX-2/mPGES1 activity, in resolution of neuroinflammation through EP2 activation (Brenneis et al, 2011). Moreover, not only AA but also EPA is metabolized by COX-2. As a result, EPA-derived eicosanoids (3-series prostaglandins, PGE3) are generated. EPA-derived 3-series prostanoids have much less pro-inflammatory activity than AA-derived eicosanoids (reviewed in (Farooqui et al, 2007). Indeed, increasing omega-3 content of membrane phospholipid of macrophages results in a decrease in PGE2 synthesis in vitro (Bagga et al, 2003). In vivo, the ratio of PGE2/PGE3 is greatest in n-3 PUFA-fed animals and lowest in those fed with linseed oil (Henderson et al, 1996). In our work, Fat-1 mice display increased COX-2 mRNA expression in the hippocampus 24 h after a LPS treatment. In addition, Fat-1 mice fed with a regular chow display decreased AA and increased EPA level in the brain. However, whether decreased AA/EPA ratio in the brain of Fat-1 lead to decreased PGE2/PGE3 production in the brain of LPS-treated mice remains to be determined.
Moreover, as another plausible mechanism for lower pro-inflammatory cytokine production and microglia phenotype modification, we found that expression levels of CX3CR1 and its ligand CX3CL1 (or fractalkine) were increased in Fat-1 mice. In the central nervous system, the fractalkine is expressed by neurons whereas its receptor is exclusively expressed by microglia. In this regard, CX3CR1-CX3CL1 signaling represents one communication pathway between microglia and neurons and has shown to control the overproduction of several pro-inflammatory cytokines (Biber et al, 2007). Evaluation of several pathological processes showed that signaling through the fractalkine receptor reduced neuronal damage (Cardona et al, 2006; Ransohoff, 2009). CX3CR1 signaling has also been shown to modulate microglial activation and protects against cognitive deficits in a mouse model of Alzheimer's disease (Cho et al, 2011). In addition, genetic disruption of CX3CL1 signaling increases the level of IL-1β in the hippocampus of mice, which is involved in the spatial memory deficit observed in these mice (Rogers et al, 2011). Thus, increased expression of the fractalkine system observed in Fat-1 mice may lead to neuroprotection and subsequent decreased action of inflammatory processes on neuronal activity. This remains to be demonstrated in the context of LPS injection.
The present study looks at the protective effect of n-3 PUFAs on the effect of inflammation on both brain microglia activity and spatial memory using a model that excludes dietary confounding factors. Our results indicate that a greater hippocampal n-3/n-6 PUFA ratio may be responsible for reduced LPS-induced neuroinflammatory activation in Fat-1 mice. Specifically, the production of IL-1β was reduced in these mice under inflammatory conditions in addition to a shift of microglia towards a more neuroprotective phenotype. The results of this study not only provide insight into how n-3 PUFAs can influence microglia activity but also suggest a role for EPA and DPA in the prevention of spatial memory deficit linked to inflammation.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Dr Guillaume Ferreira for helpful comments on the manuscript. We also thank Dr Guillaume Ferreira and Pr Muriel Darnaudery for helping with behavioral experiments. We acknowledge the technical support from V. Pitard and S. Gonzalez from the cytometry platform at the Structure Federative de Recherche TransBioMed at the University of Bordeaux Segalen. We thank J.C. Helbling and C. Tridon for transgenic mice genotyping. We also thank P. Birac, C. Tridon, and M. Cadet for taking care of the mice. This research was financially supported by INRA, ANR, and Fondation pour la Recherche Medicale (FRM). JCD is the recipient of a postdoctoral fellowship from the Région Aquitaine. This project was also supported by the grant LABEX BRAIN ANR-10-LABX-43.
References
- Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Jpn J Pharmacol. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behav Immun. 1995;9:129–148. doi: 10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch D, Bock HH, Engel T, Telgmann R, Muller-Tidow C, Varga G, et al. Apolipoprotein E induces antiinflammatory phenotype in macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1160–1168. doi: 10.1161/ATVBAHA.111.222745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, Cathcart MK. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Boudrault C, Bazinet RP, Kang JX, Ma DW. Cyclooxygenase-2 and n-6 PUFA are lower and DHA is higher in the cortex of fat-1 mice. Neurochem Int. 2010;56:585–589. doi: 10.1016/j.neuint.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Brenneis C, Coste O, Altenrath K, Angioni C, Schmidt H, Schuh CD, et al. Anti-inflammatory role of microsomal prostaglandin E synthase-1 in a model of neuroinflammation. J Biol Chem. 2011;286:2331–2342. doi: 10.1074/jbc.M110.157362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chhor V, Le Charpentier T, Lebon S, Ore MV, Celador IL, Josserand J, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, et al. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. J Neurochem. 2007;101:577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE. Transgenic increase in N-3/n-6 Fatty Acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One. 2013;8:e67791. doi: 10.1371/journal.pone.0067791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RJ, Bell JG, Park MT. Polyunsaturated fatty acid composition of the salmon (Salmo salar L.) pineal organ: modification by diet and effect on prostaglandin production. Biochim Biophys Acta. 1996;1299:289–298. doi: 10.1016/0005-2760(95)00213-8. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Uchida K, Nakajima H, Guerrero AR, Takeura N, Watanabe S, et al. The prevalence and phenotype of activated microglia/macrophages within the spinal cord of the hyperostotic mouse (twy/twy) changes in response to chronic progressive spinal cord compression: implications for human cervical compressive myelopathy. PLoS One. 2013;8:e64528. doi: 10.1371/journal.pone.0064528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX. Fat-1 transgenic mice: a new model for omega-3 research. Prostaglandins Leukot Essent Fatty Acids. 2007;77:263–267. doi: 10.1016/j.plefa.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Suenobu M, Yoshida A, Koga K, Hyodo A, Ohtsuka H, et al. Intracerebral microinjection of interleukin-4/interleukin-13 reduces beta-amyloid accumulation in the ipsilateral side and improves cognitive deficits in young amyloid precursor protein 23 mice. Neuroscience. 2012;207:243–260. doi: 10.1016/j.neuroscience.2012.01.049. [DOI] [PubMed] [Google Scholar]
- Kelly L, Grehan B, Chiesa AD, O'Mara SM, Downer E, Sahyoun G, et al. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging. 2011;32:e2311–e2315. doi: 10.1016/j.neurobiolaging.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Labrousse VF, Costes L, Aubert A, Darnaudery M, Ferreira G, Amedee T, et al. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS One. 2009;4:e6006. doi: 10.1371/journal.pone.0006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Gregoire S, et al. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS One. 2012;7:e36861. doi: 10.1371/journal.pone.0036861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- Larrieu T, Madore C, Joffre C, Laye S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J Physiol Biochem. 2012;68:671–681. doi: 10.1007/s13105-012-0179-6. [DOI] [PubMed] [Google Scholar]
- Laye S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot Essent Fatty Acids. 2010;82:295–303. doi: 10.1016/j.plefa.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Madore C, Joffre C, Delpech JC, De Smedt-Peyrusse V, Aubert A, Coste L, et al. Early morphofunctional plasticity of microglia in response to acute lipopolysaccharide. Brain Behav Immun. 2013;34:151–158. doi: 10.1016/j.bbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Zimmerman RP. Natural and induced cytotoxicity of oligodendrocytes by microglia is inhibitable by TGF beta. Glia. 1991;4:327–331. doi: 10.1002/glia.440040311. [DOI] [PubMed] [Google Scholar]
- Mingam R, Moranis A, Bluthe RM, De Smedt-Peyrusse V, Kelley KW, Guesnet P, et al. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur J Neurosci. 2008;28:1877–1886. doi: 10.1111/j.1460-9568.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranis A, Delpech JC, De Smedt-Peyrusse V, Aubert A, Guesnet P, Lavialle M, et al. Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav Immun. 2012;26:721–731. doi: 10.1016/j.bbi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Sauvant J, Combe C, Parnet P, Konsman JP. Brain cyclooxygenase-2 mediates interleukin-1-induced cellular activation in preoptic and arcuate hypothalamus, but not sickness symptoms. Neurobiol Dis. 2010;39:393–401. doi: 10.1016/j.nbd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Nair A, Hunzeker J, Bonneau RH. Modulation of microglia and CD8(+) T cell activation during the development of stress-induced herpes simplex virus type-1 encephalitis. Brain Behav Immun. 2007;21:791–806. doi: 10.1016/j.bbi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SK, Tong JY, Kang JX, Ma DW, Bazinet RP. The fat-1 mouse has brain docosahexaenoic acid levels achievable through fish oil feeding. Neurochem Res. 2010;35:811–819. doi: 10.1007/s11064-010-0139-x. [DOI] [PubMed] [Google Scholar]
- Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, et al. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127:378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Quan N, Whiteside M, Herkenham M. Cyclooxygenase 2 mRNA expression in rat brain after peripheral injection of lipopolysaccharide. Brain Res. 1998;802:189–197. doi: 10.1016/s0006-8993(98)00402-8. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Remington LT, Babcock AA, Zehntner SP, Owens T. Microglial recruitment, activation, and proliferation in response to primary demyelination. Am J Pathol. 2007;170:1713–1724. doi: 10.2353/ajpath.2007.060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, et al. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of diet and essential fatty acids. World Rev Nutr Diet. 2001;88:18–27. doi: 10.1159/000059742. [DOI] [PubMed] [Google Scholar]
- Song C, Horrobin D. Omega-3 fatty acid ethyl-eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1beta administration. J Lipid Res. 2004;45:1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Kohman RA, Scott VJ, Boehm GW. Bacterial endotoxin-induced behavioral alterations in two variations of the Morris water maze. Physiol Behav. 2005;86:244–251. doi: 10.1016/j.physbeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ide M, Shibutani T, Ohtaki H, Numazawa S, Shioda S, et al. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res. 2006;83:557–566. doi: 10.1002/jnr.20752. [DOI] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]