Abstract
Drug addiction is associated with a relative devaluation of natural or socially-valued reinforcers that are unable to divert addicts from seeking and consuming the drug. Before protracted drug exposure, most rats prefer natural rewards, such as saccharin, over cocaine. However, a subpopulation of animals prefer cocaine over natural rewards and are thought to be vulnerable to addiction. Specific behavioral traits have been associated with different dimensions of drug addiction. For example, anxiety predicts loss of control over drug intake whereas sensation seeking and sign-tracking are markers of a greater sensitivity to the rewarding properties of the drug. However, how these behavioral traits predict the disinterest for natural reinforcers remains unknown. In a population of rats, we identified sensation seekers (HR) on the basis of elevated novelty-induced locomotor reactivity, high anxious rats (HA) based on the propensity to avoid open arms in an elevated-plus maze and sign-trackers (ST) that are prone to approach, and interaction with, reward-associated stimuli. Rats were then tested on their preference for saccharin over cocaine in a discrete-trial choice procedure. We show that HR rats display a greater preference for saccharin over cocaine compared with ST and HA whereas the motivation for the drug was comparable between the three groups. The present data suggest that high locomotor reactivity to novelty, or sensation seeking, by predisposing to an increased choice toward non-drug rewards at early stages of drug use history, may prevent the establishment of chronic cocaine use.
INTRODUCTION
Over the course of cocaine addiction, loss of control and compulsivity develop (DSM-IV, 2000; Everitt and Robbins, 2005) and the individual's behavior focuses exclusively on means to obtain and consume the drug at the expense of other sources of reinforcement. This narrowing of interest that contributes to the chronicity of addiction, has been suggested to depend upon a cocaine-induced overvaluation of the motivational properties of the drug over other natural or socially-valued reinforcers (Hyman et al, 2006). However, this relative devaluation of natural sources of reinforcement observed in cocaine addicts could potentially originate from a spontaneous lower interest in natural reinforcers before any exposure to the drug, resulting in an increased preference for the drug during the first stages of drug exposure before the onset of addiction. The latter hypothesis implies that pre-existing individual differences in the choice of cocaine over an alternative reinforcer during the early stages of exposure to cocaine may be a marker of vulnerability to addiction (Lenoir et al, 2007; Cantin et al, 2010; Ahmed, 2012; Ahmed et al, 2013).
In rats self-administering cocaine it has been demonstrated that deprivation of a sweetened solution induces an increase in instrumental responding for the drug (Carroll and Boe, 1982) whereas the availability of a sweet beverage during the session impairs, or reduces, the acquisition and maintenance of cocaine self-administration, respectively (Carroll et al, 1989). Although it has been demonstrated that the availability of alternative reinforcers alters the acquisition of cocaine self-administration, these studies did not address specifically the choice preference that rats may display toward the natural reinforcer or the drug. This can be measured in discrete-trial choice procedures, which assess the relative preference for two different rewards being offered as two mutually exclusive options, associated with the delivery of two distinct reinforcers (Griffiths et al, 1975; Aigner and Balster, 1978; Young, 1981). Recent studies by Ahmed and colleagues have demonstrated that when rats are offered the mutually exclusive choice between cocaine and saccharin, most display a preference for saccharin over the drug (Lenoir et al, 2007) although a minority of rats, about 15%, show a preference for cocaine over saccharin.
These inter-individual differences in the choice for cocaine during early stages of drug exposure in rats have been suggested to represent a novel operationalization of vulnerability to cocaine addiction whereby a spontaneous disinterest toward natural rewards expressed after a brief exposure to cocaine is suggested to facilitate the subsequent development of addiction (Ahmed, 2010). However, vulnerability to addiction is a multifaceted construct, (Everitt et al, 2008; Belin and Deroche-Gamonet, 2012) with several factors contributing differentially to the distinct stages of drug use that ultimately leads to addiction. These range from the individual propensity to use drugs to the increased motivation toward the drug and eventually the loss of control over drug intake that becomes compulsive.
We and others have identified behavioral traits in rats, such as high anxiety, that predict both increased motivation for cocaine (Homberg et al, 2002) and increased vulnerability to switch from controlled to escalated cocaine self-administration (Dilleen et al, 2012). These factors contributing to the development of addiction-like behaviors have been shown to be, at least partly, dissociable (Belin et al, 2008, 2011; Molander et al, 2011) from factors that instead predict an increased sensitivity to the associative and motivational properties of cocaine (Flagel et al, 2008; Robinson and Flagel, 2009; Meyer et al, 2012b) and a greater propensity to acquire cocaine self-administration (Belin et al, 2008), namely the sign-tracking (Tomie et al., 1989, 2008) and high locomotor response to novelty traits (Piazza et al, 1989), respectively.
Despite the heuristic value of choice procedures for the understanding of the psychobiological substrates of addiction, it remains to be established whether the spontaneous choice preference for cocaine is associated with behavioral traits of either increased sensitivity to the drug or vulnerability to develop addiction-like features of drug self-administration.
We therefore investigated, in a longitudinal study in rats, whether individual propensity to choose cocaine over a non-drug, alternative reinforcer that is not biologically essential, namely saccharin, is associated with traits of increased vulnerability to use cocaine, such as high locomotor response to novelty, or to lose control over and relapse to, cocaine self-administration such as high anxiety and sign-tracking.
MATERIALS AND METHODS
Animals
Sixty adult male Sprague Dawley rats from Charles River (Lyon, France), weighing 225 g upon arrival, were housed two per cage under a reversed 12-h light/dark cycle, lights on at 1900h. After intravenous surgery, rats were individually housed. Animals had ad libitum access to water and were fed with 20 g/rat/day of standard chow pellets throughout the experiment except during the choice procedure when they had ad libitum access to the food.
All experiments were carried out in accordance with institutional and international standards of care and use of laboratory animals (UK Animals (Scientific Procedures) Act, 1986; and associated guidelines; the European Communities Council Directive (86/609/EEC, 24 November 1986) and the French Directives concerning the use of laboratory animals (décret 87-848, 19 October 1987)).
Surgery
Rats were implanted with chronic intravenous jugular catheters as previously described (Belin and Everitt, 2008). The indwelling catheter (internal diameter: 0.28 mm; external diameter: 0.61 mm; dead volume: 12 μl) was inserted through the right jugular vein into the right atrium and exited dorsally between the scapulae. Rats were given 12 days to recover from the surgery before any behavioral test. During the period of recovery, rats received an antibiotic treatment for 7 days (0.2 ml Baytril s.c.) and catheters were flushed daily with 0.1–0.2 ml heparanized saline to maintain their patency (50 U/ml in 0.9% sterile saline; Sanofi-Aventis, Germany).
Apparatus
Locomotor reactivity to novelty
Novelty-induced locomotor reactivity was measured in four white open fields (50 × 50 × 50 cm) placed on an infra-red white floor (1 × 1 m, Viewpoint Life Science, France), that was located in a bright room (555.5±7.84 lux). Horizontal locomotor activity was recorded using a video-tracking system (Viewpoint Life Science) in 1 min blocks.
Anxiety
Anxiety was measured on an elevated-plus maze (EPM; Viewpoint Life Science) constituted of a central platform (10 × 10 cm) surrounded by two open arms and two enclosed arms (45 cm long × 10 cm width, walls 45 cm high) in the shape of a cross, elevated 80 cm above an infra-red white floor (1 × 1 m). Entries and time spent in the open and closed arms as well as locomotor activity were monitored by a video-tracking system (Viewpoint Life Science) in 30 s blocks. The illumination in the open arms, closed arms and central platform was 49.5±0.65, 28±0.91, and 40±0.00 lux, respectively.
Operant chambers
The set-up consisted in 12 boxes made of plexiglass and metal enclosed in wooden, sound-attenuating, ventilated cubicles (Med Associated, Sandown Scientific). Autoshaping, cocaine preference, and cocaine self-administration procedures took place in the same chambers, but with different configurations, to reduce the impact of similar testing environment. In all procedures, experimental contingencies were controlled and data collected with a PC window-compatible software (MedPC IV, Med Associates).
Autoshaping
Small chambers (31.8 cm long × 25.4 cm width × 26.7 cm high) were equipped with a house light and a magazine, connected to a dispenser that distributed 45 mg dustless precision pellets (Bio Serv), which was placed on the same wall as a retractable lever above which a light was positioned. An inactive, non-restractable lever was placed on the opposite side of the CS lever.
Cocaine self-administration
Self-administration chambers have been previously described (Murray et al, 2012). Chambers had higher walls than for the autoshaping procedure (31.8 cm long × 25.4 cm width × 34.3 cm high) and were equipped with two non-retractable levers used as devices to record responding. A cue light was located above each lever and a white house light was located at the top of the chamber to allow its complete illumination. Animals were placed daily in the chamber and their implanted catheter was connected to a pump-driven syringe by a silastic tubing shielded with a metal spring and extended with a Tygon tubing. Infusion speed was 20 μl/s.
Cocaine vs saccharin preference
The same chambers as described for cocaine self-administration were used except that one lever was replaced by a wheel. A light cue was positioned above the wheel and a retractable dipper delivered small volumes of saccharin solution in the magazine. A clicker and a tone, placed on the wall opposite to the lever, wheel and magazine, were used as signals of drug or saccharine availability.
General Procedure
Forty-eight rats (experiment 1) were tested in the different behavioral tasks according to the timeline summarized in Figure 1. In a separate experiment (experiment 2), 12 rats were tested in the choice procedure with reversed contingencies to control for the effect of the nature of the instrumental response on the choice between cocaine and saccharin.
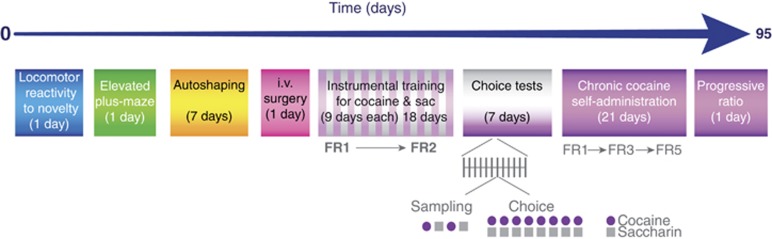
Figure 1.
Time course of the experiments. After 1 week of habituation to the facility, rats were tested for their locomotor reactivity to a novel inescapable environment in an open field. Following 8 days without test, the level of anxiety was assessed in an elevated-plus maze and after 11–12 days off, the sign-tracking phenotype was evaluated using an autosaping paradigm. Then, rats were implanted with a catheter in the right jugular vein and, following a week of recovery, they were trained to lever press for an i.v. infusion of 0.8 mg/kg cocaine and to turn a wheel to gain access to 0.2% of saccharin during 9 days under a fixed ratio (FR) that was increased from 1 to 2. Once the animals had acquired both instrumental responses, they were tested for their preference for cocaine over saccharin during seven sessions consisting in twelve trials (gray vertical bars), each one being composed of four samplings, where the rats could respond either for cocaine (black circles) or for saccharin (gray squares) alternately followed by eight tests where the two rewards were available but mutually exclusive. A second cohort of 12 rats were trained with the reversed contingencies to control potential effect of the manipulanda on the choice between saccharin and cocaine. Finally, following 17 days of cocaine self-administration under an FR5, the animals were tested for their motivation for cocaine in a progressive ratio schedule. Gray numbers at the top represent the number of days elapsed between tests and at the bottom the schedules are indicated.
After 2 weeks of habituation to the facility rats in experiment 1 started training with the initial test consisting in an exposure to an inescapable unknown environment to measure their locomotor reactivity to novelty. Rats were placed individually in an open field for 2 h. Testing was carried out during the light phase (between 2000 and 0830 h) to maximize behavioral differences (Belin et al, 2011).
Anxiety
A week after exposure to the open field, rats were tested on the EPM. Each rat was placed on the central platform of the EPM and allowed access to the four arms for 5 min (Molander et al, 2011). Three rats were excluded from all the between-subject and dimensional analyses involving anxiety because they fell from the maze (n=2) or because of a failure in data recording (n=1).
Autoshaping
Ten days after EPM testing, rats were habituated to dustless precision pellets (25 pellets per rat) in their home cage then to the magazine in the testing boxes by delivery of 50 pellets under a 30-s variable interval (VI) schedule for 2 sessions. Then, rats underwent a Pavlovian-conditioning training consisting of 25 presentations of a retractable lever (CS lever) and a cue light for an 8-s duration immediately followed by the delivery of a pellet. Presentations were initiated based on a 90-s variable interval schedule. The cue light was turned off and the lever retracted following reward delivery. Lever presses and head entries into the magazine during the 8-s CS presentation were used as indices of sign vs goal-tracking, respectively (Flagel et al, 2011).
Cocaine vs saccharin preference
The protocol has been adapted from a previous study (Lenoir et al, 2007). After 2 weeks of recovery from i.v. surgery, rats were trained daily to oral consumption of saccharin and intravenous self-administration of cocaine, nine sessions each, for a total of 18 days. The nature of the reinforcer was signalled at the beginning of each session by a click or a 10-ms tone (counterbalanced between rats) and by the presence of the wheel or the lever in the operant chamber. For each half turn of the wheel, animals gained access to a 0.2% saccharin solution for a period of 50 s signalled by the cue light above the wheel. On separate sessions, pressing the lever resulted in an infusion of cocaine (0.25 mg/100 μl/infusion) followed by a 50-s time-out period signalled by the cue above the lever.
For each reinforcer, a fixed ratio (FR) 1 schedule was applied for the first 6 days followed by 3 days with an FR2 schedule. Sessions ended after either 30 deliveries of the reinforcer or 2 h elapsed. Preference for cocaine was tested during sessions composed of 12 discrete trials, separated by 10-min intervals. Trials started by the illumination of the operant chamber, the emission of a sound (click and/or tone) and the presentation of the lever when cocaine was available. At the beginning of each trial, rats could respond either for cocaine (Coc) or for saccharin (Sac) on the following schedule: Coc-Sac-Coc-Sac during four sampling tests. After two consecutive responses on the appropriate device, the reward was delivered and the corresponding cue light was turned on. Then, during eight preference tests, both reinforcers were available, but mutually exclusive. Rats had to choose between turning the wheel and pressing the lever to earn the corresponding reward. If rats failed to respond within 5 min or responded successively on two different devices then the trial was reset. During inter-trial intervals, the house light was switched off and the lever retracted. In experiment 2, we controlled any effect of the device on the preference for cocaine by testing a new cohort of 12 rats on a discrete-trial choice procedure where lever press allowed access to saccharin and wheel turn resulted in cocaine infusions.
Cocaine self-administration
The self-administration procedure has been previously described (Belin et al, 2009; Belin and Deroche-Gamonet, 2012). After the discrete-trial choice procedure, rats underwent daily self-administration (SA) sessions composed of three drug components (40 min each) signalled by the house light on and separated by 15 min of drug-free periods signalled by the house light switched off. During the ‘no-drug' periods, lever presses were without scheduled consequences. During the ‘drug' periods, press on one lever turned on the white cue light above it and turned on the infusion pump. The cue light remained on for a total of 5 s. Presses on the other lever had no scheduled consequences. Each infusion (0.25 mg/100 μl/5.7 s) was followed by a 40-s time-out period. During the first 3 days, an FR1 schedule of reinforcement was applied followed by an FR3 (one session) and finally by an FR5 for the rest of the experiment.
After 17 days of self-administration, motivation for the drug was tested in a progressive ratio schedule of reinforcement (Belin and Deroche-Gamonet, 2012). During this session, drug availability was signalled by the illumination of the chamber. The ratio of responses per infusion was increased after each infusion according to the following progression: 10, 20, 30, 45, 65, 85, 115, 145, 185, 225, 275, 325, 385, 445, 515, 585, 665, 745, 835, 925, 1025, 1125, 1235, 1345, 1465, and 1585. The maximal number of responses that a rat performed to obtain one infusion (the last ratio completed) is referred to as the break point. The session ceased after either 6 h or when a period of 1 h elapsed since the previously earned infusion.
Drugs
Cocaine hydrochloride (Coopération Pharmaceutique Française) was dissolved in sterile 0.9% NaCl. Saccharin solutions (Sigma-Aldrich) were mixed fresh daily and dissolved in tap water at a final concentration of 0.2% as previously described (Lenoir et al, 2007).
Data Analyses
For each behavioral measure, the nature of the distribution of the population was tested. Then, animals were ranked according to their performance and, given that no objective physical criteria could be applied on the distributions to identify subpopulations (such as a bimodal distribution, see Belin et al, 2011 for further details), the upper and lower quartiles were selected for between-subject analyses as previously described (Belin et al, 2008, 2011; Dilleen et al, 2012). This approach identifies homogenous populations, maximizes differences between these populations, limits type I errors, and prevents type II errors (Cain et al, 2005).
For the locomotor reactivity to novelty, rats were ranked according to their total traveled distance during the 2-h session in the open field. In the autoshaping paradigm, the average number of CS-lever presses during the sessions of Pavlovian conditioning with stable performance (ie, the last three sessions) was used as the index of si (OA) of the EPM ((time spent in open arms)/(time spent in open and closed arms) × 100). The number of lever presses for cocaine and wheel turns for saccharin were recorded during the discrete-trial choice and cocaine preference was measured by the percentage of cocaine choice ((number of cocaine infusions)/(number of cocaine infusions+number of accesses to saccharin)). A percentage above 50% was an index of cocaine preference whereas a percentage under 50% indicated a preference for saccharine. A percentage of 50% indicated indifference between cocaine and saccharin. A summary of the behavioral tests and associated measures is provided in Supplementary Table S1.
Statistical Analyses
Figures show group means ±SEM. Statistical analysis were carried out with Statistica (StatSoft). Pearson's Chi2 test was used to analyze traits representativity and Pearson's correlation analysis to assess the dimensional relationship between traits. The variables used were total distance run over the 2-h exposure to an open field (locomotor reactivity to novelty), percentage of time spent in the OA of the EPM (anxiety), number of lever contacts during the 8-s compound CS presentation averaged over the last three sessions of autoshaping (sign-tracking) and the percentage of choices toward cocaine in the last session of the exclusive choice procedure (cocaine choice).
Repeated-measures analyses of variance (ANOVA) with behavioral traits as the between-subject factor and time as the within-subject factor were used to analyze main group effects and interactions. Upon confirmation of main effects, Newman–Keuls post hoc tests were applied for pairwise comparisons.
Cocaine preference was tested with a Student's t-test for a comparison of single means to the fixed value of 50%.
For all analyses, statistical significance was accepted at p<0.05.
RESULTS
Anxiety, Locomotor Reactivity to Novelty, and Autoshaping Are Independent Dimensions
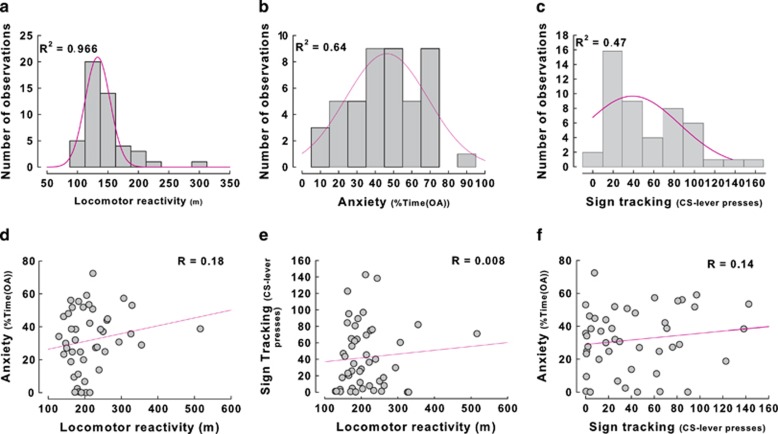
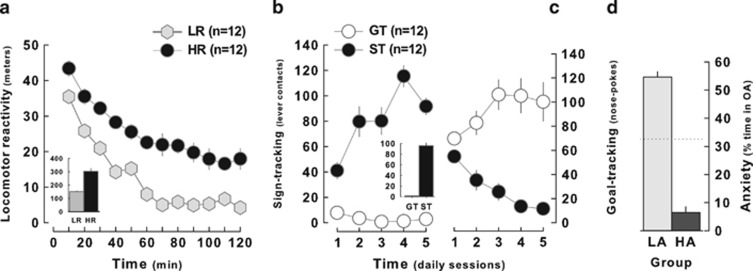
Sensation seeking, anxiety, and sign-tracking were characterized by normal distributions (R2s=0.97, 0.64, and 0.47, respectively) (Figure 2a–c) that were not correlated with each other (Supplementary Table S2) (sensation seeking × anxiety: R=0.17; sensation seeking × sign-tracking: R=0.06; anxiety × sign-tracking: R=0.14) (Figure 2d and e). However, marked inter-individual differences were revealed such that high responders (HR, n=12) displayed much higher locomotor response to novelty than low responders (LR, n=12) (main effect of group: F1,22=44.2, p<0.01, time: F11,242=95.87, p<0.01 and group × time interaction: F11,242=2.26, p<0.05) (Figure 3a). Similarly, in an autoshaping procedure, sign-trackers (ST) progressively increased the interactions with the CS lever, as shown by the growing number of lever contacts over sessions, whereas goal-trackers (GT) were never interested by the CS-lever stimulus, but instead developed a rigid approach of the goal, ie, the magazine (group × approach location: F1,22=103.21; p<0.01; group × session × approach location: F4,88=25.127; p<0.01) (Figure 3b). On the EPM, rats with high level of anxiety (HA) spent significantly less time in the open arms compared with low anxious (LA) rats (effect of group: F1,22=267.02, p<0.01) (Figure 3c).
Figure 2.
Locomotor reactivity to novelty, anxiety, and sign-tracking are uncorrelated behavioral traits. (a) Sensation-seeking, (b) anxiety and (c) sign-tracking followed a normal distribution. These behavioral traits represented distinct dimensions as shown by the lack of correlation between (d) anxiety and locomotor reactivity to novelty, (e) sign-tracking and locomotor reactivity to novelty, and (f) anxiety and autoshaping.
Figure 3.
Inter-individual differences in locomotor reactivity to novelty, sign-tracking, and anxiety. (a) High responders (HR, n=12) displayed slower locomotor habituation than low responders (LR, n=12) to a novel unescapable environment (insert: total traveled distance during the session). (b) Sign-trackers (ST, n=12) spent more time interacting with the food-associated stimulus (left) whereas goal-trackers (GT, n=12) displayed more interest for the magazine (right). The insert represents the average number of press on the CS lever. (c) High anxious rats (HA, n=12) spent less time in the open arms (OA) of the elevated-plus maze (EPM) than low anxious (LA, n=12) rats.
These behavioral traits were apparently not overlapping in that HA and ST rats displayed similar locomotor reactivity to novelty as LA and GT rats, respectively (ST vs GT: main effect of time: F11,242=92.81; p<0. 01 and group × time interaction: F1,22 <1; HA vs LA: main effect of time: F11,242=110.75; p<0.01 and group × time interaction: F1,22=1.13, p>0.2) (Supplementary Figure S1a and b) while in the autoshaping procedure neither HR and LR nor HA and LA rats presented a differential goal or sign-tracking phenotype as shown by the similar increase in the number of lever contacts and visits to the magazine over the sessions (HR vs LR: response × time interaction: F4,88=3.79; p<0.01 and trait × response × time interaction: F1,22<1; HA vs LA: response × time interaction: F4,88=7.13; p<0.01, trait × response × time interaction: F4,88=1.62; p>0.1) (Supplementary Figure S1c and d). Similarly, when compared on their anxiety as measured on the EPM, HR did not differ from LR rats (effect of group: F1,21<1) and the trend toward a lower anxiety observed in ST as compared with GT did not reach statistical significance (F1,22=2.51, p>0.1) (Supplementary Figure S1e).
Inter-individual Differences in the Acquisition of Cocaine and Saccharin Self-administration
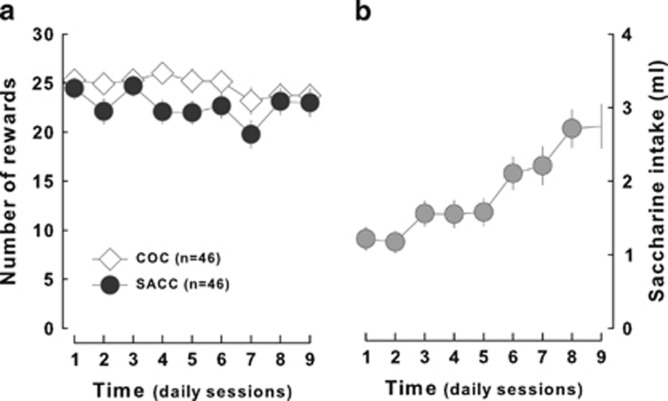
Rats were then implanted with a catheter in the jugular vein and were subjected to an instrumental training with either cocaine or saccharin as reinforcers. Thus on alternative days, pressing a lever resulted in the delivery of an infusion of 0.8 mg/kg cocaine whereas turning a wheel was rewarded by the delivery of sweetened water (0.2%, delivery of a maximum of 3 ml for 50 s). Both instrumental responses were acquired as early as the first session and led to a daily level of access to rewards, similar between the two reinforcers, that was stable throughout the training (main effect of reinforcer: F1,45=2.63, p>0.1; reinforcer × time interaction: F8,360=1.54, p>0.1) (Figure 4a). For each 10-min discrete trial, contrary to cocaine which was infused immediately after the lever press, without any additional behavioral requirement, saccharin was consumed by repeated licking of a spout coming back and forth in the magazine during 50 s provided the animal maintained his head in the magazine. Over sessions, the volume of saccharin intake per access increased progressively to rapidly reach an asymptote (at the eighth session) that reflected that the animals consumed as much as they possibly could (main effect of time: F8,360=15.86; p<0.01) (Figure 4b). As soon as session 2 of training, the access to saccharin (number of opportunities to drink saccharin) was correlated with the quantity of saccharin drunk by the animals (all R>0.32, p's<0.05), reflecting a relationship between preparatory and consummatory responses for saccharin.
Figure 4.
Acquisition of the instrumental response for saccharin and cocaine. (a) Rats displayed no difference in the acquisition of the instrumental response for cocaine (COC, light diamonds) or saccharin (SACC, dark circles) in the discrete-trial choice procedure. In this test, rats pressed a lever to self-administer cocaine and turned a wheel to gain access to saccharin. (b) Moreover, they learned how to earn maximal quantity of saccharin as their intake (gray circles) progressively increased.
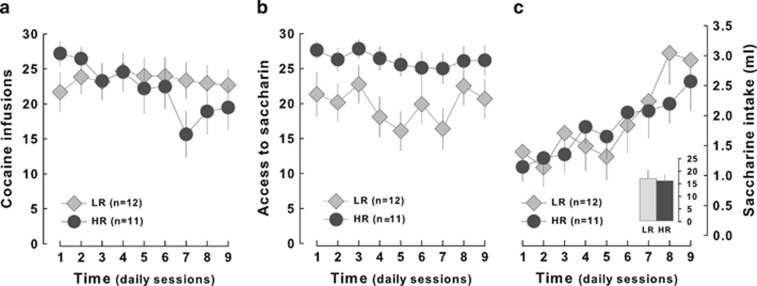
We then assessed the influence of the different behavioral traits on the acquisition of cocaine vs saccharin self-administration. Interestingly, HR and LR rats differed in their overall access to cocaine vs saccharin rewards (trait × reinforcer interaction: F1,22=7.19, p<0.05) (Figure 5a and b) in that HR rats displayed a tendency to access saccharin more often than they infused cocaine (F1,11=4.77; p=0.051) while LR rats maintained a higher level of cocaine infusions than they had access to saccharin over the course of the training (F8,88=2.25, p<0.05). Additionally, over the course of time, HR rats displayed a progressive reduction in their daily cocaine infusions as compared with LR rats (trait × reinforcer interaction: F8,176=2.25, p<0.05) (Figure 5a and b). However, HR and LR rats displayed a similar increase in saccharin intake over the sessions (trait × session interaction: F8,168<1) (Figure 5c), thereby suggesting that they did not differ in their consummatory response.
Figure 5.
Dissociation between low and high responders in their responding for saccharin and cocaine. (a) High responders (HR, n=11, light gray diamonds) earned a similar number of cocaine infusions as compared with LR (n=12, dark gray diamonds but displayed a progressive decrease in their cocaine intake over time. (b) HR rats tended to access saccharin more often than LR animals but (c) saccharin consumption increased at a similar rate for the two groups (insert: total amount of saccharin consumed).
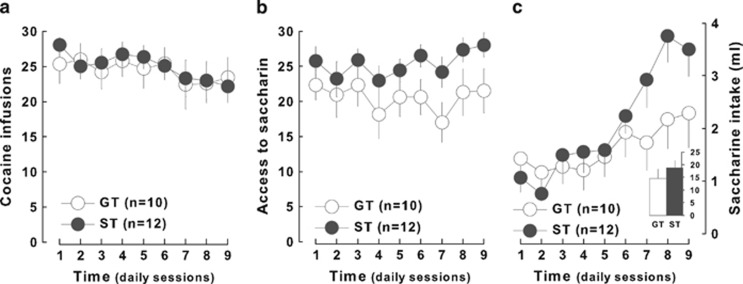
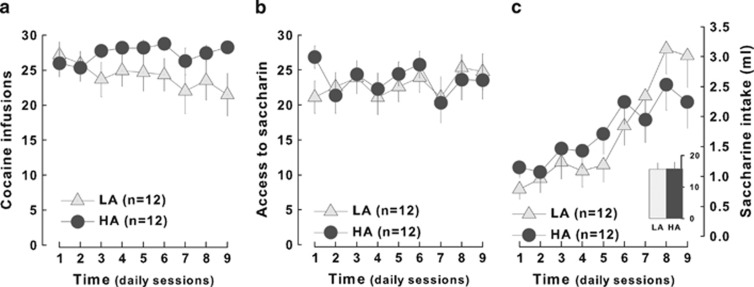
As opposed to HR and LR, no differences could be observed between ST and GT or HA and LA rats for the propensity to acquire cocaine SA with unit doses of 0.8 mg/kg (main effects of groups: F1,22, NS) (Figures 6a and 7a). ST and GT rats, however, obtained a similar access to saccharin (trait × reinforcer interaction: F1,20=1.52, p>0.2) (Figure 6b) but displayed a marked difference in saccharin intake as revealed by the greater increase in drinking in ST than in GT rats (trait × time interaction: F8,160=3.66; p<0.01) (Figure 6c), a difference that was not observed between HA and LA rats (F1,2 2<1) (Figure 7c).
Figure 6.
Goal- and sign-trackers differ in their consummatory response for saccharin. Goal- (GT, n=10, white circles) and sign-trackers (ST, n=12, dark gray circles) earned a similar number of (a) cocaine infusions and (b) access to saccharin. (c) However, ST presented a marked increase in saccharin consumption as compared with GT.
Figure 7.
High and low anxious rats show similar acquisition of operant responding for cocaine and saccharin and a comparable saccharin intake. (a) High anxious (HA, n=12, dark gray circles) animals presented a similar number of daily cocaine infusions as compared with low anxious rats (LR, n=12, light pink triangles). (b) Furthermore, the two groups did not differ in their responding for saccharin and (c) consumed the same amount of sweetened solution.
Rats Prefer Cocaine over Saccharin
Once cocaine and saccharin SA were acquired, ie, after nine sessions, rats were tested for their relative preference between the two reinforcers in a discrete-trial choice procedure. Rats were allowed to sample alternatively each reinforcer twice to assess cocaine and saccharin rewarding values before eight consecutive choice preference trials where both cocaine and saccharin were available but mutually exclusive.
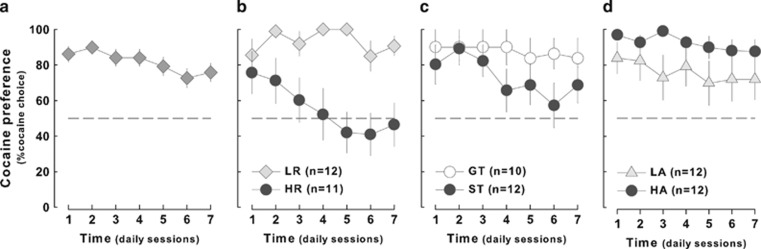
The overall population of rats showed an overall significant preference for cocaine over saccharin on each of the 7 days of testing (t45=8.24, p<0.01) (Figure 8a). This preference was not due to the nature of the instrumental responses associated with each reinforcer as the animals from a second, independent, cohort trained with opposite instrumental contingencies, ie, to lever press for saccharin and to turn the wheel for cocaine (see SOM results and Supplementary Figure 2a and b), also showed a marked preference for cocaine from the fourth session onward (t10=2.81, p<0.05) (Supplementary Figure S2c, insert). The preference for cocaine (on every choice session) was predicted, at the population level, by the mean number of cocaine infusions received during each of the last 2 days of training before the introduction of the choice (Rs from 0.44 <Rs<0.76, 0.11<R2s<0.34, all p's<0.1) but neither by the access to saccharin nor by saccharin intake. Of marked interest, the most robust predictor of the preference for cocaine over saccharin was the total distance travelled during the stress-induced locomotor activity test that yielded a negative correlation factor of −0.65<R<−0.39 for each choice session after day 1.
Figure 8.
Rats readily choose cocaine but high responders prefer saccharin. The overall population of rat preferred cocaine over a sweet solution of saccharin (a). (b) Nevertheless, high responders (HR, n=11) progressively lost their interest for cocaine whereas low responders (LR, n=12) did not. (c) Sign- (ST, n=12) and goal-trackers (GT, n=10) exhibited similar and constant cocaine preference as well as (d) low (LA, n=12) and high anxious animals (HA, n=12). The dashed line at 50% of choice toward cocaine represents no preference between cocaine and saccharin.
‘High Responder' Rats Do Not Prefer Cocaine
As suggested by the dimensional relationship between locomotor reactivity to novelty and cocaine choice the preference for cocaine over saccharin was dependent upon specific behavioral traits. Thus, although a marked preference for cocaine was displayed both by HR and by LR rats during the first session of choice, the former developed a progressive disinterest for cocaine from the second session (main effect of trait: F1,21=12.37, p<0.01 and trait × time interaction: F6,126=3.23, p<0.01) (Figure 8b), being the only group under investigation not to show a preference for cocaine by the end of the choice procedure (GT vs ST rats: effect of trait: F1,21<1 and trait × time interaction: F6,126=1.79, p>0.1, Figure 8c and HA vs LA: effect of trait: F1,22=2,27, p>0.1 and trait × time interaction: F6,132<1, Figure 8d).
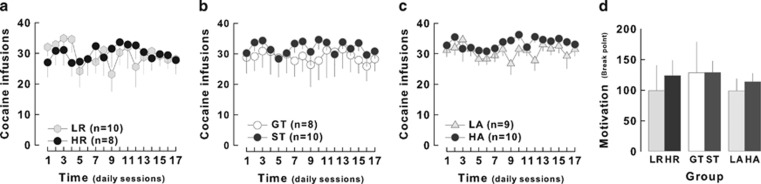
The diminishing preference for cocaine developed by HR rats in the course of the choice procedure was not attributable to a differential motivation for the drug. Indeed, after the last choice session all rats were trained to self-administer cocaine daily for 17 additional days and were tested on the eighteenth day under a progressive ratio schedule of reinforcement. HR rats acquired cocaine self-administration under an FR5 schedule at a similar rate as LR rats over the 17 days (main effect of trait: F1,16<1) (Figure 9a). In the progressive ratio challenge, HR rats (n=8) displayed break points similar to those shown by LR rats (n=10) (124±24 and 99±40, respectively) (effect of trait: F1,14<1) (Figure 9d). Similarly, neither sign-tracking nor anxiety influenced the rate of cocaine intake under FR5 (effect of trait: F1,16<1; F1,18=4.39, NS for ST vs GT and HA vs LA, respectively) (Figure 9b and c) or the break point during the progressive ratio challenge (effect of trait: F1,15<1; F1,17<1 for ST vs GT and HA vs LA, respectively) (Figure 9d).
Figure 9.
Locomotor reactivity to novelty, sign-tracking, and anxiety are not associated with altered self-administration or motivation for cocaine. After the assessment of cocaine preference, there was no effect of behavioral traits on the early phase of cocaine self-administration under an FR5 schedule of reinforcement. (a) For 17 days, high (HR, n=8) and low responders (LR, n=10) self-administered cocaine at the same rate, (b) and so did sign- (ST, n=10) and goal-trackers (GT, n=8) and (c) low (LA, n=12) and high (HA, n=12) anxious rats. (d) Motivation for cocaine was measured on the eighteenth day of self-administration by the break point during a progressive ratio session. HR and LR, as well as ST and GT and HA and LA, did not show any significant difference in their break point.
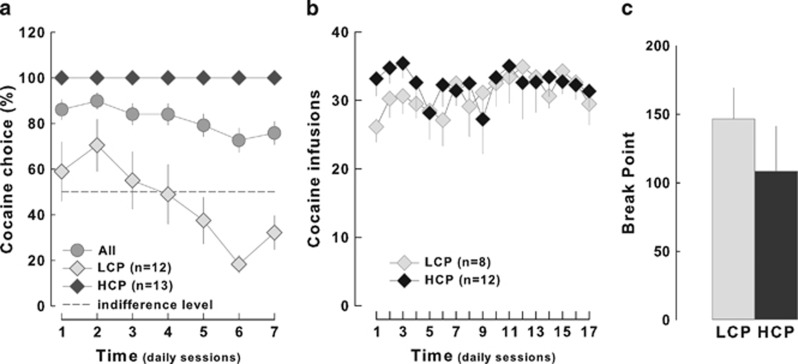
Interestingly, when rats were selected according to the upper and lower quartile of the population ranked for the preference for cocaine as high cocaine preferers (HCP, n=12, that include 7 LR and 1 HR rats) or low cocaine preferers (LCP, n=12, that include 7 HR and 1 LR rats), respectively, the magnitude of the preference for cocaine (Figure 10a) predicted neither an increase in the rate of cocaine intake (main effect of group: F1,18<1 and group × time interaction: F16,288=1.02, NS) (Figure 10b) nor a differential motivation for the drug after 2 weeks of daily exposure [F1,18<1] (Figure 10c).
Figure 10.
Preference for cocaine predicts neither an increase in cocaine intake nor a higher motivation for cocaine. (a) Rats selected on the basis of their high (HCP, n=12) or low (LCP) cocaine preference displayed (b) a similar rate of cocaine self-administration across sessions and (c) a comparable break point during the progressive ratio schedule.
DISCUSSION
Cocaine addiction is accompanied by a marked disinterest in sources of reinforcement other than the drug itself, a process that may contribute to worsening the severity of the pathology and impede the response to treatments (Ahmed et al, 2013). In rats, inter-individual differences in the preference for cocaine during early stages of drug exposure have been suggested to represent a novel operationalization of vulnerability to cocaine addiction (Ahmed, 2010).
In the present study, we used multiple behavioral assays in a longitudinal approach to investigate whether the sensitivity to an alternative reinforcer was associated with behavioral traits—elevated response to novelty (Piazza et al, 1989; Belin et al, 2008), high anxiety (Dilleen et al, 2012), and enhanced sensitivity to the salience of environmental stimuli (Saunders et al, 2013)—that have themselves been linked to distinct stages of the addiction process, namely the vulnerability to acquire cocaine SA, the propensity to lose control over cocaine intake and the vulnerability to relapse to cocaine seeking, respectively.
These three behavioral dimensions were not correlated with each other suggesting that they may represent independent measures, in agreement with previous observations (Homberg et al, 2002; Robinson and Flagel, 2009; Molander et al, 2011). Unlike other dimensional subgroups, HR and LR rats displayed opposing propensities to acquire instrumental responding for cocaine at 0.8 mg/kg vs saccharin. HR rats tended to access more saccharin rewards than cocaine infusions in independent self-administration sessions throughout the training whereas LR rats maintained a higher level of cocaine infusions than access to saccharin. Despite these differences in instrumental responding, the two groups displayed no differences with regard to the quantity of saccharin ingested suggesting their consummatory response for the sweet solution was similar. These observations suggest that, at least for the 0.25 mg/infusion dose used here, HR rats were less motivated by cocaine in an instrumental setting where the context is also associated with the opportunity, on alternative days, to access saccharin. Interestingly, this observation suggests that the increased propensity of HR rats to acquire self-administration of stimulant drugs (Piazza et al, 1989; Belin et al, 2008) may reflect facilitated instrumental conditioning (Mitchell et al, 2005) that is highly dependent upon the setting and may be disrupted by the contextual cues predicting the opportunity to obtain an alternative reinforcer. An alternative explanation may be that under fixed ratio schedules, a relatively low rate of self-infusions might reflect a higher sensitivity to the reinforcing properties of cocaine infused at the 0.8-mg/kg unit dose (Spealman and Goldberg, 1978). However, this latter explanation seems unlikely as it would be very difficult to reconcile with the progressive disinterest in cocaine HR rats developed over the course of the choice sessions. Additionally, it would predict a lower rate of cocaine SA in HR rats during subsequent sessions of exclusive access to cocaine, as well as an increased motivation under a progressive ratio schedule of reinforcement, behavioral features that were not observed in the present study.
ST rats did not differ from GT rats in their propensity to acquire cocaine SA, in line with what has been previously reported (Saunders et al, 2013). However, ST and GT rats, which displayed a similar rate of access to saccharin, markedly differed in their consummatory responses—ST rats increased their saccharin intake over time much more than GT rats. Together with the observation that, for the averaged data, preparatory responses and consummatory responses for saccharin were correlated, it may be suggested that inter-individual differences in autoshaping may be associated with a dissociation between these two psychological components of behavior (Berridge et al, 2009). Additionally, the progressive development of higher rates of saccharin intake observed in ST rats may suggest a dynamic process, potentially dependent upon sensitization to the reinforcing properties of saccharin across repeated training, and may reflect loss of control (Kampov-Polevoy et al, 1995). Thus despite their drive toward the goal associated with increased dopamine transmission in the accumbens core at the onset of pellet delivery (Flagel et al, 2011), GT rats displayed less interest in consuming saccharin than ST rats which are behaviorally and neuropharmacologically bound to the CSs (Flagel et al, 2011; Meyer et al, 2012a; Robinson et al, 2014). Considering the differential contribution of dopamine and opiates in the ventral regions of the basal ganglia to preparatory and consummatory responses (Barbano and Cador, 2006, 2007; Berridge et al, 2009), the present results suggest that ST and GT rats may differ not only in their dopaminergic, but also in their opioidergic neurophysiology. These differential processes may also be influenced by the cholinergic system which has been demonstrated to control sign-tracking (Palmatier et al, 2013), likely through its control over dopaminergic neurons (Maskos et al, 2005; Avale et al, 2008) or prefrontal function (Paolone et al, 2013), eventually resulting in a modulation of attention and reward expectancy at the onset of CS presentation (Inglis et al, 1994).
Of further interest, individual differences during self-administration training did not predict subsequent performance in the mutually-exclusive choice procedure. Indeed, in the current study, the majority of a cohort of 60 outbred Sprague Dawley rats showed a marked preference for cocaine. Only HR rats, ie, that display a high locomotor response to an inescapable environment, a behavioral marker of increased propensity to acquire drug SA (Piazza et al, 1989), developed a progressive disinterest for cocaine over the free choice sessions.
The demonstration that rats prefer an i.v. infusion of 0.8 mg/kg of cocaine over the opportunity to access a non-drug reward, such as a saccharin solution, is in agreement with the human literature, but contrasts with previous results from preclinical studies (Lenoir et al, 2007; Cantin et al, 2010) which reported that about 85% of rats preferred saccharin over cocaine in a similar choice procedure. The discrepancy between these sets of results may be attributable to three, potentially interacting, differences in experimental parameters: (i) the differential nature of the instrumental response associated with each reinforcer, (ii) the configuration of the operant chamber, and (iii) the parameters dictating access to saccharin.
(i) In the present study access to each of the reinforcers was contingent upon making a distinct instrumental response whereas in the previous studies the same response (e.g., lever press), was required to obtain each of the reinforcers. Moreover, in previous studies (Lenoir et al, 2007), similar instrumental responses were used as both the preparatory and the consummatory response for cocaine, whereas it reflected only the preparatory response for saccharin, the consummatory response being expressed as a magazine head entry. Such differences in the chain of events following instrumental responding for two different reinforcers may lead to an aberrant contrast of incentive value attributed between the two manipulanda. This may stem from an engagement of ventro-striatal, dopamine-dependent learning processes during the anticipation period between the lever press and the access to saccharin (Blackburn et al, 1987, 1989a, 1989b; Bassareo and Di Chiara, 1999) that does not occur following the response on the other lever which leads to a cocaine infusion. Similarly, when the animal can lever press for both cocaine and saccharin, the constant presence of the magazine in the vincinity of the saccharin lever (Lenoir et al, 2007; Cantin et al, 2010) (ie, the goal of the saccharin-paired lever press, acting as a discriminative stimulus for saccharin) as opposed to the absence of a cocaine-associated CS at the beginning of the choice procedure, may, as suggested by Konorski (1967), facilitate the saccharin preparatory lever press response at the detriment of the cocaine-associated consummatory lever press (VanDercar, 1967).
(ii) Pavlovian approach to the magazine that was placed in the close vincinity of the saccharin-associated lever in previous studies (Lenoir et al, 2007; Cantin et al, 2010) may contribute to facilitating contact with the saccharin-associated lever to the detriment of the cocaine-associated one. Only the introduction of a seeking-taking chained schedule of reinforcement for cocaine would disantagle the potential bias of using similar manipulanda for preparatory responses for cocaine and saccharin. However, the present study, using two highly dinstinguishable instrumental responses for the two reinforcers and a spatial configuration of the operant chamber with the magazine located on the wall opposite to the saccharin-associated manipulandum, may have minimized some of these potential confounding factors.
(iii) The other major difference between the present and the previous studies (Lenoir et al, 2007; Cantin et al, 2010) relates to the access animals had to saccharin. In previous studies a full range of unit doses for single cocaine infusions were compared within choice procedures to an access to saccharin which was unmanipulated (ie, range not adjusted) and practically unrestricted in each trial. In the present study, following a response on the saccharin-associated manipulandum, rats had access to saccharin delivered per 0.01 ml by a sipper that went back and forth into the magazine for 50 s so that the animals, provided they maintained their head in the magazine for this 50 s interval, could drink up to 3 ml of the sweetened solution. Such procedure required the rats to learn to maintain their head in the magazine, as reflected by the increased saccharin intake per session that reached an asymptotic level by session 8 (Figure 3b). In these conditions, the relative value of saccharin may have been lower as compared with a unit dose of 0.8 mg/kg of cocaine than in previous studies.
Future studies will be necessary to better understand which of these experimental differences accounts for the preference for cocaine over saccharin observed here. Nevertheless, the present results illustrate that the outcome of choice procedures is dependent upon experimental parameters and that, at least under certain conditions, rats indeed prefer cocaine over saccharin, even after a brief history of cocaine SA.
The finding that rats readily prefer cocaine over a food reward is a very important result because it helps reconcile studies in preclinical models with the human literature. The experimental parameters in the present experiments may therefore be very useful to probe the influence of alternative reinforcers on the propensity to develop addiction in preclincal models that have heuristic value with regard to the human condition. The preference for cocaine observed here could not be attributable to the nature of the instrumental response associated with each of the two reinforcers because rats acquired cocaine and saccharin SA at a similar rate and displayed a marked preference for cocaine in two independent experiments in which the instrumental contingencies were counterbalanced.
Neither high anxiety nor sign-tracking predicted differential choice preference between cocaine and saccharin. HA and ST rats more readily choose cocaine over saccharin from the first choice session. However, HR rats progressively reduced their preference for cocaine over repeated sessions, a between-group effect that was supported by the negative correlation between novelty-induced locomotor activity and the percentage of cocaine choice in the last six sessions. High locomotor reactivity to novelty has been initially suggested to be an operationalization of sensation seeking (Dellu et al, 1996) that is dissociable, both behaviorally and neurobiologically from novelty seeking, as measured using a novelty-induced conditioned place preference procedure (Bardo et al, 1996; Belin et al, 2011). Early work from Piazza et al (1989) demonstrating that HR rats would self-administer stimulants at doses that were not reinforcing in LR rats led to the speculation that high locomotor reactivity to novelty was a marker of vulnerability to addiction (Piazza and Deroche-Gamonet, 2013). However, despite their increased propensity to acquire drug self-administration (Belin et al, 2008), HR rats, trained to self-administer cocaine at a unit dose similar to the one used in the present study, seem to be resilient to addiction as revealed by their very low addiction severity score in a multisymptomatic model of cocaine addiction (Belin et al, 2008, 2011). Thus unlike highly impulsive or high novelty preference rats, neither of which differing from their littermates in their propensity to acquire cocaine self-administration (Belin et al, 2008, 2011; Besson et al, 2013), HR rats seem to resist to the transition from controlled to compulsive cocaine intake. The present results extend this notion by demonstrating that HR rats, with a short history of cocaine self-administration at a unit dose similar to the one used in previous choice studies (Lenoir et al, 2007), are highly sensitive to rewarding alternatives in the drug taking context. This observation is supported by previous work showing that HR rats exhibit higher sensitivity to the reinforcing properties of food than LR rats (Dellu et al, 1996), indicating that the former are more responsive to rewards in general and that their higher propensity to acquire instrumental conditioning is not restricted to drugs. The development of indifference between cocaine and saccharin in HR rats could not be solely attributable to their lower intake during the training phase as supported by the negative relationship between the locomotor response to novelty and the choice for cocaine, which suggests that a preexisting neurobiological mechanism may contribute to this behavioral response. Additionally, HR rats differed from LR rats neither in their rate of cocaine self-administration for 17 sessions during which cocaine was the only available reinforcer under an FR5 schedule of reinforcement nor in their break point during a progressive ratio session, in agreement with our previous studies (Belin et al, 2008, 2011).
In the current study, we show that rats prefer cocaine over saccharin under the appropriate experimental settings, thereby demonstrating that the previously-reported preference toward choice for natural rewards over cocaine in rodents is not a universal phenomenon. The present study further demonstrates that the propensity to self-administer drugs is not related to the vulnerability to develop addiction. Indeed, HR rats were the only subpopulation tested in which cocaine intake was diminished by the potential opportunity to obtain an alternative reinforcer in the self-administration setting, demonstrating a progressive loss of preference for cocaine over saccharin when the choices are mutually exclusive. Taken together, these observations suggest that HR rats—exhibiting high locomotor responses to novelty—may be a valuable model to study resilience to the maintenance of cocaine intake in complex environments with alternative reinforcers.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Dr Serge Ahmed for his input on the initial phase of the project as well as Khadra Escriva and Laurence Turi for their precious help in the animal facility. This work was funded by an INSERM AVENIR and Agence Nationale de la Recherche (ANR) ANR12 SAMA00201 grant to DB, the région Poitou-Charentes, an AXA research fund fellowship to ABR, and a Ministère de la Recherche et de la Technologie grant to NV. AM was supported by the Behavioural and Clinical Neuroscience Institute of Cambridge.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
AUTHOR CONTRIBUTIONS
DB and NV designed the experiment. ACM, ABR, and NV wrote the behavioral programs. NV performed the experiment. NV, ED, and DB analyzed the data. NV, ABR, ED, and DB designed the figures. NV, ACM, ED, and DB wrote the paper.
Supplementary Material
References
- Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–587. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Aigner TG, Balster RL. Choice behavior in rhesus monkeys: cocaine versus food. Science. 1978;201:534–535. doi: 10.1126/science.96531. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association 2000Diagnostic and Statistical Manual of Mental Disorders4th edn, Text Revision (DSM-IV TR)American Psychiatric Association: Washington, DC [Google Scholar]
- Avale ME, Faure P, Pons S, Robledo P, Deltheil T, David DJ, et al. Interplay of beta2* nicotinic receptors and dopamine pathways in the control of spontaneous locomotion. Proc Natl Acad Sci USA. 2008;105:15991–15996. doi: 10.1073/pnas.0807635105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano M, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Bardo M, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med. 2012;2:pii: a011940. doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine-seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking', ‘wanting', and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Pelloux Y, Dilleen R, Theobald DE, Lyon A, Belin-Rauscent A, et al. Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology. 2013;38:1963–1973. doi: 10.1038/npp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Fibiger HC. Dopamine and preparatory behavior: I. Effects of pimozide. Behav Neurosci. 1987;101:352–360. doi: 10.1037//0735-7044.101.3.352. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Fibiger HC. Dopamine and preparatory behavior: III. Effects of metoclopramide and thioridazine. Behav Neurosci. 1989;103:903–906. doi: 10.1037//0735-7044.103.4.903. [DOI] [PubMed] [Google Scholar]
- Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Dopamine and preparatory behavior: II. A neurochemical analysis. Behav Neurosci. 1989;103:15–23. doi: 10.1037//0735-7044.103.1.15. [DOI] [PubMed] [Google Scholar]
- Cain M, Saucier D, Bardo M. Novelty seeking and drug use: contribution of an animal model. Exp Clin Psychopharmacol. 2005;13:367–375. doi: 10.1037/1064-1297.13.4.367. [DOI] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS ONE. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M, Lac ST, Nygaard SL. A concurrently available nondrug reinforcer prevents the acquisition or decreases the maintenance of cocaine-reinforced behavior. Psychopharmacology. 1989;97:23–29. doi: 10.1007/BF00443407. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17:563–567. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats—biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, et al. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl) 2012;222:89–89. doi: 10.1007/s00213-011-2626-4. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2008;56:139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Discrete-trial choice procedure: effects of naloxone and methadone on choice between food and heroin. Pharmacol Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- Homberg JR, van den Akker M, Raaso HS, Wardeh G, Binnekade R, Schoffelmeer AN, et al. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci. 2002;15:1542–1550. doi: 10.1046/j.1460-9568.2002.01976.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Day JC, Fibiger HC. Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience. 1994;62:1049–1056. doi: 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Overstreet DH, Rezvani AH, Janowsky DS. Saccharin-induced increase in daily fluid intake as a predictor of voluntary alcohol intake in alcohol-preferring rats. Pharma Bio Behav. 1995;57:791–795. doi: 10.1016/0031-9384(94)00389-0. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative Activity of the Brain: An Interdisciplinary Approach. University of Chicago: Chicago; 1967. [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed S. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency-modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2012;219:999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Cunningham CL, Mark GP. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander AC, Mar AC, Norbury A, Steventon S, Moreno M, Caprioli D, et al. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl) 2011;215:721–731. doi: 10.1007/s00213-011-2167-x. [DOI] [PubMed] [Google Scholar]
- Murray JE, Everitt BJ, Belin D. N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol. 2012;17:437–440. doi: 10.1111/j.1369-1600.2011.00330.x. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Marks KR, Jones SA, Freeman KS, Wissman KM, Sheppard AB. The effect of nicotine on sign-tracking and goal-tracking in a Pavlovian conditioned approach paradigm in rats. Psychopharmacology (Berl) 2013;226:247–259. doi: 10.1007/s00213-012-2892-9. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33:8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76:Pt B:450–Pt B:459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine ‘craving': role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Drug Self-Administration by Laboratory Animals: Control by Schedules of Reinforcement. Annual Rev Pharma Toxi. 1978;18:313–339. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- Tomie A, Brooks W, Zito B. Sign-tracking: the search for reward. Contemporary Learning Theories: Pavlovian Conditioning and the Status of Traditional Learning Theory. 1989. pp. 191–223.
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDercar DH, Schneiderman N. Interstimulus interval functions in different response systems during classical discrimination conditioning of rabbits. Pscychon Sci. 1967;9:9–10. [Google Scholar]
- Young JS. Discrete-trial choice in pigeons: effects of reinforcer magnitude. J Exp Anal Behav. 1981;35:23–29. doi: 10.1901/jeab.1981.35-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.