Abstract
Generally, rewards that are received sooner are often preferred over future rewards, and the time between the choice and the reception of the reward is an important factor that influences our decisions, a phenomenon called delay discounting (DD). In DD, the medial prefrontal cortex (MePFC) and striatal dopamine neurotransmission both play an important role. We used repetitive transcranial magnetic stimulation (rTMS) to transiently activate the MePFC to evaluate its behavioral effect on the DD paradigm, and subsequently to measure its effect on striatal dopamine. Twenty-four right-handed young healthy subjects (11 females; age: 22.1±2.9 years) underwent DD following 10 Hz-rTMS of the MePFC and vertex stimulation (control condition). Thereafter, 11 subjects (5 females; age: 22.2±2.87 years) completed the PET study at rest using [11C]-(+)-PHNO following 10 Hz-rTMS of the MePFC and vertex. Modulation of the MePFC excitability influenced the subjective level of DD for delayed rewards and interfered with synaptic dopamine level in the striatum. The present study yielded findings that might reconcile the role of these areas in inter-temporal decision making and dopamine modulation, suggesting that the subjective sense of time and value of reward are critically controlled by these important regions.
INTRODUCTION
Time is a critical factor when individuals make decisions and consider the outcomes associated with their choices. Every day, we have to decide between options that have immediate or delayed consequences. We might, for example, restrict our habits opting for an immediate loss of pleasure for the future benefits of better physical health and appearance. Thus, individuals have to voluntarily postpone impulsive urges for immediate gratification and persist in goal-directed behavior to achieve positive outcomes in the future (Wittmann and Paulus, 2008). Generally, rewards that are received sooner are often preferred over future rewards, and the time between the choice and the reception of the reward is an important factor that influences our decisions. A delayed outcome of a choice reduces the subjective value of the reward, a phenomenon called delay discounting (DD) (Kirby and Santiesteban, 2003; Wittmann et al, 2007). Practically speaking, individuals who choose immediate gratification at the expense of long-term interest are judged to be impulsive. Using delay-discounting paradigms to quantify dysfunctions of impulse control, it has been shown that children with attention deficit hyperactivity disorder (Barkley et al, 2001), individuals with Parkinson's disease (Housden et al, 2010) and neuropsychiatric disorders (Cardinal et al, 2004), smokers (Baker et al, 2003), and substance-dependent individuals (Madden et al, 1999) show increased discounting of delayed rewards.
Functional imaging studies using DD tasks are beginning to examine which brain areas are associated with impulsivity and self-control (McClure et al, 2004; Tanaka et al, 2004; Kable and Glimcher, 2007; Ballard and Knutson, 2009). Interestingly, some of these studies have shown that the neural activity in several brain regions (particularly medial prefrontal cortex (MePFC), posterior cingulate cortex, and the ventral striatum ) participates in the subjective valuation of delayed monetary rewards (Kable and Glimcher, 2007). In other words, the neural activity in these regions tracked changes in the subjective value of the delayed reward, and activation of these regions (particularly, the MePFC) varied when only the delayed reward changed. This suggests that the MePFC makes an important contribution to the subjective sense of time and the value for reward, two critical factors in economic inter-temporal decision making.
There is substantial anatomical evidence showing that MePFC fibers project to the ventral tegmental area and pars compacta of the substantia nigra, thus having direct influence on dopaminergic neuron in the ventral mesencephalon (Beckstead, 1979; Sesack et al, 1989). In fact, while cortical fibers from the dorsal prefrontal cortex terminate primarily in the caudate nucleus, axonal inputs from the MePFC and dorsal anterior cingulate terminate mainly within subregions of the ventral striatum (Selemon and Goldman-Rakic, 1985; Haber et al, 1995). This anatomical substrate supports the hypothesis that the MePFC may share reward-related information with ventral striatum through the dopaminergic system (McClure et al, 2004; Kable and Glimcher, 2007; Xu et al, 2009), which may be modulated by rTMS.
DA neurotransmission has a specific role in computing how the temporal proximity of a reward relates to its subjective value (ie, rate of temporal discounting) (Pine et al, 2010). Using an inter-temporal choice task, pharmacologically enhancing DA activity controlled how the timing of a reward was incorporated into the construction of its ultimate value, suggesting a mechanism through which DA may influence human choice and temporal discounting, and traits such as impulsiveness that may account for several behavioral disorders associated with a hyperfunctioning DA system (Pine et al, 2010).
Despite that these functional neuroimaging studies have provided important insights into the neural control of decision-making processes associated with delayed discounting (DD), imaging alone suffers from the limitation that it can only provide neuronal correlates of cognitive performance and often cannot determine a causal relation between biological observations and behavioral performance (Rushworth et al, 2002). Thus, here, we used repetitive transcranial magnetic stimulation (rTMS) to transiently activate the MePFC: (i) first to evaluate its behavioral effect during performance of the DD paradigm, and (ii) subsequently to measure its effect on striatal dopamine. Our first working hypothesis was that if in a region like the MePFC the neural activity was correlated with the subjective valuation of a delayed reward, the stimulation of this area should have influenced the rate of temporal discounting, influencing the level of impulsivity. Thus, if rTMS of the MePFC reduced impulsivity, individuals would increase their preference for delayed larger rewards. Instead, if rTMS increased impulsivity, individuals would increase their preference choice for immediate small rewards. Our second working hypothesis was that if stimulation of the MePFC affected the rate of temporal discounting and impulsivity level, we should expect changes in rTMS-induced DA release in the striatum. In particular, we predicted that an increased impulsivity with preference for immediate smaller rewards should be associated with dopaminergic changes in the ventral striatum, whereas reduced impulsivity with preference for future larger rewards would be associated with no dopaminergic changes in the ventral striatum.
To test these hypotheses, we used 10 Hz rTMS which has been shown previously to excite and activate the underlying cortex and to induce release of DA in the striatum (Strafella et al, 2001; Strafella et al, 2003; Cho and Strafella, 2009; Ko and Strafella, 2012). To measure striatal DA neurotransmission, we used positron emission tomography (PET) combined with a newly developed, high-affinity radiotracer, ie, [11C]-(+)-PHNO. This tracer is a D2/D3 agonist, binding specifically to the active form of the receptor, thus permitting an evaluation of the functionally relevant form of this receptor in the striatum. Recent studies using [11C]-(+)-PHNO have shown a strong binding signal in the striatum, globus pallidus (GP), substantia nigra, and anterior thalamus (Freedman et al, 1994; Narendran et al, 2006; Willeit et al, 2006; Graff-Guerrero et al, 2008) (as compared with [11C]raclopride), opening a new possibility for investigating reward-related behavior. In-vivo study of [11C]-(+)-PHNO suggested that this radioligand has fourfold higher preference for D3 than D2 receptors (Narendran et al, 2006). This observation was supported by studies in nonhuman primates showing that the [11C]-(+)-PHNO binding in the GP and midbrain is selectively displaceable with the D3 preferential agonist BP897 (Narendran et al, 2006) and antagonist SB-277011 (Ginovart et al, 2006). Altogether, these reports provided strong evidence that [11C]-(+)-PHNO has a higher affinity for D3 over D2 and is extremely sensitive to detect dopaminergic changes in D3-abundant subcortical regions such as limbic striatum and GP.
To stimulate the cortical areas, we opted to use a double-cone TMS coil to activate the target regions equally in both the left and right hemisphere. High-frequency rTMS (10 Hz) was applied to the MePFC and over a control site (ie, vertex) in 24 young healthy subjects and discounting rate was tested using the DD task. During the DD task, subjects were asked to choose between different amounts of monetary reward available with varying time delays (smaller-immediate vs larger-delayed option) based on their preference. Individual discounting level (k value) for future reward was calculated based on hyperbolic function (Richards et al, 1999) as a main outcome. Eleven subjects completed the [11C]-(+)-PHNO PET study to measure the displacement effect of brain stimulation over MePFC and control site (ie, vertex) on striatal DA.
SUBJECTS AND METHODS
Participants
Twenty-four right-handed young healthy subjects were enrolled (11 females; age, 22.1±2.9 years) (Supplementary Table S1). All subjects were naïve to rTMS. Exclusion criteria included history of psychiatric and/or neurological disorder (particularly epilepsy), any previous exposure to stimulant drugs, pregnancy, and migraine. Subjects were also screened for depression and anxiety using the Beck Depression Inventory (BDI) (exclusion criterion of a score of >10) and Short Anxiety Screening Test (SAST) (exclusion criterion of a score of >24), respectively. We assessed the self-reported impulsivity by means of the Barratt Impulsivity Scale-11 (BIS) (Spinella, 2007). To rule out structural lesions in the brain and to identify the anatomical target for the rTMS stimulation, a T1-weighted MRI image was obtained for all subjects (n=24) using a 3T high-resolution MRI (GE Discovery MR750 3T, FSPGR with repletion time=6.7 ms, echo time=3.0 ms, flip angle=8 mm, slice thickness=1 mm, NEX=1, matrix size=256 × 192).
Written informed consent was obtained in all cases before study enrollment. The study protocols were approved by the Ethical Committee of the Center for Addiction and Mental Health Research, University of Toronto.
Study Design
For the behavioral study with the DD task, each subject (n=24) underwent two rTMS sessions: MePFC (active condition) and vertex (control condition) in the same day. The task started 3 min after the completion of stimulation for all subjects. To minimize the carry-over effect of the prior stimulation, there was at least a 30-min interval between the two stimulation conditions. Fourteen subjects agreed to participate in the PET imaging study, however, 11 subjects (5 females; age, 22.2±2.87 years) completed the PET scans and 3 participants dropped out because of the nausea induced by the [11C]-(+)-PHNO D2/D3 agonist. Each subject underwent a [11C]-(+)-PHNO PET scan on two separate days. On one day, the subject received rTMS over MePFC stimulation; on the other day, the subject received stimulation over the control area (ie, vertex). The rTMS sessions were counterbalanced. Only binding potentials (BP) within the basal ganglia were considered for further analysis, because this is the brain structure where receptor-specific [11C]-(+)-PHNO binding is mainly detected. A reduction in BP is indicative of an increase in extra-cellular dopamine concentration (Endres et al, 1997; Laruelle et al, 1997).
DD Task
The DD task is a behavioral analytic approach to understand how each individual makes a choice between a smaller reward given immediately and a larger reward given after a time delay, thus assessing the degree of cognitive impulsivity or self-control (Dixon et al, 2005). The task was composed of 120 trials; in each trial, the amounts of monetary reward for immediate and delay options are decided by the fixed k value and the delay time based on the hyperbolic function of delay discount, V=A/(1+kD), where V is the value of the delayed outcome (ie, the indifference value), A is the delayed reward, D is the length of the delay, and k expresses the steepness of the discount function (Mitchell, 1999; Richards et al, 1999; de Wit et al, 2002). On the basis of this function, higher k values are associated with preference for immediate small-size reward and lower k values are an expression of delayed large-size reward. Thus, low k values are an index of minor discounting for future reward. Subjects were instructed that they have to make preferential judgments about hypothetical rewards shown on a computer screen. All reward choices were made by pressing either the ← or → key on keyboard with the subject's dominant hand (right hand for all subjects). The trial order was randomized for each session across the subjects.
The k values were estimated separately in small (range: 1–100CAD), medium (range: 200–500CAD), and large (range: 600–1000CAD) reward magnitude as the geometric mean between the lowest implied indifference k value in which subjects chose the delayed option, and the highest implied indifference k value in which subjects chose the immediate option (Kirby et al, 1999; Monterosso et al, 2007). The geometric mean is a type of mean which indicates the central tendency of a set of numbers and is used because the task required subjects to express preferences (Monterosso et al, 2007). We expect that changes in the individual k value will vary in a relative manner of reward amount and delay period. If the response consistency is over 66% for one response (small immediate or large delay option) within a given k value, then that k value was assigned to immediate choice preference or delay choice preference.
Location of the Target Site
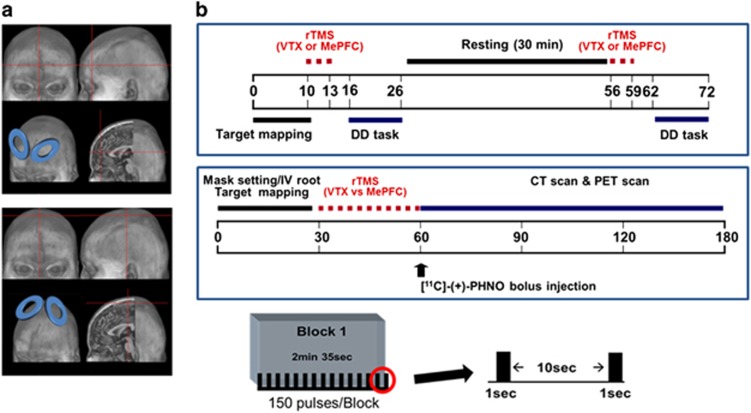
To target the MePFC and the vertex, we used a procedure that takes advantage of the standardized stereotaxic space of Talairach and Tournoux (Talairach and Tournoux, 1988) and frameless stereotaxy (Strafella et al, 2001). The coordinates selected for targeting the MePFC (BA 10; x=0, y=59, z=12) are similar to those described in previous studies (Kable and Glimcher, 2007). For the control site, we used the vertex (x=0, y=−20, z=85) (Pascual-Leone et al, 1996). The Talairach coordinates were converted into each subject's native MRI space using the reverse native-to-Talairach transformation (Figure 1a). The positioning of the TMS coil over these locations, marked on the native MRI, was performed with the aid of Brainsight, a frameless stereotaxic system (Rogue Research, Montreal, QC, Canada), for each stimulation session.
Figure 1.
Stimulation target areas and protocol. (a) The MePFC (upper figure) and vertex (lower figure) target area mapped on Colin brain (b) experimental procedures for behavioral (upper figure) and PET imaging (lower figure) studies. For each rTMS session, 15 10-pulse trains of 1-s duration were delivered at a stimulation frequency of 10 Hz, with a between-train interval of 10 s.
rTMS Protocol
rTMS was carried out with the Magstim Rapid2 magnetic stimulator (Magstim, UK), using a double-cone coil (P/N 9902–00; Magstim). This coil structure uses two angled windings to improve coupling to the head, increasing its effective stimulating power to relatively deep brain areas (Allison et al, 1996; Terao et al, 2000). This type of coil was chosen to stimulate the target regions (MePFC and vertex) equally in both the left and right hemispheres. Stimulus intensities, expressed as a percentage of the maximum stimulator output, were set at 80% of the active motor threshold. Active motor threshold was defined from the tibialis anterior muscle, with AgCl surface electrodes fixed on the skin with a belly-tendon montage, as the lowest stimulus intensity able to elicit five motor evoked potentials of at least 200 uV averaged over 10 consecutive stimuli delivered over the motor cortex at intervals longer than 5 s. During the determination of active motor threshold, subjects were instructed to maintain a steady muscle contraction of 20% of maximum voluntary contraction. One rTMS session was applied for each cortical site (ie, MePFC and vertex) during the behavior study with the DD task, and five rTMS sessions were delivered for each PET imaging study (during MePFC and vertex stimulation). Each stimulation session was separated by a 5-min interval. For each rTMS session, 15 10-pulse trains of 1-s duration were delivered at a stimulation frequency of 10 Hz, with a between-train interval of 10 s (Figure 1b). Thus, a total of 150 pulses were delivered for the behavior study and 750 pulses were delivered preceding the start of the PET acquisition.
PET Imaging
Subjects were scanned with [11C]-(+)-PHNO using a high resolution PET CT, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN, USA) operating in 3D mode with an in-plane resolution of approximately 4.6 mm full width at half-maximum. The radiosynthesis of [11C]-(+)-PHNO ([11C]-(+)-4-propyl-9-hydroxynaphthoxazine) has been described in detail elsewhere (Wilson et al, 2005). Concisely, [11C]-propionyl chloride was reacted with 9-hydroxynaphthoxazine to generate a [11C]-amide which is subsequently reduced by lithium aluminum hydride. Purification by HPLC and formulation give radiochemically pure [11C]-(+)-PHNO as a sterile, pyrogen-free solution suitable for human studies.
To minimize subject's head movements in the PET scanner, we used a custom-made thermoplastic facemask together with a head-fixation system (Tru-Scan Imaging, Annapolis). Following the acquisition of a scout view for accurate positioning of the subject, a low dose (0.2 mSv) CT scan for attenuation correction was conducted before the PET scan. After completion of the rTMS session, [11C]-(+)-PHNO was injected into the left antecubital vein over 60 s and dynamic scanning was acquired for 90 min. For each 3D sinogram, data were normalized with attenuation and scatter corrected before applying fourier rebinning to convert the 3D sinograms into 2D sinograms. The 2D sinograms were then reconstructed into image space using a 2D filtered back projection algorithm, with a ramp filter at Nyquist cutoff frequency. After reconstruction, a Gaussian filter with a 5 mm FWHM was applied and the images calibrated to nCi/cc. The spatial resolution of the reconstructed images was 2 × 2 × 2 mm (X × Y × Z).
PET Image Analysis
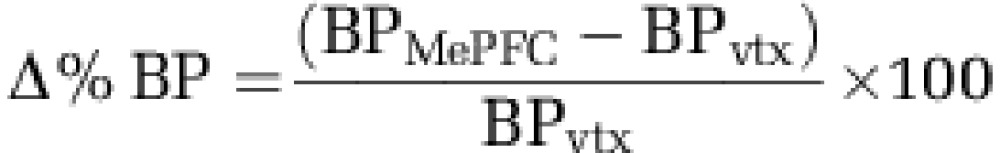
The motion-corrected PET data were analyzed using the in-house image analysis platform ROMI (Rusjan et al, 2006). The detailed image analysis procedure was described elsewhere (Rusjan et al, 2006). This included (i) transforming a standard brain template with a cerebellar ROI to match individual high-resolution MR images, (ii) co-registering each subject's MR image to the PET image, (iii) refining ROIs from the transformed template based on the gray matter probability of voxels in the individual MR images, (iv) transforming the individual refined ROI to the PET space using the matrix obtained during the MRI to PET co-registration, (v) extracting time activity curve of the reference region, the cerebellum, from PET images in original space (Supplementary Figure S1). After the ROMI procedure, parametric [11C]-(+)-PHNO PET BP map was calculated in the native PET space with simplified reference tissue method (Lammertsma and Hume, 1996) using the cerebellar time activity curve value as reference. For statistical analysis, parametric BP images were transformed into standardized stereotaxic space using individual MRI. Finally, normalized images were smoothed with a Gaussian function at 4 mm full width half-maximum. The image preprocessing for the statistical analysis was carried out with SPM 2 (Wellcome Department of Imaging Neuroscience, London). Statistical maps in the striatum were threshold at a level of P<0.05 FDR corrected with an extent threshold of at least 50 contiguous voxels to test significant changes in [11C]-(+)-PHNO binding following MePFC rTMS (as compared with control site stimulation). For the nucleus accumbens (NAcc), we used the WFU-PickAtlas (SPM extension toolbox) to generate volume of interest image of this brain region. Left and right NAcc volume images were imported into MarsBar (SPM extension tool box, MRC Cognition and Brain Sciences Unit, Cambridge, UK). BPs were extracted from parametric BP image for each stimulation condition. The relative changes in BP (Δ%BP) were calculated according to this formula where BPMePFC is BP of MePFC stimulation condition and BPvtx is BP of vertex stimulation condition.
 |
A regression analysis was performed to evaluate the relationship between displacement in DA and changes in individual discounting level. The significance level for all statistical analysis was set at P<0.05.
RESULTS
We found that high-frequency rTMS over the MePFC (compared with vertex-rTMS) significantly affected DD rate and reduced log-transformed k value (ln(k)) compared with control site stimulation (ln(k): −4.3±1.5 for MePFC vs −3.8±1.2 for vertex, t=3.25, P<0.01) (Figure 2). This reduction in ln(k) was about 19.0.±32.5% indicating a less stiff discounting tendency for future rewards. The simple preference choice toward the larger-delayed option was significantly different between the two stimulation conditions. In fact, although MePFC-rTMS increased the preference toward larger-delayed rewards (P<0.05), it decreased the choices for the immediate small options (P<0.05). There were no changes on the response consistency following rTMS ruling out a possible confounding factor influencing the decision-making process.
Figure 2.
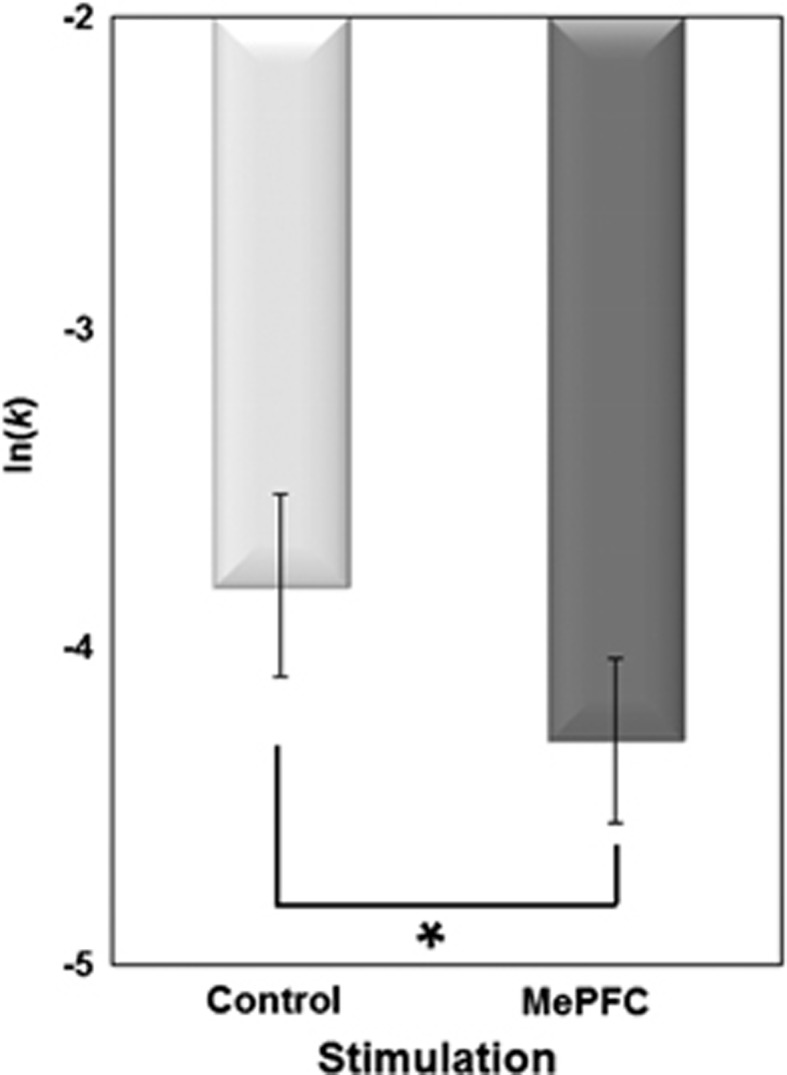
Effect of rTMS on DD ln(k) for each rTMS condition (n=24). rTMS over the MePFC (compared with vertex-rTMS) significantly affected DD rate and reduced log-transformed k value (ln(k)) compared with control site stimulation (P<0.01).
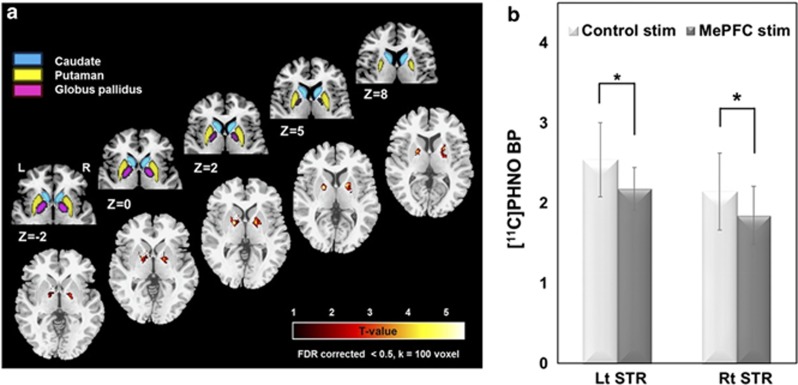
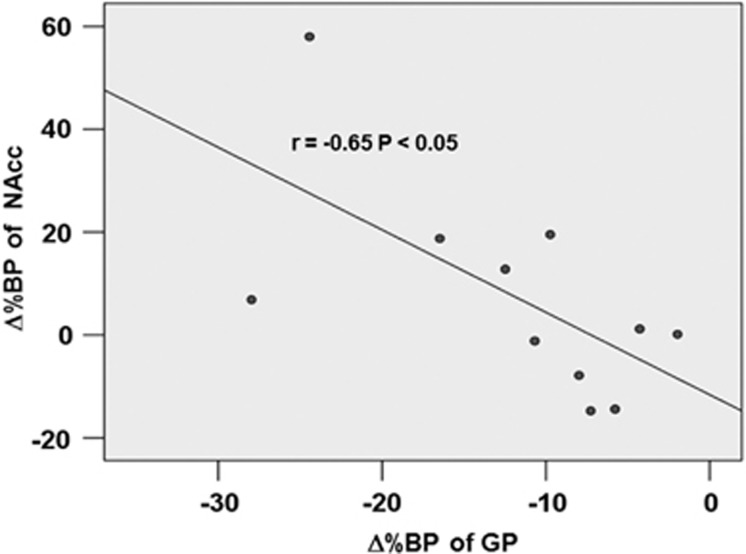
During PET imaging, MePFC-rTMS (compared with vertex-rTMS) displaced DA in the striatum with a reduction in [11C]-(+)-PHNO BP specifically in the bilateral dorsal putamen and bilateral dorsal/ventral GP (Figure 3a, Table 1), thus reflecting release of DA in these subcortical areas. The Δ%BP was −13.1±8.1% in the left and −13.0±8.8% in the right striatal cluster (Figure 3b). We looked, as well, for changes in the region of the NAccand interestingly, although we did not observe a significant rTMS-induced reduction in [11C]-(+)-PHNO BP (ie, DA release), a correlation analysis revealed a significant inverse relationship between Δ%BP of the GP and Δ%BP of the NAcc in the left hemisphere (r=−0.65, P<0.05) (Figure 4). This suggests that MePFC rTMS-induced displacement of DA in the dorsal striatum paralleled the lack of DA release in the NAcc.
Figure 3.
[11C]-(+)-PHNO PET imaging results (n=11). (a) Anatomical representation of the basal ganglia (upper row) and MePFC-rTMS induced displacement of DA in the striatum with a reduction in [11C]-(+)-PHNO BP specifically in the bilateral dorsal putamen (DPu) and bilateral dorsal/ventral globus pallidus (GP) (compared with vertex-rTMS) (lower row). Color bar indicates t-statistics (b). The bars display BPs extracted from statistically significant clusters showing the [11C]-(+)-PHNO BP reduction between MePFC and vertex control stimulations in left and right striatum. The mean Δ%BP was −13.1±8.1% in the left and −13.0±8.8% in the right striatal cluster.
Table 1. Basal Ganglia Regions with Dopamine Displacement.
| Brian Region |
Coordinatesa |
T-score | Cluster size | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Lt Dorsal putamen | −24 | 6 | 2 | 5.2 | 251 |
| Lt Globus pallidus | −19 | −2 | 4 | 8.7 | |
| Rt Dorsal putamen | 22 | 5 | 10 | 4.9 | 253 |
| Rt Globus pallidus | 16 | 0 | 4 | 6.2 | |
MNI space
Figure 4.
Correlation showing the inverse relationship between Δ%BP of GP and Δ%BP of the NAcc in the left hemisphere (P<0.05). MePFC rTMS-induced displacement of DA in the dorsal striatum paralleled the lack of consistent DA release in the NAcc.
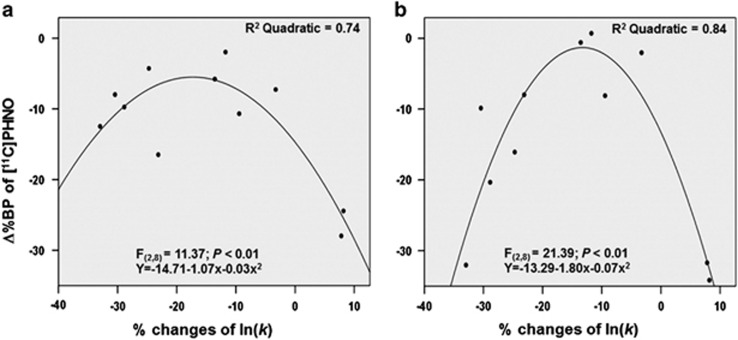
Next, because we were interested in how behavioral changes induced by MePFC-rTMS were associated with changes in striatal DA level, we conducted a regression analysis to test this relationship. A quadratic relationship with an inverted U-shaped curve was found between [11C]-(+)-PHNO Δ%BP and % changes of ln(k) in the bilateral GP (R2=0.74, F2,8=8.17, P<0.01 for left; R2=0.84, F2,8=21.39, P<0.01 for right) (Figure 5a and b). In particular, discounting level interacted with striatal dopamine displacement, and participants who showed preferences toward future rewards tended to occupy in general the upper portion of the inverted U-shaped curve, exhibiting on average less striatal DA release. Thus, suggesting a modulatory role of rTMS over MePFC both on rate of discounting (ie, increased preference of delayed rewards) and striatal DA release.
Figure 5.
Dopamine-behavioral relationships showing changes in DD rate in the left (a) and right (b) GP. A quadratic relationship was found between [11C]-(+)-PHNO Δ%BP and % changes of ln(k) in the bilateral GP (R2=0.74, F2,8=8.17, P<0.01 for left; R2=0.84, F2,8=21.39, P<0.01 for right).
No significant relationship between the behavioral measures (BDI, SAST, and BIS) and rTMS effects were observed.
DISCUSSION
In summary, stimulation of MePFC influenced preference choice for future larger rewards supporting the original hypothesis that this brain area participates in the subjective valuation of delayed monetary rewards. In particular, although rTMS modulation of excitability of MePFC affected individual discounting level and guided the individual preference to delayed rewards, these behavioral changes were not associated with rTMS-induced release of DA in the NAcc, as proposed in our initial working hypothesis. Interestingly, DA release was observed mainly in the dorsal striatum, bilaterally. The changes in synaptic DA in the left dorsal striatum and the lack of DA release in the ipsilateral NAcc appeared interrelated, and the magnitude of release of DA was less strong in those individuals with rTMS-induced preference for future rewards.
Repetitive TMS may have influenced the subjective preference for future larger rewards by a number of possible mechanisms. It is well established that 10-Hz rTMS has an excitatory effect on the underlying cortex and is able to control release of DA in the striatum (Strafella et al, 2001; Strafella et al, 2003; Ko and Strafella, 2012). During these inter-temporal choices, individuals must choose between rewards of differing magnitude and delay, based on the relationship of the timing of rewards and their utility. Within this framework, striatal DA plays an important role in controlling how the timing of a reward is incorporated into the construction of its ultimate value (Kable and Glimcher, 2007; Pine et al, 2010). Investigations using neuropsychological and pharmacological approaches studying time perception in animals and humans have confirmed the hypothesis that fronto-striatal circuits, modulated by the striatal dopaminergic system, are crucial for inter-temporal reward processing (Matell and Meck, 2004; Wittmann and Paulus, 2008). Imaging experiments have shown how areas of MePFC, reciprocally connected to the dorsal striatum and in turn conveying information to the GP, may contribute to the neurobiological substrates of different temporal information related to reward processing (Stevens et al, 2007). The involvement of these regions, consistent with our own results, suggests that rTMS-induced activation of the target region (ie, MePFC) and related DA release in the bilateral dorsal striatum (and not in the NAcc) may reduce impulsivity level and influence choice preferences for future larger rewards. The fact that rTMS did not induce any changes in synaptic DA in the NAcc, a region consistently reported in previous studies to be associated with increased impulsive behavior (Kable and Glimcher, 2007; Ballard and Knutson, 2009) is coherent with our current observation and raises the possibility that rTMS-induced dopaminergic changes in the ventral striatum could have led to completely opposite results (ie, increased preference for immediate rewards).
Thus, by manipulating MePFC and bilateral dorsal striatal DA with rTMS, the present study yielded findings that might reconcile the role of these areas in the subjective valuation of delayed monetary rewards, suggesting that in economic inter-temporal decision making, subjective sense of time and value for reward are critically controlled by these important regions.
Using [11C]-(+)-PHNO, several studies have examined the effect of dopamine concentrations in human subject. One study, after treatment with 2 mg/kg amphetamine i.v, reported significant displacement of [11C]-(+)-PHNO bindings in different striatal regions (ventral striatum 25%, caudate 13%, and putamen 21%) (Willeit et al, 2008). A more recent study using an oral dose of 0.3 mg/kg of amphetamine also reported similar amount of [11C]-(+)-PHNO binding changes in the same regions (ventral striatum 21%, caudate 12%, putamen 16%, and GP 11%) (Shotbolt et al, 2012). Thus, the magnitude of % changes observed in our acute MePFC-rTMS challenge appears quite consistent with these reports following administration of amphetamine.
On the basis of the current findings, we could postulate that rTMS (and other related brain stimulation techniques) of the MePFC could possibly be considered as therapeutic intervention of refractory form of impulse control disorders in different neuropsychiatric and drug-abuse conditions. This would require eventually future additional PET imaging studies to investigate the effect of MePFC stimulation on prefrontal dopamine by using high-affinity radiotracers able to measure extrastriatal dopamine (eg [11C]FLB 457).
FUNDING AND DISCLOSURE
This study was supported by the Canadian Institutes of Health Research (MOP 110962). A.P.S. is supported by the Canada Research Chair program. S.S.C. is supported by a fellowship from Parkinson Society Canada. In the last 5 years, Z.J.D. received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc and a travel allowance through Merck. Z.J.D. has also received speaker funding through Sepracor Inc, AstraZeneca and served on the advisory board for Hoffmann-La Roche Limited and Merck and received speaker support from Eli Lilly. This work was supported by the Ontario Mental Health Foundation (OMHF), the Canadian Institutes of Health Research (CIHR), the Brain and Behaviour Research Foundation, the Temerty Family and Grant Family and through the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute. The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
AUTHOR CONTRIBUTIONS
A.P.S. and S.S.C. designed the experiments; S.S.C., Y.K., K.A., and I.O. performed the experiments; S.S.C. analyzed data and P.R. helped in quantification analysis of PET data; A.P.S. and S.S.C wrote the manuscript; A.E.L., Z.J.D., and S.H. helped in shaping the manuscript and discussion.
Supplementary Material
References
- Allison T, McCarthy G, Luby M, Puce A, Spencer DD. Localization of functional regions of human mesial cortex by somatosensory evoked potential recording and by cortical stimulation. Electroencephalogr Clin Neurophysiol. 1996;100:126–140. doi: 10.1016/0013-4694(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J Abnorm Child Psychol. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Beckstead RM. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. J Comp Neurol. 1979;184:43–62. doi: 10.1002/cne.901840104. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Jacobs EA, Sanders S, Guercio JM, Soldner J, Parker-Singler S, et al. Impulsivity, self-control, and delay discounting in persons with acquired brain injury. Behavioral Interventions. 2005;20:101–120. [Google Scholar]
- Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, et al. Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab. 1997;17:932–942. doi: 10.1097/00004647-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, et al. Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther. 1994;268:417–426. [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, et al. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, et al. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15 (7 Pt 1:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housden CR, O'Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Intact reward learning but elevated delay discounting in Parkinson's disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology. 2010;35:2155–2164. doi: 10.1038/npp.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Santiesteban M. Concave utility, transaction costs, and risk in measuring discounting of delayed rewards. J Exp Psychol Learn Mem Cogn. 2003;29:66–79. [PubMed] [Google Scholar]
- Kirby KNP, Nancy M, Bickel WarrenK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Ko JH, Strafella AP. Dopaminergic neurotransmission in the human brain: new lessons from perturbation and imaging. Neuroscientist. 2012;18:149–168. doi: 10.1177/1073858411401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4 (3 Pt 1:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK, Jacobs EA. Discounting of delayed rewards in opioid-dependent outpatients: exponential or hyperbolic discounting functions. Exp Clin Psychopharmacol. 1999;7:284–293. doi: 10.1037//1064-1297.7.3.284. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30:8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002;87:2577–2592. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, et al. Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab. 2012;32:127–136. doi: 10.1038/jcbfm.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella M. Normative data and a short form of the Barratt Impulsiveness Scale. Int J Neurosci. 2007;117:359–368. doi: 10.1080/00207450600588881. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Hum Brain Mapp. 2007;28:394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126 (Pt 12:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme: Stuttgart; 1988. [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Hanajima R, Machii K, Furubayashi T, Mochizuki H, et al. Predominant activation of I1-waves from the leg motor area by transcranial magnetic stimulation. Brain Res. 2000;859:137–146. doi: 10.1016/s0006-8993(00)01975-2. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, et al. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, et al. High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry. 2006;59:389–394. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9 -ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Xu L, Liang ZY, Wang K, Li S, Jiang T. Neural mechanism of intertemporal choice: from discounting future gains to future losses. Brain Res. 2009;1261:65–74. doi: 10.1016/j.brainres.2008.12.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.