Abstract
Changes in cerebral cortical anatomy have been tied to the clinical course of attention deficit hyperactivity disorder (ADHD). We now ask if alterations in white matter tract microstructure are likewise linked with the adult outcome of childhood ADHD. Seventy-five young adults, 32 with ADHD persisting from childhood and 43 with symptom remission were contrasted against 74 never-affected comparison subjects. Using diffusion tensor imaging, we defined fractional anisotropy, a metric related to white matter microstructure, along with measures of diffusion perpendicular (radial) and parallel (axial) to the axon. Analyses were adjusted for head motion, age and sex, and controlled for multiple comparisons and medication history. Tract-based analyses showed that greater adult inattention, but not hyperactivity–impulsivity, was associated with significantly lower fractional anisotropy in the left uncinate (standardized β=−0.37, t=3.28, p=0.002) and inferior fronto-occipital fasciculi (standardized β=−0.37, t=3.29, p=0.002). The ADHD group with symptoms persisting into adulthood had significantly lower fractional anisotropy than the never-affected controls in these tracts, differences associated with medium to large effect sizes. By contrast, the ADHD group that remitted by adulthood did not differ significantly from controls. The anomalies were found in tracts that connect components of neural systems pertinent to ADHD, such as attention control (inferior fronto-occipital fasciculus) and emotion regulation and the processing of reward (the uncinate fasciculus). Change in radial rather than axial diffusivity was the primary driver of this effect, suggesting pathophysiological processes including altered myelination as future targets for pharmacological and behavioral interventions.
INTRODUCTION
ADHD is far from being a problem confined to childhood, but rather it persists into adulthood in 20–40% of individuals (Faraone et al, 2006). Problems with attention tend to be more prominent than hyperactivity–impulsivity in adults and are a major cause of impairment for the individuals in the workplace and in interpersonal relationships (Birnbaum et al, 2005; Faraone et al, 2006; Pingault, 2011). Understanding the neural basis of the variable outcome is not only a public health priority but could also help stimulate novel treatment approaches to promote remission from ADHD.
ADHD is increasingly conceptualized as the result of disruption to multiple neural systems rather than reflecting dysfunction within one neuropsychological domain (Casey and Durston, 2006; Castellanos and Proal, 2012; Durston et al, 2011; Nigg and Casey, 2005; Sonuga-Barke, 2005). Thus it is likely that the variable clinical course of the disorder will reflect the development of multiple neural systems. In support of this concept, we recently found that severity of adult inattentive symptoms of ADHD was linked with differing developmental trajectories within the cortical components of multiple neural networks supporting attention, cognitive control, and the default mode network (Shaw et al, 2013). A logical next step is to examine the properties of the white matter tracts that constitute the structural connections within these neural systems. This can be attained using diffusion tensor imaging (DTI), which probes tissue microstructure through the diffusion of water within the brain (Basser et al, 1994). The technique provides measures of white matter microstructure such as fractional anisotropy (FA), and more focused measures, specifically diffusion parallel (axial diffusivity) and perpendicular (radial diffusivity) to axons (Basser and Pierpaoli, 1996). FA is determined by a complex amalgam of the physical determinants of white matter microstructure, including axonal packing, myelination, and the coherence of fiber direction (Basser and Pierpaoli, 1996). Although a direct mapping from FA to a specific tissue characteristic is thus not possible, the metric does capture important aspects of white matter architecture that allow us to delineate the physical basis for structural connectivity.
White matter tract morphology is also of interest to those seeking to define the neural processes related to the variable clinical outcome of neurodevelopmental disorders such as ADHD. It is possible that the degree of disruption to white matter microstructure in adults with a history of childhood ADHD will reflect the severity of current adult symptoms. In testing this model, an approach that treats symptoms as a continuous variable is preferred as this maximizes power to detect symptom-related changes. This approach also allows a separate examination of the symptom dimensions that is necessary given their different natural histories and our finding that cortical development is linked with adult inattention but not hyperactivity–impulsivity. A second possibility is that a history of childhood ADHD leaves a ‘mark' on white matter microstructure that persists regardless of the course of ADHD in adulthood. The only study to examine adults with a variable outcome from their childhood ADHD found such remnants of childhood status when data were analyzed on the basis of childhood rather than adult diagnostic status (Cortese et al, 2013). An even more stringent approach is to define trait markers in white matter as the anomalies that are found even among adults who have remitted from ADHD. In these adults the contribution of ‘state'-related anomalies reflecting current symptom status is negated, helping dissect out the contribution of an enduring ‘trait'. Here we employ both the approaches.
Progress has been made in charting the neurocognitive basis of the variable clinical course of ADHD. Change in effortful, executive function has been found to track with symptom severity, whereas lower-level processes may reflect the onset of ADHD regardless of its course (Rajendran et al, 2013). This link between cognition and clinical course may be more pronounced in early rather than late childhood (Coghill et al, 2014), and this developmental heterogeneity may explain the limited power of cognitive measures in predicting outcome (van Lieshout et al, 2013). Neuroanatomic imaging studies generally find that cerebral, cerebellar, and hippocampal anatomies are more atypical in adults with persisting symptoms and tend to normalize in those who remit (Halperin and Schulz, 2006; Mackie et al, 2007; Plessen et al, 2006; Shaw et al, 2013). This normalization may be only partial and thus some anomalies that reflect a childhood history of ADHD have been reported in adults (Cortese et al, 2013; Proal et al, 2011). The functional imaging literature similarly finds prominent state-related anomalies sometimes against a background of trait deficits. Thus, whereas decreased thalamo-cortical activation during response preparation in adults reflected their childhood history of ADHD, the level of coordinated activity within this network was linked with the severity of their current, adult symptoms (Clerkin et al, 2013). Similarly, anomalous connectivity within the default mode network in adults reflected both the severity of current ADHD symptoms and a childhood history of ADHD, depending on the region chosen as the center of the network (Mattfeld et al, 2014).
Here, we aim to extend this work by defining white matter microstructure in a group of young adults who have a variable outcome from their childhood ADHD. First, we asked whether anomalies in white matter microstructure would be ‘state related', associated with the severity of adult symptoms of inattention rather than hyperactivity–impulsivity, as we found for cortical development (Shaw et al, 2013). Second, we tested for possible ‘trait' markers that could reflect the history of childhood ADHD in this adult cohort. Finally, we explored whether change in radial or axial diffusivity was more prominent as these measures point to partly distinct pathophysiological mechanisms.
MATERIALS AND METHODS
Participants were drawn from a longitudinal ongoing study into brain development at the intramural programs of the National Institutes of Health. This imaging study examined the adult status of 75 individuals with a childhood history of ADHD. Sixty-four entered the study as children and a childhood diagnosis of DSM-IVR ADHD had been determined using the Parent Diagnostic Interview for Children and Adolescents (Reich, 2000). For the 11 participants who entered the study as young adults, collateral information to confirm a history of childhood ADHD was obtained from parents or health-care providers. For all individuals, the assessment of adult ADHD symptoms was obtained through clinical interviews (either PS or WS) using the clinician-administered ADHD Rating Scale, version IV, providing examples and prompts appropriate for late adolescent and young adult groups (DuPaul et al, 1998). The interviewer rated each of the nine possible symptoms of inattention and nine symptoms of hyperactivity/impulsivity. In line with DSM-5, attention deficit/hyperactivity disorder, combined presentation, was diagnosed when an individual has both five or more symptoms of inattention and five or more symptoms of hyperactivity/impulsivity. The inattentive or hyperactive/impulsive presentations are diagnosed when symptoms are confined to these domains. Presence of other psychiatric diagnoses was established through the Structured Clinical Interview for DSM Axis I Disorders. Medication histories were obtained from participants.
Contrasts were made against 74 subjects who never had ADHD, referred to as the comparison group, who were drawn from a study of typical brain development. The ADHD and comparison groups were matched on sex, age of assessments, and intelligence. All comparison subjects were free of Axis I DSM-IV mental disorders. Intelligence quotient was estimated using age-appropriate versions of the Wechsler intelligence scales. General exclusion criteria were a full-scale IQ of <80, evidence of neurological disorders known to affect brain structure, current substance dependence, or psychotic disorders. Institutional review boards of the National Institutes of Health approved the research protocol, and written informed consent was obtained from participants.
Neuroanatomic Methods
All data were collected on a 3-T HDx MRI system (GE Healthcare, Milwaukee, WI) with gradients capable of 40 mT/m and using an eight-channel phase array coil. DTI data were acquired with a single-shot dual-spin-echo echo-planar imaging sequence, with the following parameters: TE=85 ms, TR=18.5 s, FOV=240 mm, 96 × 96 matrix, slice thickness=2.5 mm, gap=0 mm, and acceleration factor=2. A custom set of diffusion directions and weightings were acquired for a total of 80 volumes: 10 volumes at b-value=0 s/mm2, 10 volumes with evenly distributed directions at b-value=300 s/mm2, and 60 volumes with evenly distributed directions at b-value=1100 s/mm2.
The diffusion data were processed using the TORTOISE software package (Pierpaoli et al, 2010). Preprocessing steps included rigid body motion correction, eddy–current distortion correction, B0 distortion correction, and bicubic upsampling to 1.5 mm isotropic resolution. The diffusion tensor was computed by nonlinear least squares fitting (Basser et al, 1994). All data were visually inspected and individuals who had more than one volume showing artifacts were excluded. The degree of head motion was determined from the absolute value of the three translation parameters that were averaged over the registered volumes and combined using the Euclidean norm. A similar procedure defined a rotation index for each subject. Data sets were excluded if there was >3 mm of combined translation or >0.03 radians of rotation. In addition, data sets with extreme radial or axial diffusivity values in white matter tracts were excluded. Finally, some typical subjects were removed to equate the groups in head motion, sex, and age. As a result, 149 of the initial 200 DTI data sets were retained.
DTI-TK software was used to register the diffusion tensors into a common adult template space (Zhang et al, 2007; Zhang et al, 2009). This spatial normalization software uses the directional information of the diffusion tensors to align the data of all subjects, incorporates sequential rigid, affine, and nonlinear registration steps, and has been ranked as the top-performing tool in its class (Wang et al, 2011). Tract-specific analyses were also performed using this software (Zhang et al, 2010). This defines 11 major white matter tracts through alignment to a template that captures the average shape and diffusion properties of tracts parcellated via modeling of sheet-like white matter fasciculi (Zhang et al, 2010). This software calculates the diffusion measures locally (FA, radial diffusivity, and axial diffusivity), which are then projected onto a medial model, or a mesh of the tract, the model being a sheet-like structure that represents the 3D tract. We estimate the diffusion metric at the point with the maximum FA value along the spokes that correspond to each vertex of the mesh (similar to Tract-Based Spatial Statistics (Smith et al, 2006)). The 11 tracts examined were the bilateral uncinate, inferior fronto-occipital, superior longitudinal, inferior longitudinal, corticospinal fasciculi, and the corpus callosum.
Analyses
We regressed inattention and hyperactive–impulsive symptoms in the ADHD group against FA for the 11 tracts, and a Bonferroni correction for multiple comparisons was applied (0.05/11=0.0045). In adjusted analyses, age, sex, and head motion parameters were entered as covariates; thus the model was:
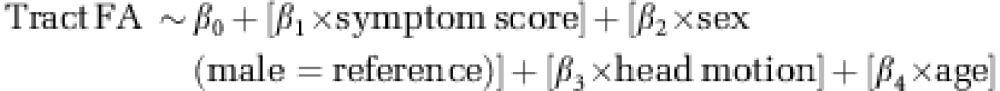 |
In a further regression, the ADHD participants were categorized into persistent and remitted groups, and the FA values for each group were contrasted against the reference, never-affected comparison group. In these regressions, the never-affected group was taken as the reference category. Bonferroni adjustment was made for multiple comparisons (22 planned contrasts for FA; p<0.0023). Group differences were expressed as effect sizes (where d∼0.2 is a small, d∼0.5 is a medium, and d∼0.8 is a large effect size).
Finally, to facilitate comparison with previous studies (Proal et al, 2011), we reanalyzed the data on the basis of childhood diagnoses, comparing the group with a history of childhood ADHD against the never-affected comparison group.
RESULTS
Clinical
The comparison and clinical groups did not differ significantly in age, estimated intelligence, or sex (Table 1). Rates of daily psychostimulant were significantly higher and there was a trend to higher rates of comorbidity in the persistent compared with the remitted ADHD group.
Table 1. Demographic and Clinical Characteristics of the Participants.
| Persistent ADHD N=32 | Remitted ADHD N=43 | Never-affected comparison N=74 | ||
|---|---|---|---|---|
| Age (years) at assessment: mean (SD) | 23.3 (3.7) | 24.1 (3.9) | 24 (3.3) | F(2,146)=0.51, p=0.61 |
| IQ: mean (SD) | 111 (12) | 114 (14) | 113 (11) | F(2,127)=0.89, p=0.41 |
| Sex: males; females | 13; 19 | 26; 17 | 44; 30 | X(2)2=3.77, p=0.15 |
| Symptoms, mean number (SD) | ||||
| Inattention | 5.7 (1.9) | 1.8 (1.4) | NA | t(73)=10.5, p<0.0001 |
| Hyperactivity–impulsivity | 4.3 (2.5) | 1.5 (1.4) | NA | t(73)=6.0, p<0.0001 |
| Comorbidity | ||||
| Any comorbidity | 9 (28%) | 5 (11%) | N/A | Any comorbidity vs none, Fisher's exact test p=0.08 |
| Depression | 4 | 1 | ||
| Dysthymia | 1 | 1 | ||
| GAD/panic disorder | 2 | 2 | ||
| Anxiety NOS | 2 | 2 | ||
| Psychostimulant treatment | ||||
| Taking psychostimulant medication | 16 (50%) | 2 (5%) | N/A | Fisher's exact test p<0.0001 |
Abbreviations: GAD, Generalized anxiety disorder; NOS, not otherwise specified.
Missing data are indicated in the degrees of freedom.
Tract-Based Analyses
In dimensional analyses, there was a significant association between inattentive symptoms and decreased FA in the inferior fronto-occipital longitudinal fasciculi bilaterally and the left corticospinal and uncinate fasciculi—see Table 2 and Figure 1. These links survived adjustment for age, sex, and head motion within the left inferior fronto-occipital and left uncinate fasciculi, with trends for the left corticospinal and right inferior fronto-occipital tracts. There were no significant associations between hyperactive–impulsive symptoms and FA that survived correction for multiple comparisons. However, a nominally significant association emerged for the right superior longitudinal fasciculus (Supplementary Table 1).
Table 2. The Standardized β Coefficients, Associated t and p Values for the Regression of Inattentive Symptoms in the Group with a History of Childhood ADHD against White Matter Metrics for the 11 Major Tracts.
| Tracts |
Inattentive symptoms |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Fractional anisotropy: unadjusted |
Fractional anisotropy: adjusted |
Radial diffusivity: adjusted |
Axial diffusivity: adjusted |
|||||||||
| Std β | t | P | Std β | t | P | Std β | t | P | Std β | t | P | |
| L inferior fronto-occipital | −0.42 | 3.94 | 0.0002a | −0.37 | 3.29 | 0.002a | 0.36 | 4.53 | 0.00001a | 0.14 | 1.57 | 0.12 |
| R inferior fronto-occipital | −0.39 | 3.64 | 0.001a | −0.32 | 2.71 | 0.009 | 0.35 | 4.26 | 0.0004a | 0.08 | 0.87 | 0.39 |
| L uncincate | −0.46 | 4.45 | 0.00003a | −0.37 | 3.28 | 0.002a | 0.35 | 4.43 | 0.00002a | 0.12 | 1.42 | 0.16 |
| R uncincate | −0.30 | 2.67 | 0.009 | −0.21 | 1.72 | 0.09 | 0.29 | 3.51 | 0.001a | 0.15 | 1.79 | 0.08 |
| L corticospinal | −0.35 | 3.17 | 0.002a | −0.33 | 2.84 | 0.006 | 0.10 | 1.21 | 0.23 | 0.06 | 0.69 | 0.49 |
| R corticospinal | −0.31 | 2.77 | 0.007 | −0.23 | 1.98 | 0.05 | 0.05 | 0.62 | 0.53 | −0.03 | 0.38 | 0.7 |
| L superior longitudinal | −0.30 | 2.65 | 0.01 | −0.26 | 2.1 | 0.04 | 0.30 | 3.55 | 0.001a | 0.22 | 2.58 | 0.01 |
| R superior longitudinal | −0.32 | 2.87 | 0.005 | −0.28 | 2.29 | 0.02 | 0.31 | 3.7 | 0.0003a | 0.20 | 3.24 | 0.02 |
| L inferior longitudinal | −0.13 | 1.1 | 0.29 | −0.08 | 0.59 | 0.56 | 0.12 | 1.41 | 0.16 | 0.22 | 2.62 | 0.01 |
| R inferior longitudinal | −0.05 | 0.44 | 0.66 | 0.01 | 0.09 | 0.93 | 0.16 | 1.86 | 0.07 | 0.32 | 3.81 | 0.0002a |
| Corpus callosum | −0.29 | 2.54 | 0.01 | −0.25 | 2.14 | 0.04 | 0.15 | 1.76 | 0.08 | 0.17 | 1.91 | 0.06 |
Adjustment is made for age, head motion parameters, and sex.
Survives Bonferroni adjustment for multiple comparisons.
Results in bold indicate those surviving Bonferroni adjustment for multiple comparisons.
Figure 1.
The top panel shows the 11 major tracts. The left fronto-occipital (in blue) and uncinate (in red) showed a significant association with inattentive symptoms that survived adjustment for multiple comparisons; the superior longitudinal fasciculus (pink); corticospinal tracts (light blue); and corpus callosum did not. Scatter plots with regression lines (and 95% confidence intervals) show the significant association between inattention and fractional anisotropy, and radial but not axial diffusivity in these tracts.
Changes in radial diffusivity were the primary contributor to this link between white matter microstructure and inattentive symptoms—Table 2. Thus, there were significant links between inattentive symptoms and radial diffusivity in both adjusted and unadjusted analyses for the bilateral inferior fronto-occipital, uncinate, and superior longitudinal fasciculi. A significant association between axial diffusivity and inattentive symptoms was found for the right inferior longitudinal fasciculus only.
In the planned categorical contrasts, the remitted ADHD group did not differ significantly from the never-affected comparison group in any tract—Table 3. However, the persistent ADHD group showed reduced FA relative to the never-affected comparison group in the bilateral uncinate fasciculi and the right inferior fronto-occipital fasciculus, which held following adjustment for age, head motion and sex, and for the left corticospinal and left inferior fronto-occipital in unadjusted analyses only. These group differences were associated with medium to large effect sizes. The persistent ADHD also showed a nominally significant reduction in FA in the superior longitudinal fasciculi relative to the comparison group. Again, changes in radial, not axial diffusivity, accounted for these effects. A comparison of the remitted and persistent ADHD groups followed the pattern of results found in the analyses based on symptom scores. Thus the persistent ADHD group had lower FA than the remitted group in adjusted analyses in the bilateral inferior fronto-occipital, the right superior longitudinal and left uncinate fasciculi (Supplementary Table 2).
Table 3. Group Differences in Tract Tensor Values.
| Tracts |
Fractional anisotropy |
Contrast against controls |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Controls |
Remitted ADHD |
Persistent ADHD |
Fractional anisotropy |
Radial diffusivity |
Axial diffusivity |
||||||||||
| Mean (SD) | Mean (SD) | Mean (SD) |
Persistent vs controls: unadjusted |
Persistent vs controls: adjusted |
Remitted vs controls: unadjusted |
Remitted vs controls: adjusted |
Persistent vs controls: adjusted |
Persistent vs controls: zadjusted |
|||||||
| Std β | d | Std β | d | Std β | d | Std β | d | Std β | d | Std β | d | ||||
| L inferior fronto-occipital | 0.444 (0.019) | 0.443 (0.018) | 0.431 (0.022) | −0.27a | 0.61 | −0.24 | 0.55 | −0.03 | 0.07 | −0.02 | 0.05 | 0.32a | 0.73 | 0.17 | 0.38 |
| R inferior fronto-occipital | 0.451 (0.016) | 0.450 (0.014) | 0.439 (0.02) | −0.30a | 0.68 | −0.28a | 0.63 | −0.01 | 0.02 | −0.01 | 0.02 | 0.29a | 0.67 | 0.05 | 0.11 |
| L uncincate | 0.406 (0.024) | 0.403 (0.025) | 0.383 (0.027) | −0.37a | 0.86 | −0.32a | 0.73 | −0.05 | 0.11 | −0.05 | 0.11 | 0.31a | 0.71 | 0.09 | 0.21 |
| R uncincate | 0.400 (0.021) | 0.394 (0.016) | 0.384 (0.024) | −0.31a | 0.72 | −0.28a | 0.54 | −0.13 | 0.26 | −0.13 | 0.27 | 0.29a | 0.66 | 0.13 | 0.29 |
| L corticospinal | 0.421 (0.016) | 0.418 (0.013) | 0.411 (0.016) | −0.26a | 0.60 | −0.23 | 0.52 | −0.09 | 0.18 | −0.09 | 0.19 | 0.04 | 0.09 | 0.001 | 0.00 |
| R corticospinal | 0.437 (0.016) | 0.433 (0.014) | 0.429 (0.017) | −0.21 | 0.22 | −0.17 | 0.38 | −0.12 | 0.24 | −0.11 | 0.24 | −0.01 | 0.01 | −0.07 | 0.14 |
| L superior longitudinal | 0.438 (0.016) | 0.438 (0.017) | 0.429 (0.02) | −0.21 | 0.46 | −0.17 | 0.38 | −0.004 | 0.01 | 0.01 | 0.01 | 0.29a | 0.65 | 0.20 | 0.44 |
| R superior longitudinal | 0.493 (0.022) | 0.494 (0.022) | 0.478 (0.024) | −0.26 | 0.58 | −0.24 | 0.54 | 0.03 | 0.01 | 0.03 | 0.06 | 0.30a | 0.69 | 0.15 | 0.34 |
| L inferior longitudinal | 0.322 (0.031) | 0.328 (0.033) | 0.328 (0.034) | 0.08 | 0.17 | 0.09 | 0.21 | 0.08 | 0.17 | 0.09 | 0.18 | 0.08 | 0.17 | 0.29a | 0.66 |
| R inferior longitudinal | 0.313 (0.031) | 0.311 (0.03) | 0.315 (0.033) | 0.02 | 0.05 | 0.06 | 0.13 | −0.03 | 0.06 | −0.02 | 0.04 | 0.12 | 0.27 | 0.32a | 0.76 |
| Corpus callosum | 0.455 (0.015) | 0.459 (0.014) | 0.455 (0.017) | −0.10 | 0.22 | −0.08 | 0.17 | 0.02 | 0.05 | 0.02 | 0.04 | 0.08 | 0.18 | 0.14 | 0.31 |
Columns 2–4 show the mean (SD) of the fractional anisotropy for each tract. The next columns give the standardized β coefficients for the planned contrasts of the persistent and remitted ADHD groups against the never-affected comparison, ‘control' group (unadjusted and adjusted) with the associated effect size (d). The final columns show radial and axial diffusivities for the persistent against the comparison group; there were no significant differences between the remitted and comparison group.
Survives Bonferroni adjustment for multiple comparisons.
Results in bold indicate those surviving Bonferroni adjustment for multiple comparisons.
The pattern of results held when the presence of comorbidity was entered as a covariate and when those who were taking psychostimulant medication regularly were excluded (Supplementary Table 3).
Finally, in tract-based analyses using childhood diagnostic status (childhood ADHD contrasted against the never-affected comparison group) there were nominally significant reductions in FA in the bilateral inferior fronto-occipital, uncinate, and corticospinal tracts. However, none of these group difference survived adjustment for covariates and multiple comparisons (Supplementary Table 4).
DISCUSSION
We find atypical white matter microstructure among adults whose childhood symptoms of ADHD persist, delineating a major contributor to the abnormal structural connectivity seen in the disorder (Konrad and Eickhoff, 2010b). Specifically, decreased FA was found in the left inferior fronto-occipital and uncinate fasciculi that reflected primarily the severity of adult inattentive but not hyperactive–impulsive symptoms. Whereas the persistent ADHD group differed significantly from the never-affected comparison group, the remitted ADHD group did not. The dominant change resided in diffusion perpendicular and not parallel to the axon; that is, in radial and not axial diffusivity.
The findings both replicate and extend previous studies (Casey et al, 2007; Cortese et al, 2013; Konrad et al, 2010a; Makris et al, 2008). Reduced FA within the inferior fronto-occipital tract that connects visual association cortex to prefrontal and auditory regions is a consistent finding in adult ADHD (Cortese et al, 2013; Konrad et al, 2010a). This tract is implicated in attention set–shifting abilities, semantic processing and reading, cognitive skills which are either impaired in ADHD or found in frequently comorbid learning disabilities (Doricchi et al, 2008; Epelbaum et al, 2008). Decreased FA of the uncinate fasciculus, which joins the orbitofrontal cortex and subcortical limbic regions, has also been reported both in children and adults with ADHD (Konrad et al, 2010a; Nagel et al, 2011). Given that this tract connects core components of systems generating and regulating emotions as well as the processing of reward and salience, its altered microstructure in ADHD is notable given the high prevalence of emotional dysregulation ADHD throughout the life span (Shaw et al, 2014). Fasciculi that showed decreased FA in unadjusted analyses only included the left corticospinal tract. Our study is thus consonant with prior reports of anomalies within these tracts and provides a plausible substrate for the deficits in motor control seen in ADHD, although we note that no significant association with hyperactive–impulsive symptoms was found (Cole et al, 2008; Mostofsky et al, 2006; van Ewijk et al, 2012). Indeed, the only associated with hyperactive–impulsive symptom emerged in the right superior longitudinal fasciculus. This is consonant with previous findings of decreased FA in this tract in those with persistent ADHD (Konrad et al, 2010a; Makris et al, 2008; van Ewijk et al, 2012). This tract connects the inferior parietal lobule and dorsolateral prefrontal cortex, central components of the neural systems subserving the selection of stimuli for attentional focus and working memory, and is implicated in the production of complex motor acts (Oechslin et al, 2009). In summary, the principle anomalies are identified within neural systems that support facets of attentional control (the inferior fronto-occipital fasciculus) and ‘hot' cognitive–affective processes (the uncincate fasciculus), each perhaps contributing to the complex symptom profile of ADHD (Casey and Durston, 2006; Sonuga-Barke, 2005).
We found that inattention, but not hyperactivity–impulsivity, was predominately linked with white matter architectural anomalies. This is congruent with our previous finding that atypical cortical development during adolescence was detected only when considering the final endpoint of adult inattentive, but not hyperactive–impulsive symptoms (Shaw et al, 2013). Such links with adult symptom severity were not reported in the only other DTI study to include adults with childhood ADHD who had either remitted or persistent ADHD (Cortese et al, 2013). The discrepancy may reflect analytic approaches as the previous study used DSM-based categorical analyses, whereas we include analyses treating symptoms as continuous variables, thus enhancing the ability to detect symptom-related changes. Our study also benefits from advances in DTI, particularly the use of 60 noncollinear gradient directions, which allows a more precise determination of the diffusion tensor ellipsoid and thus white matter architecture than the six-direction procedure used in prior adult DTI studies.
The link between white matter microstructure and adult inattention can be interpreted in several ways. First, the disrupted structural connectivity arising from white matter microstructural anomalies might cause inattention. In support of this model, increases in mainly prefrontal white matter FA occur in tandem with the typical development of many cognitive skills, including working memory and information-processing abilities (Nagy et al, 2004; Tamnes et al, 2012). Moreover, the FA of long association tracts correlates with visuospatial attention in healthy adults and the severity of inattentive symptoms in those with ADHD (Tuch et al, 2005; van Ewijk et al, 2012). Alternatively, anomalies in white matter microstructure and the symptoms of ADHD might arise from a common etiological mechanism such as dysregulated modulation of cortical plasticity, in turn perhaps linked with atypical dopaminergic function (Liston et al, 2011). By this model, abnormalities of cortical plasticity that endure into adulthood, perhaps reflected by atypical neuroanatomic trajectories, could lead to both persistent symptoms and disrupted white matter microstructure as common end points. By contrast, if early childhood cortical plasticity anomalies rectify during adolescence, perhaps captured by normalizing neuroanatomic trajectories, this might result in both clinical remission and more typical adult white matter microstructure.
We can only speculate on the genomic and molecular mechanisms at play. Polymorphisms of the dopaminergic system have been associated with the persistence of childhood ADHD into adulthood, specifically of the dopamine transporter gene and dopamine D4 receptor (Franke et al, 2010). The variants implicated appear to be functional, and impact on cortical and striatal dimensions (Durston et al, 2005; Shaw et al, 2007). Dopaminergic tone may also influence white matter microarchitecture. Thus manipulations of dopamine precursors produces parallel changes in the levels of both dopamine and myelin-related proteins, and the dopamine D3 receptor is expressed in oligodendrocyte precursors (Bongarzone et al, 1998; Joseph and Dyer, 2003). In addition, genetic variation within genes controlling myelin-related protein expression have been linked with abnormal white matter structure in substance dependence, which commonly co-occurs with ADHD (Moeller et al, 2004). Thus, our findings provide another context for the interpretation of ongoing large-scale studies into the genomic architecture of ADHD.
This study can also inform future searches for trait markers for ADHD. Some have defined ‘trait' markers in adult cross-sectional data through analyses based on childhood diagnoses When we employed this approach we found trends to decreased FA in multiple tracts, congruent with an earlier study (Cortese et al, 2013). However, when we use the more stringent definition of a trait marker as anomalies present among adults who have remitted, no tract level differences were found. Future studies should evaluate the possibility of static, white matter ‘trait' anomalies in ADHD using prospectively acquired data.
This is the first adult DTI study in ADHD to report on the different components of FA, and finds that white matter changes were almost entirely attributable to shifts in radial, not axial, diffusivity. Given that FA is calculated from both radial and axial diffusivity, a significant difference in radial diffusivity will not always translate into a significant difference in FA, particularly when axial diffusivity does not differ significantly, as we found for some tracts. Moreover, decomposing FA into radial and axial diffusivity can cause problems both in regions of low FA and in areas where white matter tracts are likely to cross (Wheeler-Kingshott and Cercignani, 2009). These pitfalls are mitigated in the current study by restricting analyses to regions of high FA and the spatial extent of the findings, which incorporates regions unlikely to have a high proportion of crossing fibers. Radial diffusivity represents a complex amalgam of neuronal and glial microstructure, prohibiting a simple mapping from this metric to tissue properties (Klawiter et al, 2011; Song et al, 2002; Song et al, 2005). Nonetheless, human and animal studies link changes in myelination with increases in radial, but not axial, diffusivity. As the other contributors to radial diffusivity become apparent, they may join the processes underpinning myelination as novel targets for genomic and molecular studies in ADHD.
Change in white matter detected by DTI has proved a sensitive measure of learning and the efficacy of behavioral interventions (Zatorre et al, 2012). Interventions which boost the ability to sustain attention and regulate emotions in adults have been linked with increased fractional anisotropy in the cingulum bundle, an effect driven primarily by reductions in radial diffusivity (Tang et al, 2012). Such findings suggest that changes in white matter microstructure might serve as a biomarker to help evaluate the efficacy and potentially understand the mechanisms of novel interventions.
Embedding this large cross-sectional study within a prospectively followed cohort avoids the problems inherent in retrospective recall of childhood problems. Among the limitations of the study are the higher rates of comorbidities and psychostimulant use among those with persistent ADHD, possibly contributing to the findings. Against this possibility, results held when analyses were controlled for these factors. We also excluded those who were substance dependent at the time of the study given the impact of substance dependence on white matter microstructure (Pfefferbaum and Sullivan, 2004). A final limitation is that 11 of the 75 clinical participants entered the study as adults, and their childhood diagnosis was made in the community not in the research clinic.
This study demonstrates alterations in ADHD in white matter microstructure tracking with the severity of inattention. The findings are driven by changes in radial rather than axial diffusivity. The biophysical basis of radial diffusivity is only partly understood but includes myelination, a finding that can inform future pathophysiological studies and provide potential targets for pharmacological and behavioral interventions.
FUNDING AND DISCLOSURE
The study was funded by the Intramural Program of the National Human Genome Research Institute and the National Institute of Mental Health. The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, et al. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Curr Med Res Opin. 2005;21:195–205. doi: 10.1185/030079904X20303. [DOI] [PubMed] [Google Scholar]
- Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT. Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci. 1998;18:5344–5353. doi: 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Durston S. From behavior to cognition to the brain and back: what have we learned from functional imaging studies of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:957–960. doi: 10.1176/ajp.2006.163.6.957. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, et al. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am J Psychiatry. 2013;170:1011–1019. doi: 10.1176/appi.ajp.2013.12070880. [DOI] [PubMed] [Google Scholar]
- Coghill D, Hayward D, Rhodes S, Grimmer C, Matthews K. A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): improvements in executive functioning do not explain clinical improvement. Psychol Med. 2014;44:1087–1099. doi: 10.1017/S0033291713001761. [DOI] [PubMed] [Google Scholar]
- Cole W, Mostofsky S, Larson JG, Denckla M, Mahone E. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Imperati D, Zhou J, Proal E, Klein RG, Mannuzza S, et al. White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:591–598. doi: 10.1016/j.biopsych.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P. White matter (dis)connections and gray matter (dys)functions in visual neglect: Gaining insights into the brain networks of spatial awareness. Cortex. 2008;44:983–995. doi: 10.1016/j.cortex.2008.03.006. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power JD, Anastopouls AA, Reid R. ADHD Rating Scale-IV: Checklists, Norms and Clinical Interpretation. The Guilford Press: New York, NY, USA; 1998. [Google Scholar]
- Durston S, Fossella J, Casey B, Hulshoff Pol H, Galvan A, Schnack H, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Durston S, van Belle J, de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1178–1184. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, et al. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44:962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Franke B, Vasquez AA, Johansson S, Hoogman M, Romanos J, Boreatti-Hummer A, et al. Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology. 2010;35:656–664. doi: 10.1038/npp.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Joseph B, Dyer CA. Relationship between myelin production and dopamine synthesis in the PKU mouse brain. J Neurochem. 2003;86:615–626. doi: 10.1046/j.1471-4159.2003.01887.x. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang H-F, Budde MD, Naismith RT, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, et al. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. Eur J Neurosci. 2010;31:912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp. 2010;31:904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Cohen MM, Teslovich T, Levenson D, Casey B. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point. Biol Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and executive systems abnormalities in adults with childhood ADHD: a DT-MRI study of connections. Cereb Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Gabrieli JD, Biederman J, Spencer T, Brown A, Kotte A, et al. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain. 2014;137 (Pt 9:2423–2428. doi: 10.1093/brain/awu137. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2004;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, et al. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:283–292. doi: 10.1016/j.jaac.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Oechslin MS, Imfeld A, Loenneker T, Meyer M, Jäncke L. The plasticity of the superior longitudinal fasciculus as a function of musical expertise: a diffusion tensor imaging study. Front Hum Neurosci. 2009;3:76. doi: 10.3389/neuro.09.076.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2004;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu M, Barnett A, Basser P, Chang L, et al. 2010TORTOISE: an integrated software package for processing of diffusion MRI dataISMRM 18th Annual Meeting, Stockholm, Sweden, #1597.
- Pingault JB. Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: a 16-year longitudinal population-based study. Am J Psychiatry. 2011;168:1164. doi: 10.1176/appi.ajp.2011.10121732. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry. 2011;68:1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran K, Rindskopf D, O'Neill S, Marks DJ, Nomura Y, Halperin JM. Neuropsychological functioning and severity of ADHD in early childhood: A four-year cross-lagged study. J Abnorm Psychol. 2013;122:1179. doi: 10.1037/a0034237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J Am Acad Child Adolesc Psychiatry. 2000;39:59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gornick M, Lerch J, Addington A, Seal J, Greenstein D, et al. Polymorphisms of the dopamine d4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry. 2014;171:276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song S-K, Yoshino J, Le TQ, Lin S-J, Sun S-W, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Østby Y, Walhovd KB. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 2012;32:972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-Y, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci USA. 2012;109:10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci USA. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- van Lieshout M, Luman M, Buitelaar J, Rommelse N, Oosterlaan J. Does neurocognitive functioning predict future or persistence of ADHD? A systematic review. Clin Psychol Rev. 2013;33:539–560. doi: 10.1016/j.cpr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Liu Z, Zhang H, Escolar ML, Gilmore JH, et al. DTI registration in atlas based fiber analysis of infantile Krabbe disease. Neuroimage. 2011;55:1577–1586. doi: 10.1016/j.neuroimage.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About ‘axial' and ‘radial' diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Avants BB, Yushkevich PA, Woo JH, Sumei W, McCluskey LF, et al. High-dimensional spatial normalization of diffusion tensor images improves the detection of white matter differences: an example study using amyotrophic lateral sclerosis. IEEE Trans Med Imaging. 2007;26:1585–1597. doi: 10.1109/TMI.2007.906784. [DOI] [PubMed] [Google Scholar]
- Zhang H, Awate SP, Das SR, Woo JH, Melhem ER, Gee JC, et al. A tract-specific framework for white matter morphometry combining macroscopic and microscopic tract features. Med Image Anal. 2010;14:666–673. doi: 10.1016/j.media.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yushkevich P, Rueckert D, Gee J. A computational DTI template for aging studies. Proc Intl Soc Mag Reson Med. 2009;217:3230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



