Abstract
In this review we elaborate on two main questions concerning the management of Helicobacter pylori infection in children. First, we focus on who should be treated. In the presence of Helicobacter pylori (H. pylori)-associated peptic ulcer disease, eradication of the micro-organism is recommended. When H. pylori infection is detected by biopsy-based methods in the absence of peptic ulcer disease in a child with dyspeptic symptoms, treatment of H. pylori infection may be considered. In infected children whose first degree relatives have gastric cancer, treatment can be offered. A ‘test-and-treat’ strategy is not and has never been recommended in children. The second issue to address is what the recommended treatments are. ESPGHAN/NASPGHAN recommends that treatment tailored to susceptibility testing should be the first choice in pediatric patients. The duration of therapy should be 10-14 days. Costs, compliance and adverse effects should be taken into account. Checking the result of eradication with a reliable non-invasive test such as the 13C urea breath test, is recommended at least 4-8 weeks following completion of therapy.
Keywords: Keywords Helicobacter pylori, treatment, child
Introduction
The purpose of this review is to summarize from a practical point of view the management of Helicobacter pylori (H. pylori) infection in children according to the last joint Recommendations of the European Society of Pediatric Gastroenterology and Nutrition (ESPGHAN) and the North American Society of Pediatric Gastroenterology and Nutrition (NASPGHAN) [1], as well as to comment on the last published papers in this field.
Recommendations for H. pylori treatment in children are not comparable to those in adults, because the level of evidence for most disease outcomes is lower, fewer randomized placebo-controlled treatment trials are available for the different outcomes and often with only small numbers of cases included [2,3]. These and other differences explain why some of the recommendations for adults [4,5] may not apply for children.
Our topic will focus on two main questions in the management of H. pylori infection in children: who and how should be treated?
Who should be treated?
In the presence of H. pylori-associated peptic ulcer disease (PUD), eradication of the micro-organism is recommended
In adults, several meta-analyses demonstrate that eradication of H. pylori in patients with PUD significantly reduces the relapse rate of ulcer disease and recurrent bleeding ulcers [6,7]. In children, previous studies showed a high relapse rate in patients with PUD if they were not treated for their H. pylori infection [8] although only one randomized controlled pediatric trial in H. pylori-infected children with PUD (n=106) has been published. This trial compares the eradication rate of H. pylori and the cure rate of PUD by using three different treatment regimens, but does not report the recurrence of ulcer or bleeding ulcer in those who failed bacterial eradication [9]. However it can be assumed that H. pylori-related PUD recurrence would be prevented in children by eradication of the infection [10,11].
When H. pylori infection is detected by biopsy-based methods in the absence of PUD in children with dyspeptic symptoms, H. pylori treatment may be considered
The finding of H. pylori-associated gastritis in the absence of PUD during diagnostic endoscopy poses a dilemma for the endoscopist. According to the literature review, there is inadequate evidence supporting a causal relationship between H. pylori gastritis and abdominal symptoms in the absence of ulcer disease in children [12,13]. Therefore, eradication of the organism in the absence of ulcers may not result in improvement in symptoms. Also in adults, it is considered that only one of 12 patients with non-ulcer dyspepsia will improve by eradication of H. pylori. However, H. pylori is a risk factor for the development of gastric malignancies. The carcinogenic risk is modified by strain-specific bacterial factors, host responses and/or specific host-microbe interactions [14]. Current evidence suggests that in high risk populations such as the Chinese, the eradication of H. pylori may have the potential to decrease the risk of gastric cancer in a subset of individuals without precancerous lesions [15]. In adults with non-ulcer dyspepsia eradication of H. pylori may reduce the development of peptic ulcers [16]. A potential benefit of chronic infection with certain H. pylori strains cannot be excluded [17]. Therefore, the decision to treat H. pylori-associated gastritis in the absence of duodenal or gastric ulcers is subject to the judgment of the clinician and deliberations with the patient and family taking into consideration the potential risks and benefits of the treatment in the individual patient.
A ‘test-and-treat’ strategy is not recommended in children
The primary goal of testing is to diagnose the cause of clinical symptoms. Since it has been clearly demonstrated that H. pylori infection is not associated with specific symptoms and can even be asymptomatic, the “test-and-treat” strategy (the detection of the presence of H. pylori infection by a non-invasive test followed by treatment in the case of a positive test) cannot provide this information in children. Because of the lack of biopsies the treatment would then be based on standard treatments only but not tailored according to antibiograms which allows testing of the susceptibility or resistance of the strains. For these reasons the use of non-invasive diagnostic tests should be restricted to epidemiological studies and also to check the result of the treatment but avoided as a pre-treatment diagnostic method relating non-typical symptoms to H. pylori infection. The subordination of the treatment to biopsies through the rather invasive endoscopy technique may reduce unnecessary treatments that can result in the development of resistant strains in other microorganisms involved in other diseases, such as pulmonary and urinary.
In contrast to current guidelines for adults [4,5], the “test-and-treat” practice should not be supported in children and treatment should be granted only when H. pylori infection has been documented by histology, and, whenever possible, H. pylori strain antibiogram in order to optimize the treatment.
In children who are infected with H. pylori and whose first-degree relatives have gastric cancer, treatment may be offered
It has been shown in animal models and by several epidemiological and interventional studies that there is a strong causal relationship between H. pylori infection and the risk of gastric malignancies, including cancer and gastric marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (MALT) type [4,5]. Both of these cancer types are extremely rare during the first two decades of life. H. pylori-associated gastric cancer has not been reported in children, whereas few cases of MALT-lymphomas in H. pylori-infected pediatric patients have been described [18,19]. The World Health Organization declared H. pylori a class I carcinogen. Several meta-analyses showed that the risk for gastric cancer is increased by a factor of 2-3 in H. pylori-infected individuals. However, the risk of gastric cancer depends not only on the infection itself, but also on bacterial virulence factors [20], and other factors such as the genetic background of the host and environmental influences including diet [21]. H. pylori eradication of may have the potential to decrease the risk of gastric cancer [15,22]. Individuals with a positive family history for gastric cancer are considered a high-risk group. The risk may be particularly high in H. pylori-infected children in whom the father or the mother is affected by gastric cancer, due to genetic and environmental factors linked with the affected parent, but may also have the same bacterial strain with the same pathogenic properties [23,24]. Thus, the risk of gastric cancer may be much higher for those children with such histories than what has been estimated from epidemiological studies that lack information on relevant factors.
Within the panel, there was strong agreement that testing for H. pylori infection should be considered in children with gastric cancer first-degree relatives. There was also agreement that if H. pylori infection is confirmed in these children both with a reliable non-invasive test or with biopsy-based methods, treatment should be offered and the therapy success should be evaluated to ensure successful eradication.
Surveillance of antibiotic resistance rates of H. pylori strains in children and adolescents is recommended in the different countries and geographic areas
Several European studies have documented high resistance rates to clarithromycin and metronidazole in pediatric and adult populations [10,25-27]. Increasing rates of primary clarithromycin resistance have been reported from several countries [28,29], resistance decreasing only in Belgium [30]. Antibiotic resistance is an important factor of treatment success [25,31]. Indeed, eradication rates in children treated with standard therapy are also decreasing over time, in part related to increased antibiotic resistance. Currently, H. pylori antibiotic susceptibility data are not available in most geographic regions. Therefore, it is recommended that continuous surveillance of resistance rates be undertaken in order to effectively guide initial empiric therapy with the aim of improving treatment outcomes. This is however not enough since there can be important differences of resistance rates within the same region, Brussels being one good example [30,32].
How should the children be treated?
First-line eradication regimens are: triple therapy with a proton-pump inhibitor (PPI) + amoxicillin + clarithromyin or an imidazole; sequential therapy (ST), or bismuth salts + amoxicillin + an imidazole.
Antibiotic susceptibility testing for clarithromycin is recommended prior to initial clarithromycin-based triple therapy especially in areas/populations with a known high resistance rate (>20%) of H. pylori strains in children. The same applies for metronidazole although this is more controversial and will become also essential for quinolones.
It is recommended that the duration of triple therapy be 10-14 days. Costs, compliance and adverse effects should be taken into account. Fig. 1 summarizes the proposed algorithm of how to treat H. pylori in pediatric patients. Table 1 summarizes the different treatment schemes of H. pylori infection in children.
Figure 1.
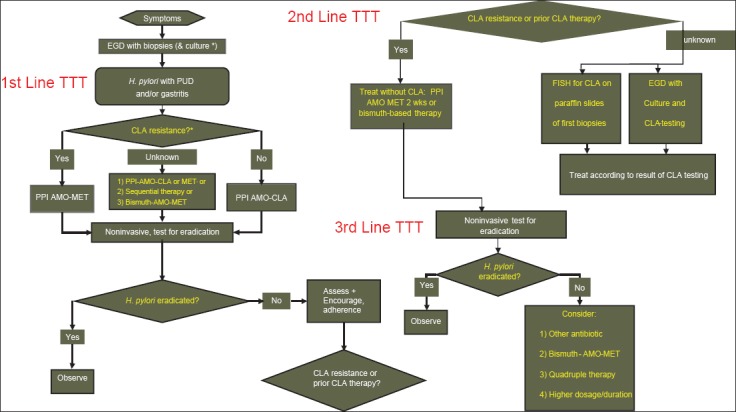
Proposed algorithm of how to treat Helicobacter pylori (H. pylori) infection in pediatric patients. AMO, amoxicillin; CLA, clarithromycin; MET, metronidazole; EGD, esophagogastroduodenoscopy; FISH, fluorescence in situ hybridization; PPI, proton pump inhibitor; PUD, peptic ulcer disease *In areas or populations with a primary clarithromycin-resistance rate of >20% or unknown background antibiotic resistance rates, culture and susceptibility testing should be performed and the treatment should be chosen accordingly ¤If susceptibility testing has not been performed or has failed, antibiotics should be chosen according to the background of the child [1]
Table 1.
Manual of treatment for Helicobacter pylori (H. pylori) eradication in children, according to the latest recommendations of our H. pylori ESPGHAN working group (unpublished data yet)
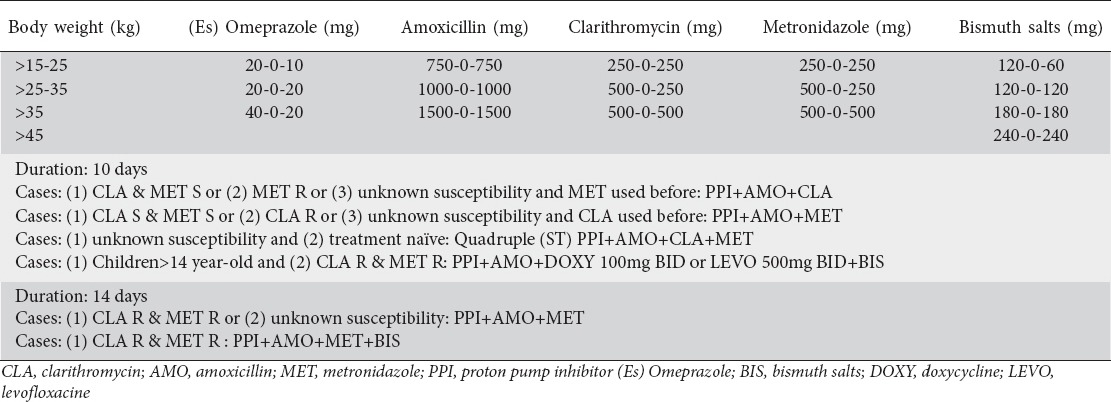
The goal of treatment is to achieve at least a 90% eradication rate on a per protocol (PP) basis at the first attempt [5]. A high initial eradication rate will prevent the development of antibiotic resistance and spreading of resistant H. pylori strains in the population. For individual patients, a high initial success rate will reduce the need for further treatments and invasive procedures, including endoscopies.
Triple therapy: PPI + 2 antibiotics
The combination of two antibiotics and a PPI has been the recommended first-line therapy since the first published pediatric guidelines. [1,33,34]. In 2000, Oderda et al [35] performed a systematic review of the published eradication treatment studies in children. Due to the marked heterogeneity and the limited number of well-designed studies it was difficult to make definitive recommendations. In 2001, the first randomized double blind trial comparing dual therapy of amoxicillin and clarithromycin with triple therapy including omeprazole in children confirmed that, in intention-to-treat (ITT) analysis, triple therapy was far superior to dual therapy with eradication rates of 74.2% versus 9.4% [36]. A meta-analysis of eradication treatment efficacy in children concluded that, in general, the methodological quality of the studies was poor and that additional well-designed randomized trials are needed [3].
The European pediatric treatment registry (PERTH) reported results in 518 children with H. pylori using 27 different regimens [37]. The results, with an overall eradication rate of 65.6%, show a lower eradication rate of H. pylori than previously reported, although the eradication rate of 79.7% was higher in children with peptic ulcers. One potential reason for this decline of the eradication rates is the high level of antibiotic resistance of H. pylori strains negatively influencing the treatment outcomes [10,25,38].
Clarithromycin resistance adversely affects eradication rates in children [39-41]. Studies in children addressing the role of susceptibility testing to target initial therapy are limited [39,40]. However, many studies in children suggest that tailoring therapy based on antibiotic susceptibility testing can enhance eradication rates [40,42-44]. In a study of 58 German children, clarithromycin and metronidazole susceptibility testing was used to guide standard triple therapy and resulted in a high eradication rate of 93% [42]. An earlier study of two consecutive groups of 75 H. pylori-infected children treated with either triple therapy including amoxicillin and clarithromycin (group 1), or antibiotic therapy guided by susceptibility testing (group 2), demonstrated a higher eradication in the group with susceptibility-guided therapy (93% versus 81%) [43]. In an open multicenter trial, 62 children (<18 years, body weight >15 kg) infected with an H. pylori strain resistant to metronidazole and clarithromycin were treated, according to body weight, with amoxicillin (75 mg/kg/day), metronidazole (25 mg/kg/day) and esomeprazole (1.5 mg/kg/day) for 2 weeks, eradication rates were on ITT 66% (41/62) [95% CI 54-78] and PP 73% (33/45) [95% CI 60-86]. They concluded that high-dose amoxicillin, metronidazole, and esomeprazole for 2 weeks is a good treatment option in children infected with a double resistant H. pylori strain [45].
Therefore, nowadays, with the high level of H. pylori-resistant strains and the lower eradication rate obtained by this first-line treatment, clarithromycin-based triple therapy can only be recommended as a first line therapy if susceptibility testing in the individual patient revealed a clarithromycin susceptible strain or if the clarithromycin resistance rate in the area is known to be low (i.e. <20%) [5,30,40]. In the absence of these conditions clarithromycin-based triple therapy cannot be recommended as first-line therapy.
ST
Declining eradication rates with these standard triple regimens have led to the development of alternate treatment options [5,38]. ST involves dual therapy with a PPI and amoxicillin for 5 days followed by 5 days of triple therapy (a PPI with clarithromycin and metronidazole/tinidazole) [40,41,46-54]. In fact, this regimen can be considered as a quadruple therapy given in sequential manner instead of combined in order to decrease the side effects and consequently improves the compliance. It is speculated that the initial use of amoxicillin reduces the bacterial load and provides protection against clarithromycin resistance.
A pooled-data analysis of all ST studies was performed [47]. The eradication rate was calculated taking into account several factors such as the presence of PUD, the PPI used, the antibiotic susceptibility and the patient’s age including children. Overall, more than 1,800 patients were treated with the ST which was found superior to 7- or 10-day triple therapies in adults but also in children and elderly patients with eradication rates constantly higher than 90% in ITT. Similar results were reported by Jafri et al [51] after analyzing data reported on 4,110 adults where 1,363 were treated by ST. They reported an eradication rate of 93.4%, significantly superior to the 76.9% eradication rate of triple therapy performed in 2,747 patients.
The meta-analysis of Gatta et al [52] evaluated the efficacy of ST in adults and children compared to triple therapy. In 13 randomized controlled trials (RCTs) enrolling 3,006 adults, the odds ratio (OR) for eradication of H. pylori with ST compared to standard triple therapy was 2.99 (95% CI 2.47-3.62) in favor of the sequential treatment. In patients with clarithromycin resistance, the OR for eradication with ST compared with clarithromycin containing triple therapy was 10.21 (95% CI: 3.01-34.58). No difference was found between regimens including tinidazole or metronidazole in the ST arm. They also analyzed 3 RCTs enrolling 260 children and adolescents where the OR for eradication was (1.98-95% CI: 0.96-4.07) in favor of the ST but the difference failed to reach statistical significance. No difference in the frequency of side effects was found. In a subsequent study evaluating probiotic supplementation, eradication of 82.5% was obtained from a group of 40 children receiving ST [46].
In a pediatric study, 74 children were randomized to receive either ST (omeprazole plus amoxicillin for 5 days, followed by omeprazole plus clarithromycin plus tinidazole for another 5 days or triple therapy (OAM) for 1 week [49]. Successful eradication was achieved in 97.3% of children receiving ST compared with 75.7% on standard triple therapy. They showed that only the A2143G mutation, involved in the resistance of H. pylori to clarirhromycin (CLA), is associated with a higher risk of treatment failure. They concluded that the ST is more effective than the classical therapy in case of CLA resistance but again their triple therapy was not tailored to antibiotic susceptibility [49].
However it is important to note that the data in children are mostly limited to Italian studies and therefore additional studies in other parts of the world, namely North America but also in different European countries are needed to confirm that the findings apply to other locations. For those reasons, we conducted a pediatric prospective, open-label, multi-center (Belgium, France and Italy) randomized study comparing a sequential regimen to a triple therapy tailored to the anti-microbial susceptibility. The eradication of H. pylori was higher with the ST in ITT and PP results vs. the triple tailored therapy (81.9 and 88.3% vs. 71.9% and 81.8%). However, the difference was not significant except for ITT in susceptible strains for both clarithromycin and metronidazole (87.8% vs 68.5%). In the group of children treated with the ST, the ITT and the PP eradication rates were significantly inferior in case of clarithromycin resistant compared to clarithromycin susceptible strains, ITT 56.2% vs. 72.7%, PP 64.3% vs. 80% [40]. Thus, clarithromycin resistance has a negative impact on eradication even with this regimen although less so compared with the standard triple therapy [40,42, 50].
A recent pediatric meta-analysis study [54], aimed at assessing the ST compared with triple therapy on H. pylori eradication rates. Ten RCTs involving a total of 857 children aged 3-18 years met the inclusion criteria. Of the 409 patients in the ST group, 318 (78%, 95% CI 73-82) were eradicated compared to 314 of the 444 patients (71%, 95% CI 66-75) in the standard triple therapy group (RR 1.14, 95% CI 1.06-1.23). ST was superior to 7-day standard triple therapy, but was not significantly better than 10-day or 14-day triple therapy. There were no significant differences between groups in the risk of adverse effects. They conclude that, the pooled evidence suggests that 10-day ST compared with standard triple therapy may be considered an option for improving eradication rates in children; although it remains at a lower level than desired [54].
Finally, in a recent prospective pediatric European multicenter study of our H. pylori-ESPGHAN working group [53], we reported the results of eradication rates for H. pylori infection in a large cohort of naïve children using a 10 days ST regimen. The overall eradication rate was 80% in the modified ITT analysis, and therefore did not fulfill the requirements of successful treatment regimen which is defined as 90% or higher [5,38]. The eradication rate significantly dropped if the strain was resistant to either clarithromycin (74%) or metronidazole (74%), or both (29%). Surprisingly, in this study, the success rate of the 10 days ST in our children harboring a fully susceptible strain reached only 86%, which is much lower than the reported 87.8% in another pediatric study [48] and 94% under the same conditions in adults [55]. This lower eradication rate cannot be explained by a lower drug dosage, because the calculated dose per kg/bodyweight of the PPI and antibiotics in our treatment regimen was higher, even in the lower range, compared to reported doses in adults. This phenomenon of lower eradication rates in children compared to adults applying the same regimen has been reported before, but was explained by a different antibiotic susceptibility [56]. Adherence to therapy is another predictor of success, and we cannot exclude that adherence was incomplete because, in our cohort, a high percentage of patients had an immigrant background and experienced language difficulties. Another explanation could be that children are more commonly infected with different strains which may not be detected if only one biopsy is taken for culture [10]. Our findings are not weakened by the fact that this was not a randomized controlled trial (RCT). The goal of a treatment trial in therapy naïve patients with H. pylori infection should be to reach the target of 95% or at least 90% eradication rate, and not to show the superiority over a previously used (unsuccessful) therapy. This has been recently emphasized by several articles and editorials [42]. We conclude that the 10 days regimen of ST with moderate to high doses cannot be recommended as first line treatment of children living in Europe. This applies even in countries with a less than 20% resistance rate for clarithromycin. Since the eradication rate was also affected by metronidazole resistance ST should not be given to immigrants from countries with a high metronidazole resistance such as Africa or Asia or to children living in these countries. Our results support ESPGHAN/NASPGHAN recommendations that treatment tailored to susceptibility testing is the first choice in pediatric patients. Further studies are needed to investigate whether with higher doses, different application times such as PPIs given three times per day or addition of probiotics the primary eradication rate can be improved in children.
However, the last hypothesis needs validation in an international study considering also the impact of populations migrating from counties where CLA-resistant rate is high (such as Southern Europe, e.g. Italy) or from countries with poorly determined CLA-resistance rates (such as Africa, Middle-East) to countries with a low CLA-resistance rate (such as Northern Europe, e g. Belgium).
Bismuth salts based therapy
Bismuth salts have been used for the eradication of H. pylori for more than 20 years. However they were progressively less used and replaced by other schemes based on PPI triple therapies. Recent data show that combinations using Bismuth salts must be considered. Bismuth based triple therapy is also recommended as an alternate first line therapy. Although there are no well designed randomized studies directly comparing this regimen with the alternate recommended first line therapies, in a study reported by the European Pediatric Treatment Registry, bismuth-containing triple therapies were more efficacious than PPI-containing ones (77% versus 64%) when used as first-line treatment [37]. Although bismuth salts have proven to be safe, inexpensive and seem to be effective, their use is hindered by the fact that bismuth salts are either banned in some countries or no longer easily available elsewhere. In adults, the efficacy and safety of 10 days of quadruple therapy with omeprazole plus a single three-in-one capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline was recently compared to 7 days standard therapy of omeprazole, amoxicillin, and clarithromycin. The bismuth containing therapy was clearly the most efficient (ITT eradication rates respectively 80% - 174/218 versus 55% - 123/222, P<0·0001) [57]. Unfortunately this treatment cannot be used in children because tetracycline is not recommended and the three-in-one big capsule is difficult to swallow. However, according to the last evidence-based guidelines from ESPGHAN and NASPGHAN for H. pylori infection in children, the use of a bismuth salts based triple therapy in association with amoxicillin and metronidazole was accepted and authorized as a first line eradication regimen.
In addition, bismuth based triple therapy may be less costly than the other options. However, concerns regarding the palatability of bismuth potentially affecting adherence should also be considered.
Several members of different countries expressed an interest for re-using Bismuth salts. In order to evaluate whether the use of Colloidal Bismuth Sub-citrate (CBS) can be an appropriate solution to circumvent the difficulty of H. pylori resistant strains and to lower the cost of eradication treatments, RCTs are needed. Indeed RCTs with bismuth-based treatments compared to classical schemes and enrolment of large numbers of patients in many centers are necessary in order to properly address this question.
Duration of treatment
Conflicting data exists regarding the benefit of longer duration of therapy for first-line regimens in adults [52,58]. A systematic review of therapy in children found no benefit from longer duration of therapy [35]. In contrast a meta-analysis of studies in children suggested that a longer duration of therapy was associated with improved eradication rates [3]. Similarly, a meta-analysis comparing ST with standard triple therapy showed higher eradication rates with a longer duration of triple therapy up to 14 days [52]. Therefore based on this data the recommended duration of therapy is 10 to 14 days taking into consideration cost, compliance and side effects. Suggested doses according to antimicrobial susceptibly testing are given in Table 1.
Probiotics treatment
The recent meta-analysis by Pacifico et al [59], states that there has been no convincing evidence on the beneficial effect of supplementation of probiotics to triple therapy for eradicating H. pylori infection in children. Probiotics are a promising additional treatment and should be carefully considered particularly in the case of eradication failure. The very few trials performed in children on the effect of probiotics alone suggest just a temporary inhibition of H. pylori that disappears with the interruption of the administration of the inhibiting factors.
Probiotic treatments seem able to reduce H. pylori therapy associated side effects and indirectly may help improvement of the eradication rate although the beneficial effects seem to be strain specific. Standardized multicenter, placebo-controlled studies in larger series of children are needed to demonstrate any benefit of probiotics in the management of H. pylori infection in children, including its effect on the severity of H. pylori gastritis. Additional work is necessary to determine the strain, dose and administration to be used. Long-term studies are also needed in children to prove whether the persistent suppressive effect of probiotics on H. pylori and its associated gastritis could prevent diseases such as gastric cancer or peptic ulcer.
A reliable non-invasive test for eradication is recommended at least 4 to 8 weeks following completion of therapy
Even when children become asymptomatic after treatment, it is recommended to evaluate the success of treatment regardless of the initial endoscopic findings. Particularly in children who had PUD, persistence of infection would warrant additional treatment. Reliable tests to monitor successful eradication include the 13C urea breath test and a monoclonal ELISA for detection of H. pylori antigen in stool, at least 2 weeks after stopping the PPI and at least 4 weeks after the completion of the antibiotic treatment. A follow-up endoscopy is not routinely indicated unless other causes of ulceration (such as eosinophilic gastroenteropathy, Crohn’s disease) are suspected, or if biopsies are needed for culture and antibiotic susceptibility testing [1].
If treatment has failed 3 options can be recommended:
-
1)
Check for compliance to treatment and understanding of the eradication scheme;
-
2)
Esophagogastroduodenoscopy with culture and susceptibility testing including alternative antibiotics to guide therapy, if not performed before;
-
3)
Fluorescence in situ hybridization (FISH) on previous paraffin embedded biopsies to guide therapy, if clarithromycin susceptibility testing has not been performed before;
-
4)
Modification of therapy by adding an antibiotic, using different antibiotics, adding bismuth and/or increasing the dose and/or duration of therapy.
Primary antibiotic resistance adversely affects treatment outcomes [38-44] as well as treatment compliance. In Belgium, a 12-year observational study, carried out in children who had failed initial therapy, showed secondary resistance following treatment in 39 of 87 strains [26]. This study suggests that secondary antibiotic resistance development in children is common. Thus, in an H. pylori-infected child who has failed initial therapy, if possible, primary culture with antimicrobial susceptibility testing should be performed to guide second-line therapy.
If primary culture and antimicrobial susceptibility testing is not available, the choice of second-line therapy must take into account the initial therapy administered and avoid re-administering of a previous given antibiotic [60]. Another option available in some centers is FISH to detect primary clarithromycin resistance on previously obtained biopsies [61]. Clarithromycin should only be used as part of second-line therapy if the strain is found to be sensitive. If it is not possible to perform a culture, then the following therapeutic regimens are suggested as second-line or salvage therapy:
Quadruple therapy: PPI + metronidazole + amoxicillin + bismuth. Quadruple therapy is the recommended second-line therapy in most guidelines [5]. However, this regimen is complicated to administer. Furthermore, bismuth salts are not universally available.
Triple therapy: PPI + levofloxacin (moxifloxacin) + amoxicillin. Evaluation of regimens using fluoroquinolones, including levofloxacin, as second-line therapy in children is limited due to its side effects in children aged less than 14 years. In adult studies this regimen appears to be effective. In a recent meta-analysis of studies in adults [61], triple therapy with levofloxacin appeared to be as efficacious as quadruple therapy for second-line treatment. However, there are concerns regarding increasing rates of resistance to quinolones [30,32,60,62,63] and as a consequence, this regimen should not be used if the child has previously received fluoroquinolones.
Although the studies on the ideal duration of therapy for second-line treatment are not conclusive, a longer duration of therapy of up to 14 days is recommended [1].
Biography
Hôpital Saint Vincent de Paul, Groupement des Hôpitaux de l’Institut Catholique de Lille (GH-ICL), Faculté de Médecine & Maïeutique, Lille, France; Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium
Footnotes
Conflict of Interest: None
References
- 1.Koletzko S, Jones NL, Goodman K, et al. on behalf of the H.pylori working groups of ESPGHAN and NASPGHAN. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53:230–243. doi: 10.1097/MPG.0b013e3182227e90. [DOI] [PubMed] [Google Scholar]
- 2.Jones NL, Sherman P, Fallone CA, et al. Canadian Helicobacter Study Group Consensus Conference: Update on the approach to Helicobacter pylori infection in children and adolescents - an evidence-based evaluation. Can J Gastroenterol. 2005;19:399–408. [PubMed] [Google Scholar]
- 3.Khurana R, Fischbach L, Chiba N, et al. Metaanalysis: Helicobacter pylori eradication treatment efficacy in children. Aliment Pharmacol Ther. 2007;25:523–536. doi: 10.1111/j.1365-2036.2006.03236.x. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O’Morain C, et al. Management of Helicobacter pylori infection: the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006;2:CD003840. doi: 10.1002/14651858.CD003840.pub4. [DOI] [PubMed] [Google Scholar]
- 7.Leodolter A, Kulig M, Brasch H, Meyer-Sabellek W, Willich SN, Malfertheiner P. A meta-analysis comparing eradication, healing and relapse rates in patients with Helicobacter pylori- associated gastric or duodenal ulcer. Aliment Pharmacol Ther. 2001;15:1949–1958. doi: 10.1046/j.1365-2036.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- 8.Drumm B, Rhoads JM, Stringer DA, Sherman PM, Ellis LE, Durie PR. Peptic ulcer disease in children: etiology, clinical findings, and clinical course. Pediatrics. 1988;82:410–414. [PubMed] [Google Scholar]
- 9.Shcherbakov PL, Filin VA, Volkov IA, Tatarinov PA, Belousov YB. A randomized comparison of triple therapy Helicobacter pylori eradication on regimens in children with peptic ulcers. J Int Med Res. 2001;29:147–153. doi: 10.1177/147323000102900301. [DOI] [PubMed] [Google Scholar]
- 10.Koletzko S, Richy F, Bontems P, et al. Prospective multicenter study on antibiotic resistance of Helicbacter pylori strains obtained from children living in Europe. Gut. 2006;55:1711–1716. doi: 10.1136/gut.2006.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohil R, Hassall E. Peptic ulcer disease in children. Best Pract Res Clin Gastroenterol. 2000;14:53–73. doi: 10.1053/bega.1999.0059. [DOI] [PubMed] [Google Scholar]
- 12.Macarthur C. Helicobacter pylori infection and childhood recurrent abdominal pain: lack of evidence for a cause and effect relationship. Can J Gastroenterol. 1999;13:607–610. doi: 10.1155/1999/286943. [DOI] [PubMed] [Google Scholar]
- 13.Tindberg Y, Nyren O, Blennow M, Granstrom M. Helicobacter pylori infection and abdominal symptoms among Swedish school children. J Pediatr Gastroenterol Nutr. 2005;41:33–38. doi: 10.1097/01.mpg.0000163734.84518.9e. [DOI] [PubMed] [Google Scholar]
- 14.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 16.Moayyedi P, Soo S, Deeks J, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2001;1:CD002096. doi: 10.1002/14651858.CD002096. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Blaser M. Inverse associations of Helicobacter pylori with asthma and allergy. Am J Epidemiol. 2007;165(Suppl 11):S100. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 18.Moschovi M, Menegas D, Stefanaki K, Constantinidou CV, Tzortzatou-Stathopoulou F. Primary gastric Burkitt lymphoma in childhood: associated with Helicobacter pylori? Med Pediatr Oncol. 2003;41:444–447. doi: 10.1002/mpo.10319. [DOI] [PubMed] [Google Scholar]
- 19.Kurugoglu S, Mihmanli I, Celkan T, Aki H, Aksoy H, Korman U. Radiological features in pediatric primary gastric MALT lymphoma and association with Helicobacter pylori. Pediatr Radiol. 2002;32:82–87. doi: 10.1007/s00247-001-0598-y. [DOI] [PubMed] [Google Scholar]
- 20.Huang JQ, Hunt RH. The evolving epidemiology of Helicobacter pylori infection and gastric cancer. Can J Gastroenterol. 2003;17(Suppl B):18B–20B. doi: 10.1155/2003/692808. [DOI] [PubMed] [Google Scholar]
- 21.Shikata K, Kiyohara Y, Kubo M, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 22.You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–983. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 23.Kivi M, Tindberg Y, Sorberg M, et al. Concordance of Helicobacter pylori strains within families. J Clin Microbiol. 2003;41:5604–5608. doi: 10.1128/JCM.41.12.5604-5608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tindberg Y, Bengtsson C, Granath F, Blennow M, Nyren O, Granstrom M. Helicobacter pylori infection in Swedish school children: lack of evidence of child-to-child transmission outside the family. Gastroenterology. 2003;121:310–316. doi: 10.1053/gast.2001.26282. [DOI] [PubMed] [Google Scholar]
- 25.Dupont C, Kalach N, Raymond J. Helicobacter pylori and antimicrobial susceptibility in children. J Pediatr Gastroenterol Nutr. 2003;36:311–313. doi: 10.1097/00005176-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Bontems P, Devaster JM, Corvaglia L, et al. Twelve year observation of primary and secondary antibiotic-resistant Helicobacter pylori strains in children. Pediatr Infect Dis J. 2001;20:1033–1038. doi: 10.1097/00006454-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Kalach N, Serhal L, Asmar E, et al. Helicobacter pylori primary resistance strains over 11 years in Fench children. Diagn Microbiol Infect Dis. 2007;59:217–222. doi: 10.1016/j.diagmicrobio.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Chisholm SA, Teare EL, Davies K, Owen RJ. Surveillance of primary antibiotic resistance of Helicobacter pylori at centres in England and Wales over a six-year period (2000-2005) Euro Surveill. 2007;12:E3–E4. doi: 10.2807/esm.12.07.00721-en. [DOI] [PubMed] [Google Scholar]
- 29.Boyanova L, Gergova G, Nikolov R, et al. Prevalence and evolution of Helicobacter pylori resistance to 6 antibacterial agents over 12 years and correlation between susceptibility testing methods. Diagn Microbiol Infect Dis. 2008;60:409–415. doi: 10.1016/j.diagmicrobio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Miendje Devi VY, Bontems P, Vanderpas J, et al. Multicenter survey of routine determinations of resistance of Helicobacter pylori to antimicrobials over the last 20 years (1990 to 2009) in Belgium. J Clin Microbiol. 2011;49:2200–2209. doi: 10.1128/JCM.02642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerrits MM, Van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis. 2006;6:699–709. doi: 10.1016/S1473-3099(06)70627-2. [DOI] [PubMed] [Google Scholar]
- 32.Megraud F. Current recommendations for Helicobacter pylori therapies in a world of evolving resistance. Gut Microbes. 2013;4:541–548. doi: 10.4161/gmic.25930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drumm B, Koletzko S, Oderda G. Medical position paper: The European Society for Pediatric Gastroenterology and Nutrition Helicobacter pylori infection in children: a consensus statement. J Pediatr Gastroenterol Nutr. 2000;30:207–213. doi: 10.1097/00005176-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Gold B, Colletti RB, Abbott M, et al. Medical position paper: The North American Society for Pediatric Gastroenterology and Nutrition Helicobacter pylori infection in children: recommendations for diagnosis and treatment. J Pediatr Gastroenterol Nutr. 2000;31:490–497. doi: 10.1097/00005176-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Oderda G, Rapa A, Bona G. A systematic review of Helicobacter pylori eradication treatment schedules in children. Aliment Pharmacol Ther. 2000;14:59–66. doi: 10.1046/j.1365-2036.2000.03102.x. [DOI] [PubMed] [Google Scholar]
- 36.Gottrand F, Kalach N, Spyckerelle C, et al. Omeprazole combined with amoxicillin and clarithromycin in the eradication of Helicobacter pylori in children with gastritis: a prospective randomized double-blind trial. J Pediatr. 2001;139:664–668. doi: 10.1067/mpd.2001.118197. [DOI] [PubMed] [Google Scholar]
- 37.Oderda G, Shcherbakov P, Bontems P, et al. Results from the pediatric European register for treatment of Helicobacter pylori(PERTH) Helicobacter. 2007;12:150–156. doi: 10.1111/j.1523-5378.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 38.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 39.Kalach N, Benhamou PH, Campeotto F, Bergeret M, Dupont C, Raymond J. Clarithromycin resistance and bacterial eradication of Helicobacter pylori in children. Antimicrob Agents Chemother. 2001;45:2134–2135. doi: 10.1128/AAC.45.7.2134-2135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bontems P, Kalach N, Oderda G, et al. Sequential therapy vs. tailored triple therapies for Helicobacter pylori infection in children: a prospective, open-label, multi-center study. J Pediatr Gastroenterol Nutr. 2011;53:646–650. doi: 10.1097/MPG.0b013e318229c769. [DOI] [PubMed] [Google Scholar]
- 41.Gisbert JP, Calvet X, O’Connor A, Megraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 2010;44:313–325. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]
- 42.Arenz T, Antos D, Russmann H, et al. Esomeprazole-based 1-week triple therapy directed by susceptibility testing for eradication of Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2006;43:180–184. doi: 10.1097/01.mpg.0000228103.89454.a2. [DOI] [PubMed] [Google Scholar]
- 43.Street M, Cellini L, Di Campli E, et al. Antibiotic resistance and antibiotic sensitivity based treatment in Helicobacter pylori infection: advantages and outcome. Arch Dis Child. 2001;84:419–422. doi: 10.1136/adc.84.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faber J, Bar-Meir M, Rudensky B, et al. Treatment regimens for Helicobacter pylori infection in children: is in vitro susceptibility testing helpful? J Pediatr Gastroenterol Nutr. 2005;40:571–574. doi: 10.1097/01.mpg.0000155567.71902.75. [DOI] [PubMed] [Google Scholar]
- 45.Schwarzer A, Urruzuno P, Iwańczak B, et al. ESPGHAN Working Group on. Helicobacter pylori infection New effective treatment regimen for children infected with a double resistant Helicobacter pylori strain. J Pediatr Gastroenterol Nutr. 2011;52:424–428. doi: 10.1097/MPG.0b013e3181fc8c58. [DOI] [PubMed] [Google Scholar]
- 46.Lionetti E, Miniello VL, Castellaneta SP, et al. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006;24:1461–1468. doi: 10.1111/j.1365-2036.2006.03145.x. [DOI] [PubMed] [Google Scholar]
- 47.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353–1357. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalach N, Serhal L, Bergeret M, Spyckerelle C, Dupont C, Raymond J. Sequential therapy regimen for Helicobacter pylori infection in children. Arch Pediatr. 2008;15:200–201. doi: 10.1016/j.arcped.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 49.Francavilla R, Lionetti E, Castellaneta SP, et al. Improved efficacy of 10-Day sequential treatment for Helicobacter pylori eradication in children: a randomized trial. Gastroenterology. 2005;129:1414–1419. doi: 10.1053/j.gastro.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Francavilla R, Lionetti E, Castellaneta S, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. J Pediatr. 2010;157:228–232. doi: 10.1016/j.jpeds.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–931. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 52.Gatta L, Vakil N, Leandro G, Di MF, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–3079. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 53.Schwarzer A, Bontems P, Urruzuno P, et al. and ESPGHAN Working group on Helicobacter pylori Sequential therapy as first line treatment in children with new diagnosed symptomatic Helicobacter pylori infection. Helicobacter. 2011;16(Suppl 1):85. (Abstract) [Google Scholar]
- 54.Horvath A, Dziechciarz, Szajewska H, et al. Meta-analysis: sequential therapy for Helicobacter pylori eradication in children. Aliment Pharmacol Ther. 2012;36:534–541. doi: 10.1111/j.1365-2036.2012.05229.x. [DOI] [PubMed] [Google Scholar]
- 55.Vakil N. Helicobacter pylori treatment: new wine in old bottles? Am J Gastroenterol. 2009;104:26–30. doi: 10.1038/ajg.2008.91. [DOI] [PubMed] [Google Scholar]
- 56.Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta-analysis. J Clin Pharm Ther. 2009;34:41–53. doi: 10.1111/j.1365-2710.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 57.Lamarque D, Burucoa C, Courillon-Mallet A, et al. Groupe d’Etudes Français des Helicobacter (GEFH) Révision des recommandations françaises sur la prise en charge de l’infection par Helicobacter pylori. HEPATO-GASTRO et Oncologie digestive. 2012;19:475–502. [Google Scholar]
- 58.Luther J, Higgins PD, Schoenfeld PS, et al. Empiric quadriplevs triple therapy for primary treatment of Helicobacter pylori infection: syqstematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105:65–73. doi: 10.1038/ajg.2009.508. [DOI] [PubMed] [Google Scholar]
- 59.Pacifico L, Frederick O, Bonci E, Romaggioli S, Baldini R, Chiesa C. Probiotics for the treatment of Helicobacter pylori infection in children. World J Gastroenterol. 2014;20:673–683. doi: 10.3748/wjg.v20.i3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007;56:1502. doi: 10.1136/gut.2007.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rüssmann H, Feydt-Schmidt A, Adler K, Aust D, Fischer A, Koletzko S. Detection of Helicobacter pylori in paraffin-embedded and in shock-frozen gastric biopsy samples by fluorescent in situ hybridization. J Clin Microbiol. 2003;41:813–815. doi: 10.1128/JCM.41.2.813-815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35–44. doi: 10.1111/j.1365-2036.2006.02737.x. [DOI] [PubMed] [Google Scholar]
- 63.Raymond J, Kalach N, Lamarque D, Burucoa C. Helicobacter pylori en France:états des lieux des résistances chez l’enfant et chez l’adulte. Arch Pediatr. 2010;17:816–817. doi: 10.1016/S0929-693X(10)70126-3. [DOI] [PubMed] [Google Scholar]


