Abstract
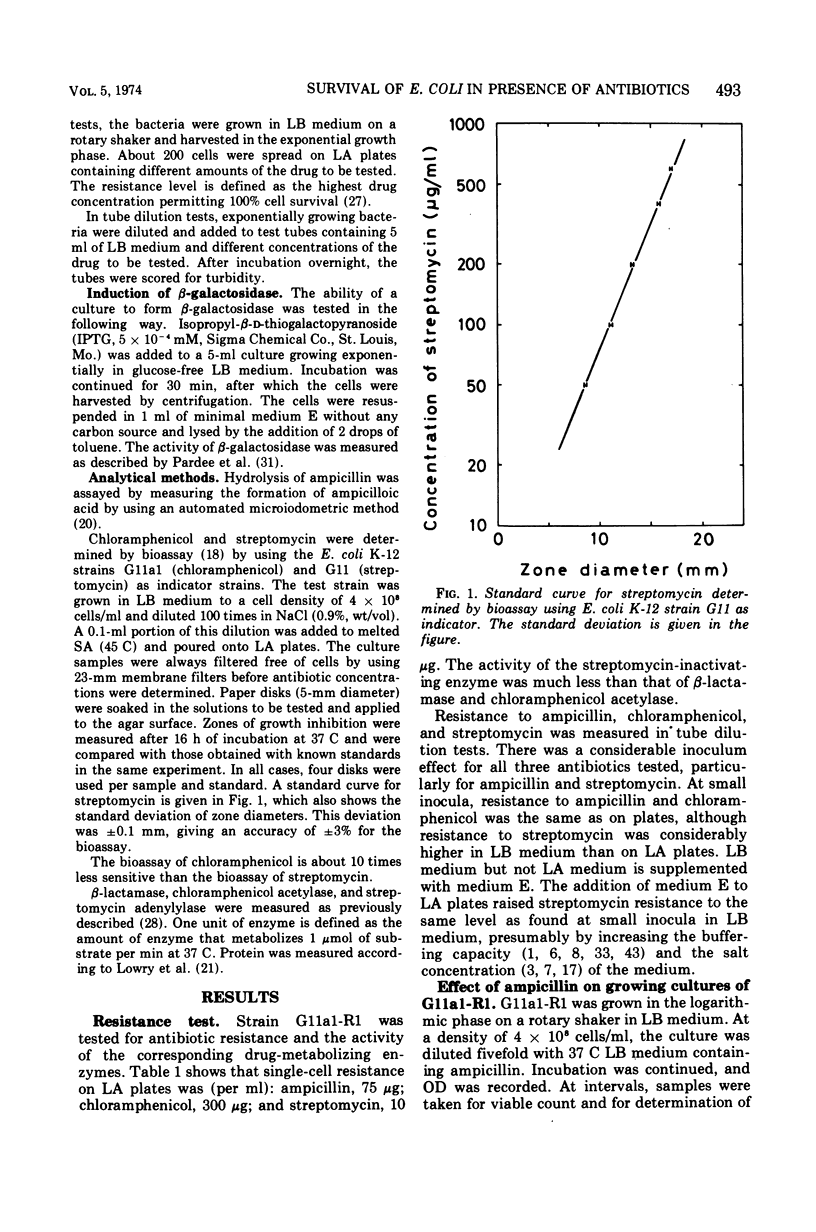
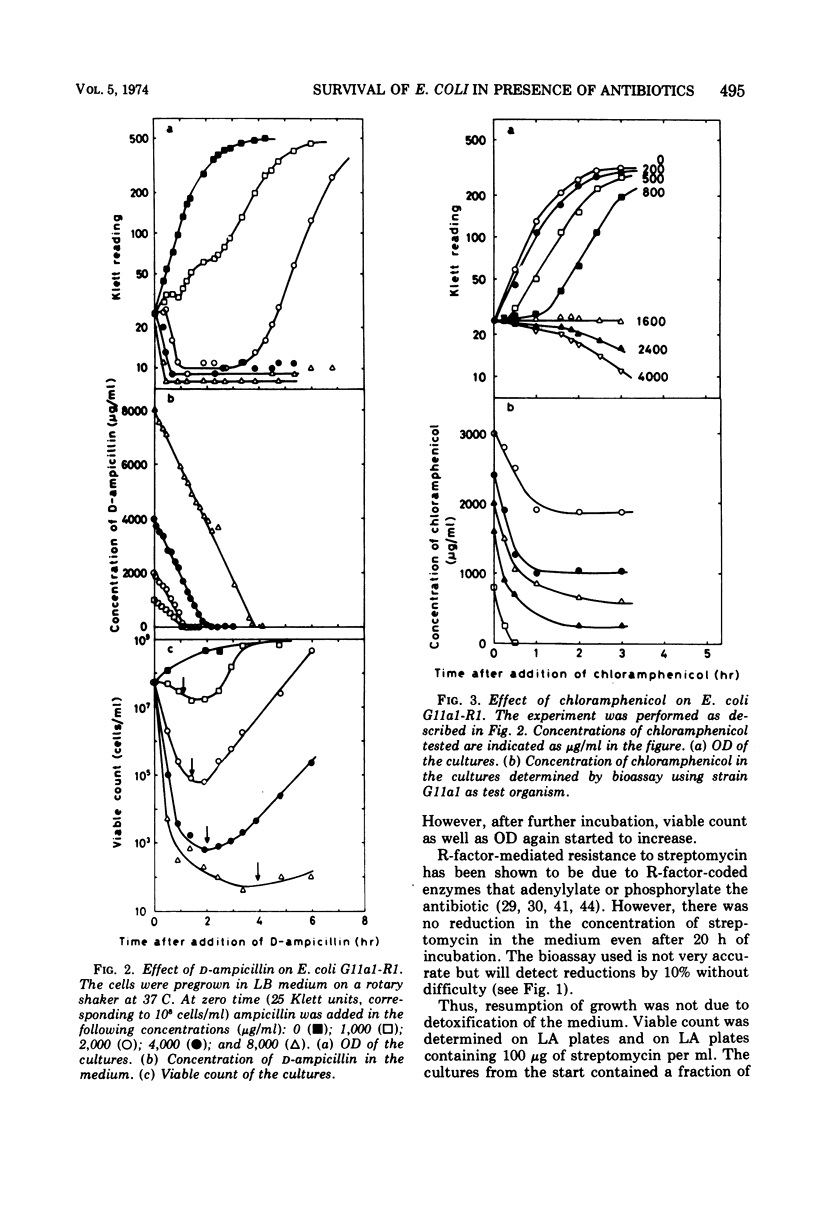
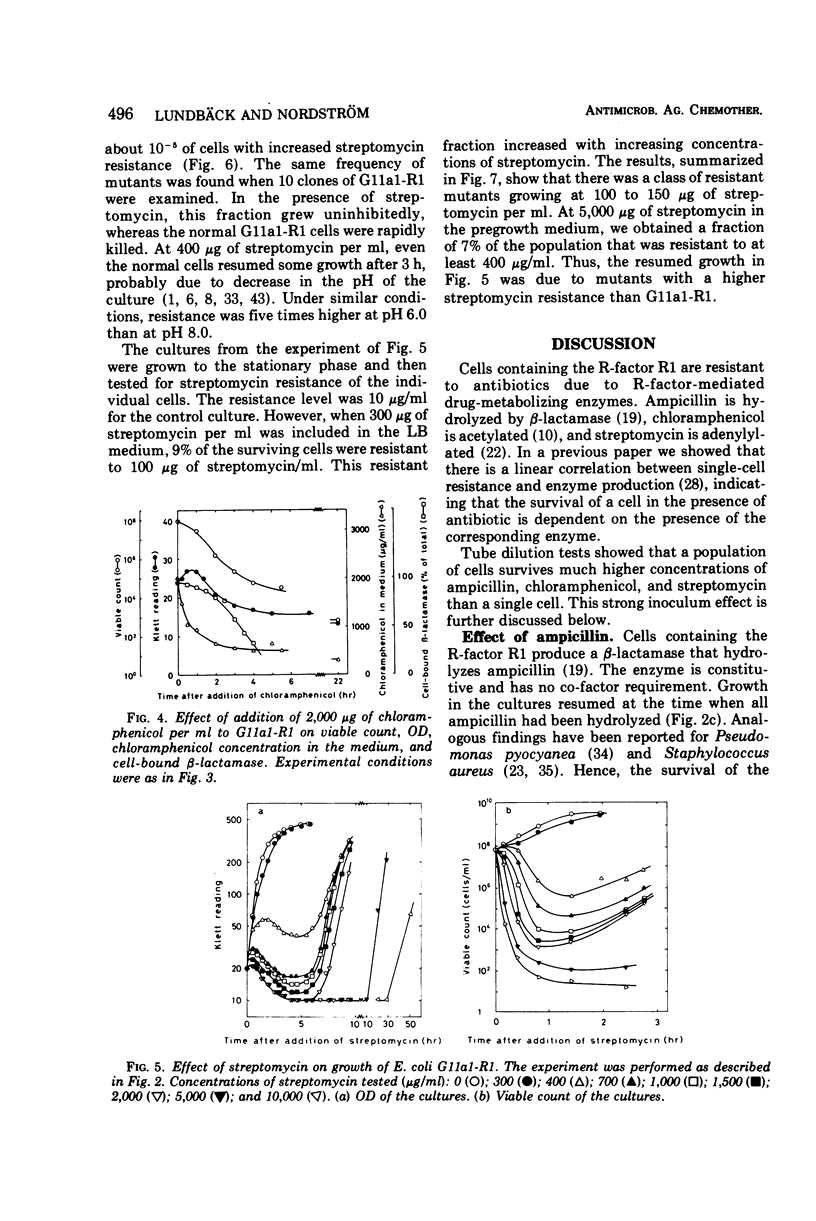
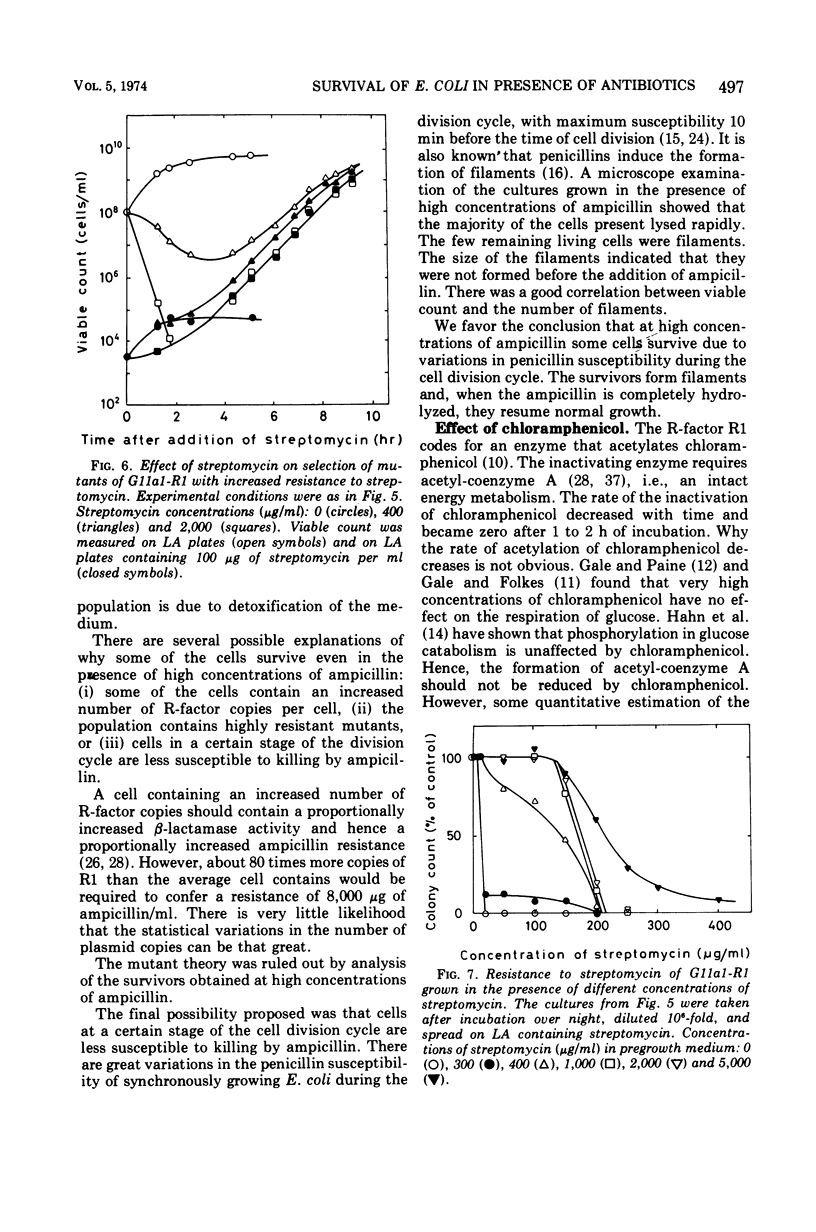
Escherichia coli K-12 carrying the R-factor R1 is resistant to ampicillin, chloramphenicol, and streptomycin. This resistance is due to R-factor-coded enzymes that metabolize the drugs. The effects of these enzymes on the survival of cell populations were studied in the presence of high concentrations of antibiotics. For all three antibiotics there were considerable inoculum effects. For ampicillin and chloramphenicol, the inoculum effect was due to detoxification of the medium, whereas streptomycin was not significantly metabolized. The survival of the population in the presence of streptomycin was due to the presence of resistant mutants. At high concentrations of ampicillin or chloramphenicol, the surviving cells were not mutants. Survival in the presence of ampicillin is presumably due to variations in resistance during the cell division cycle. The rate of acetylation of chloramphenicol decreased with time and was zero after 1 to 2 h. Treatment with high concentrations of chloramphenicol did not cause lysis of the cells but partially opened the outer membrane, causing excretion of β-lactamase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman S., Henry R. J., Housewright R. D. Studies on Streptomycin: I. Factors Influencing the Activity of Streptomycin. J Bacteriol. 1947 May;53(5):567–574. [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. CHLORAMPHENICOL. Bacteriol Rev. 1961 Mar;25(1):32–48. doi: 10.1128/br.25.1.32-48.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K., Bockrath R. C. Physiological Streptomycin Resistance in a Multiauxotroph of Escherichia coli Strain 15 T. J Bacteriol. 1970 Dec;104(3):1294–1298. doi: 10.1128/jb.104.3.1294-1298.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovick R., Bayan A. P., Canales P., Pansy F. The Influence of Certain Substances on the Activity of Streptomycin: III. Differential Effects of Various Electrolytes on the Action of Streptomycin. J Bacteriol. 1948 Jul;56(1):125–137. [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J., Howe T. G. Chloramphenicol acetyl transferase formed by wild-type and complementing R factors in Escherichia coli K12. J Gen Microbiol. 1972 Aug;71(3):575–580. doi: 10.1099/00221287-71-3-575. [DOI] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. The assimilation of amino-acids by bacteria. XV. Actions of antibiotics on nucleic acid and protein synthesis in Staphylococcus aureus. Biochem J. 1953 Feb;53(3):493–498. doi: 10.1042/bj0530493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F., PAINE T. F. The assimilation of amino-acids by bacteria; the action of inhibitors and antibiotics on the accumulation of free glutamic acid and the formation of combined of combined glutamate in Staphylococcus aureus. Biochem J. 1951 Mar;48(3):298–301. doi: 10.1042/bj0480298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHN F. E., WISSEMAN C. L., Jr, HOPPS H. E. Mode of action of chloramphenicol. III. Action of chloramphenicol on bacterial energy metabolism. J Bacteriol. 1955 Feb;69(2):215–223. doi: 10.1128/jb.69.2.215-223.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ C., ROSANO C. L. Studies on mechanism of the streptomycin reaction. I. Phosphate reversal of the dihydrostreptomycin inactivation of Escherichia coli. J Bacteriol. 1958 Jan;75(1):11–15. doi: 10.1128/jb.75.1.11-15.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström E. B., Nordström K. Automated method for determination of penicillins, cephalosporins, and penicillinases. Antimicrob Agents Chemother. 1972 Feb;1(2):100–106. doi: 10.1128/aac.1.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbäck A. K., Nordström K. Mutations in Escherichia coli K-12 decreasing the rate of streptomycin uptake: synergism with R-factor-mediated capacity to inactivate streptomycin. Antimicrob Agents Chemother. 1974 May;5(5):500–507. doi: 10.1128/aac.5.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison G. E. Kinetics of death induced by penicillin and chloramphenicol in synchronous cultures of Escherichia coli. Nature. 1968 Jul 27;219(5152):405–407. doi: 10.1038/219405a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Nordström K. Increased resistance to several antibiotics by one mutation in an R-factor, R 1a. J Gen Microbiol. 1971 May;66(2):205–214. doi: 10.1099/00221287-66-2-205. [DOI] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- SABATH L. D., ABRAHAM E. P. SYNERGISTIC ACTION OF PENICILLINS AND CEPHALOSPORINS AGAINST PSEUDOMONAS PYOCYANEA. Nature. 1964 Dec 12;204:1066–1069. doi: 10.1038/2041066a0. [DOI] [PubMed] [Google Scholar]
- SABATH L. D., FINLAND M. Inactivation of methicillin, oxacillin and ancillin by Staphylococcus aureus. Proc Soc Exp Biol Med. 1962 Dec;111:547–550. doi: 10.3181/00379727-111-27850. [DOI] [PubMed] [Google Scholar]
- SENEZ J. C. Some considerations on the energetics of bacterial growth. Bacteriol Rev. 1962 Jun;26:95–107. [PMC free article] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Synergy of antibacterial substances by apparently known mechanisms. Antimicrob Agents Chemother (Bethesda) 1967;7:210–217. [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S. The enzymatic acetylation of chloramphenicol by the multiple drug-resistant Escherichia coli carrying R factor. J Biol Chem. 1967 Oct 25;242(20):4722–4730. [PubMed] [Google Scholar]
- Takasawa S., Utahara R., Okanishi M., Maeda K., Umezawa H. Studies on adenylylstreptomycin, a product of streptomycin inactivation by E. coli carrying the R-factor. J Antibiot (Tokyo) 1968 Aug;21(8):477–484. doi: 10.7164/antibiotics.21.477. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]


