Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that occurs due to spasms of the neurons, resulting in loss of memory and behavioral changes. In particular, synaptic loss has been described as an early event in the pathogenesis of AD. The increasing evidences have suggested the role of many matrix metalloproteinase (MMPs) in central nervous system (CNS) pathology. Many studies showed that MMPs enzymes are important for the pathophysiological process during Alzheimer’s disease (AD). It is usually believed that the synaptic dysfunction and synapse loss contribute to the cognitive deficits of patients with AD. Cerebrovascular events such as blood-brain barrier (BBB) disruption lead to neuronal damage as well as neuroinflammation. BBB dysfunctions are observed at an early post injury time point, and are associated with activation of proteases, such as MMPs especially MMP-9 which is actively engage in a neuronal injury in the most of the neurodegenerative disorders. BBB opening is accompanied by astrocytic activation, BBB injury and dysregulation of cerebral blood flow. Activated MMPs disrupt neurovascular unit (NVU) which may starve the neurons and affect the synapse function by altering synaptic plasticity and ultimately lead to cognitive decline. However, how MMPs implicated in synaptic dysfunction what are the mechanism associated with this disparity needs to discuss for better understanding the role of MMP-9 in pathogenesis of AD. In this review, we focused on the role of astrocytes and MMP-9 in synaptic dysfunction. We also, underlined possible pharmacological strategies for drug development that might offer more insight into the pathogenesis of cerebrovascular disease such as stroke and Vascular dementia.
Keywords: Astrocyte, Neurovascular unit, synapse remodeling, MMPs, Alzheimer’s disease
Introduction
MMPs are implicated in several important physiological events and promote the migration of neural precursors to injury site [1] and neuronal MMP-2 degrades and inactivates a neurite function [2]. Oligodendrocytes utilize MMP-9 to extend processes along an astrocyte extracellular matrix, which is relevant to the initial steps of myelination [3–4]. In addition, MMPs also contribute to the pathogenesis of several CNS diseases such as Alzheimer’s disease (AD), and stroke [5–6]. It is known fact that MMPs are also involved in the brain tissue injury during focal cerebral ischemia in rodents [7–8]. MMPs possess the ability to stimulate numerous pro-inflammatory mediators such as chemokine CXCL-8, interleukine 1β (IL-1β) or tumor necrosis factor α (TNF-α).Matrix metalloproteinases (MMPs) breakdown the collagen type IV (the component of basal membranes). The neurovascular unit (NVU) is a physiological and functional unit encompassing endothelial cells, pericytes, smooth muscle cells, astrocytes and neurons. The NVU interacts with other cell types to create the BBB; this barrier maintains CNS homeostasis and is also thought to regulate CNS blood flow and synaptic activity [9–10]. On the other hand effects of these processes lead to neuronal damage, neuroinflammation as well as blood-brain barrier (BBB) disruption. BBB dysfunctions are observed to be associated with activation of proteases, such as MMPs. BBB opening is accompanied by edema formation using astrocytic activation. Rascher et al. [11] reported that disruption of the extracellular matrix is strongly associated with increased BBB permeability in pathological states. n the central nervous system (CNS), all members of CNS cells such as neurons and glia produce MMPs[12]. Events such as BBB disruption, astrocyte activation and increase in MMP activity leads to abnormal micro vascular and neuronal function in the brain.
Alzheimer’s disease (AD) is the most common form of dementia and characterized by severe neurodegenerative changes, such as cerebral atrophy as well as the loss of neurons and synapses[13–14]. The central nervous system (CNS) has its own resident immune system, in which glial cells (microglia, astrocytes, and oligodendrocytes) serve as a supportive and nutritive role for neurons [15]. The activation of glial cells is remarkably involved in neuroinflammation associated with neurodegenerative diseases such as AD [16]. Microglia, astrocytes and neurons are responsible for the inflammatory process. Reactive astrocytes also contain sufficient amounts of different forms of amyloid beta (Aβ42) during pathogenesis[7–18].Furthermore, the role of MMPs (MMP-9 and MMP-2) is critical for understanding its oddity in synapse remodeling in AD pathology [19–20]. Abovementioned reports strongly infer the role of MMPs in neuronal damage and neurovascular disruption but their role in synaptic remodeling is needed to be discussed.
Astrocytes
Astrocytes are the most frequent cells in the central nervous system which are generally thought to emulate the metabolic activity of neurons and neurotransmitters around synapses [21]. Astrocytes play a crucial role in maintaining physiological condition; whenever there is damage or distress they act as neuronal partners or neuronal supportive cells [22–23]. Available reports have highlighted that activated microglia promotes astrocytic activation in pathological condition. There are various cytokines amongst them areinterleukin-1 (IL-1), IL-6 and tumor necrosis factor alpha (TNF-α) which is release in the pathological conditions [24]. IL-1 which has been reported to be mainly is produced by microglia shares close association with disease pathology and main mediators of inflammation during AD pathology[25]. Singh et al. [26] has also provided collective information on cross talk between microglia and astrocytes in neuropath logical conditions. Increased IL-1 expression has been detected in reactive microglia surrounding amyloid plaques in AD [27]. Ben well et al.[28] showed that IL-1 injection into rat brain resulted in astrocytic activation indicated by GFAP upregulation. Moreover, it has been shown that IL-1 also induces nuclear hypertrophy and intercellular adhesionmolecule-1 (ICAM-1) expression in astrocytes [29–30].
Astrocyte in Alzheimer’s disease
Glial cells (astrocytes, oligodendrocytes and microglia) are significantly abundant in the brain; and are pathologically they are linked to AD. Altered functions of these glial cells have been recognized in AD patients. Moreover, these cells are also altered in animal and cell culture models of AD in the same way they were found in clinical condition. Large numbers of reports available that imply that Aβ induce alteration in the glial cells [31]. Microglia plays an important role in response to the brain injury and infection. The activated microglias assemble around amyloid plaques and degenerate neurons thereby contributing to the neurodegenerative process in AD by producing toxins and inflammatory cytokines[31]. The severe changes in glial cells in AD may promote neuronal degeneration and later on promoting neuronal plasticity and neuronal survival. However, microglia may also remove Aβ, a potential beneficial action of these immune cells [32]. In addition, although synapses degenerate in pathological condition, the remaining synapses may increase in size as compensatory mechanism which is supported by astrocytes[33]. Moreover, the production of neurotrophic factors such as basic fibroblast growth factor acts as a possible causative factor which increases astrocytes associated with Aβ deposits and these neurotropic factors as well as certain cytokines may stabilize the neurodegenerative process in AD[34].
Astrocyte and synapse function
Astrocytes play a role in the formation, maintenance, and pruning of synapses during development. Astrocytes exerta powerful influence on synaptic remodeling and pruning of the healthy adult CNS. Neuron can be activated by astrocyte by various neurotransmitters or neuromodulators, such as glutamate, nitric oxide (NO) [35]. On the other hand, the activated astrocytes affect neuronal function and contribute to the development of various neurodegenerative diseases such as AD. Activation of astrocyte may often lead to loss of the buffering function and contribute to pathological condition. An additional function of microglia includes a significant contribution to synaptic remodeling events [36–37].Although the extent to which this action is directly causative is still in debate[38–39]. However, the various reports have suggested that microglia play a significant role in nursing synaptic function and this might contribute to synapse remodeling. Microglias have been reported to be present extensively in the thalamus, cerebellum, olfactory bulb, and hippocampus during postnatal synaptic remodeling [40]. Work reported by Tremblay et al. [41] demonstrated that sensory input from 8–10 days postnatal alters microglial contact with dendritic spines and thus, alters the subsequent developmental loss of the synapses.
Synapse and dementia
The synapse is an organization of pre-synapse and post-synapse which allows a neuron to communicate electrical or chemical signal to another neuronal cell and are essential for maintenance of neuronal function. Synapse dysfunction is an early and critical incident of Alzheimer's disease (AD) and; there is a strong correlation between the extent of synapse dysfunction and cognitive function such as dementia[42, 14].Hence, it has been proposed that synapse loss underlies the memory impairment in AD. Proper synapse function is important for neuronal viability, and their disruption may account for the cell loss in the later phases of the AD. Evidences suggested that synapses deteriorated between neurons as a result of pathology causes dementia. Reports suggest that a significant decrease in synaptic density in the cortex and hippocampus of AD brain[43–14].Biochemical analysis has also shown a loss of both pre-synaptic and post-synaptic proteins[4]. Indeed, loss of synaptic proteins and other synaptic components appears to be an early circumstance in pathogenesis of AD[45–46].
Astrocyte and MMP
Astrocytes are the most frequent cells in central nervous system which are generally associated with the metabolic activity of neurons and neurotransmitters around synapses [47]. Astrocytes within the CNSplay a major role in the regulation of brain micro environment. In the brain, astrocytes are the main sources of MMPs as well as the ECM components [48–49]. Astrocytes play crucial roles in maintaining of physiological function when there are damages in neurons. Activated astrocytes are found nearby amyloid plaques in the AD brain. Several evidences suggest that they may mediate degradation in the extracellular space [50] and may regulate amyloid plaque degradation [51]. MMPs are expressed in atrocytes [52], and their activity is induced in the presence of Aβ[53]. Accumulating evidence suggest that proteases are key regulators of Aβ metabolism and MMPs could play a role in astrocyte-mediated Aβ degradation. As candidate proteases MMPs are ideally placed to regulate extracellular Aβ levels as MMPs are secreted and activated in the extracellular compartment. Levels of MMP-2 and MMP-9 are significantly elevated during neuronal injury in neurological disease [54–55].In the healthy brain, MMPs are mainly secreted by astrocytes; which are involved in angiogenesis, tissue remodeling, and neurite extension. Due to their permeating presence and capacity to secrete both MMPs and TIMPs, astrocytes have the potential to play a central role in tissue remodeling processes as astrocytes are a source of MMP-9 in ischemic injury [56].
MMPs and neuroinflammation
Matrix metalloproteinase (MMPs) area group of extracellular enzymes. MMPs are involved in function to remodel the pericellular environment, primarily through the cleavage of extra cellular matrix (ECM) proteins. Reports also suggest that changes in MMPs expression have also been associated with neuropathology which involves both neurons (neurodegeneration) and glial cells (astroglia and microglia)[57]. Data from the different study suggest that MMPs may be involved in a variety of cellular functions in the brain. Activation of MMP-9 may contribute to the pathogenesis of several neurological diseases such as stroke, AD, multiple sclerosis, and malignant glioma[7]. Several neuroinflammatory factors including cytokines and oxidative stress have been shown to up-regulate MMP-9 in astrocytes in vitro. We also reported the expression pattern of MMP-9 and MMP-2 in adult mice brain and address changes that these enzymes unregulated and triggered by homocysteine (Hcy) administration[55].
MMP-9, NMDA and synaptic plasticity
The N-methyl-D-aspartate receptor (NMDAR), a glutamate receptor, is the predominant molecular device for controlling of synaptic plasticity and memory function. Gorkiewic et al. [58] demonstrated that enzymatic activity of MMP-9 increased transferring of NR1 subunit-containing NMDA receptors. Moreover the influence of MMP-9 on structural plasticity of dendritic spines which are known to bear excitatory synapses. MMP-9 is potent to cleave synaptic proteins without causing large changes in ECM structure, to modify behavior of important synaptic receptors and to change morphology of synapses. Thus, MMP-9 actively engaged in synaptic remodeling[59]. Previous report shows that beta-dystroglycan (beta-DG), a trans-membrane protein, is a synaptic target for MMP-9 which is cleaved upon neuronal stimulation. Michaluk et al. [60] reported that stimulation of neuronal cultures caused an increase in MMP-9 activity which coincides with cleavage of beta-DG and that this cleavage can be blocked by over-expression of tissue inhibitor of metalloproteinases-1 (TIMP-1). They also showed the involvement of MMP-9 activity in cleavage of beta-DG in vivo, as well as the co-localization of both proteins at the same asymmetric synapses in the hippo-campus.MMP-9 has recently emerged as one of the important molecules involved in synaptic plasticity; however the exact role and the functions of this protease at the neuronal junction’s i.e. synapse is still not clear and should obtain attention.
Role of MMPs and ECM remodeling in learning and memory
Proteases of the matrix metalloproteinase (MMPs) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, angiogenesis, learning and memory, as well as in pathological processes, such as intracerebral hemorrhage[61]. Since now, mainly two secreted types of MMPs, (MMP-2, and MMP-9) are most frequently examined in the brain. Most of the MMPs are secreted as inactive pro-form which are activated when cleaved by extracellular proteinases. In the developing nervous system MMPs play important role in neuronal development. Tissue remodeling proteins, such as matrix metalloproteinases (MMPs) and their endogenous tissue inhibitors (TIMPs) are inflammatory mediators that play important role in the AD brain [55]. MMP-2 is mostly detected in various parts of brain structures including astroglia and some pyramidal neurons in the cortex and Purkinje cells in the cerebellum. However, MMP-9 is expressed in the hippocampus, cerebellum, and cortex, predominantly in neurons [62]. MMPs knockout of particular strain also significantly affect the neuronal injury and neuropathology, demonstrating that MMPs function as the crucial mediators of neuronal disease [63]. Interestingly, several MMP knockouts rodents show deficits in learning and memory [64]. Consistent with these notions, MMPs likely mediate the structural changes of neuronal structure such as dendritic spines as well as axon/dendrite structures in neurological diseases. Dendritic spine morphology and synaptic potentiation are dynamically modulated by proteins of the ECM remodeling; which has an important role in synaptic plasticity [65].
Synapse, MMP-9 and long term potentiation
Many synapses in the mature CNS are consist of glutamate receptors including N-methyl-D-aspartic acid- (NMDA-) type and alpha-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic- acid- (AMPA) type receptors. These synapse receptor are mainly involved in synaptic plasticity. Moreover this receptor actively associates with Long term potentiation which forms memory brain regions such as cortex and hippocampus. In the brain, MMPs are secreted by neurons and glial cells in an inactive (pro) form, and they become proteolytically active when several regulatory steps that result in removal of the propeptide are triggered in response to specific stimuli. Moreover, In situ zymography assay also revealed an occurrence of MMP-9 with synaptic glutamate receptors [66]. LTP is a widely used cellular model for synaptic plasticity for a extended time period which is thought to be necessary for learning and memory function. In other reports it has also proved that MMP-9 knockout mice show behavioral impairments in hippocampus based learning and memory[64]. Furthermore, hippocampal slice cultures from MMP-9 knockout mice show impaired LTP and thereby impair memory function, which is restored by the application of recombinant MMP-9. In other studies, brief induction of MMP-9 and MMP-3 in hippocampal region was observed when animals were used for Morris water maze test. These out comes, further supporting a role for MMPs in hippocampus based learning and memory [67]. MMP-mediated synaptic potentiation can be inhibited in hippocampal slices by blocking integrin signaling, which suggests that MMP-9 regulates synaptic plasticity and LTP through integrin’s[68].Alternatively, MMP-9 could regulate LTP by enhancing movement of glutamate receptors in excitatory synapses. Therefore, itestablished that modification of the ECM components might facilitate structural and functional plasticity in synapses. Michaluk et al. [69]showed that addition of recombinant MMP-9 in hippocampal cultured neurons increased the diffusion of NMDA receptors but not AMPA receptors suggest that MMP-9 influence the function of NMDA receptor. They further also proved that alteration in the ECM structure takes place through modification of an integrin dependent pathway but not through NMDA receptors [70]. On the other hand, Frischknecht et al. [71] have shown that removal of the ECM through enzymatic process increased the exchange of synaptic AMPA receptors but not NMDA receptors. It is thus confirmed that exchange of AMPA and NMDA receptors through membrane is differentially regulated by the ECM and MMPs in synapses. This functional change may contribute to practical difference of AMPA and NMDA receptors in synaptic plasticity. Previous studies have shown that MMP-9 rapidly becomes active at presynaptic sites in response to LTP. [72]. Thus presynaptic MMP-9 proteolysis in response to LTP induction is possibly critical for dendritic spine remodeling and functions are necessary to support long-term synaptic plasticity [73].
Therapeutic targets and future directions
Astrocyte mediated MMPs activation of Blood-brain barrier (BBB) disruption by matrix MMPs leads to apoptotic cell death of hippocampal neuron and synapse thus causes memory impairment. Therefore, targeting neuronal cell death by blocking MMP activation will act as a potential therapeutic agent for preserving BBB integrity following brain injury in humans could be one of the promising approach to treat a disease related to vascular pathology. Evidence also suggests that the Aβ 25–35 fragment stimulates the MMP-9-TIMP-1 pathway. Thus, targeting elimination of amyloid deposition and following anti-inflammatory treatment against MMPs could be considered as a therapeutic measure in AD.
Conclusion
In the pathogenesis of Alzheimer’s disease (AD), synaptic loss has been considered as an early event involving many matrix metalloproteinase (MMPs). In this review we have highlighted the important role of astrocyte and MMPs in synaptic dysfunction. In conclusion, these collective evidences will pave the way in drug development for improving clinical outcome after cerebrovascular disease such as stroke and vascular dementia.
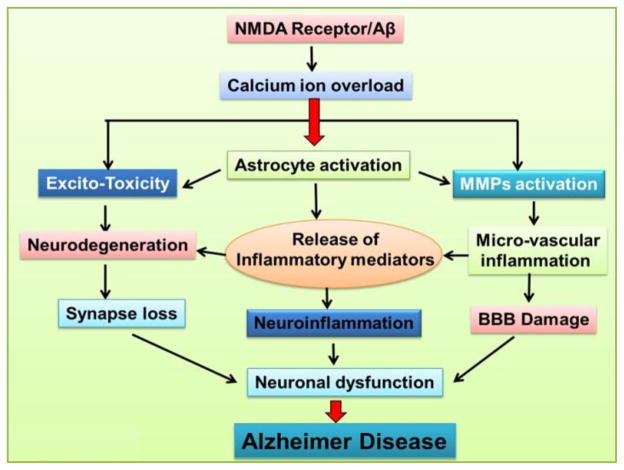
Fig 1. Possible mechanism of N-methyl-D-aspartate receptor and Aβ in Alzheimer Disease.
Deposition of amyloid plaques (Aβ) is characterized in Alzheimer’s disease (AD) which affects NMDAr resulting in Calcium-ion overload. High level of calcium-ion overload triggers critical events for neuronal dysfunction i.e. excitotoxicity, astrocyte activation and MMPs activation. Excitotoxic cell death (ECD) is an event due to increased intracellular Ca2+ by over-stimulation of NMDA receptor which results in synapse loss due to neurodegeneration. Aβ plays a role in inducing many alterations in glial cells by encouraging calcium-ion overload. The activated glial cells assemble around amyloid plaques or degenerating neurons and contribute in the neurodegenerative process in AD by causing neuroinflammation due to release of toxins or inflammatory mediators like cytokines. Astrocytes compete with the metabolic activity of neurons and neurotransmitters around synapses and considered as the major source of MMPs. MMPs are expressed in astrocytes and their activity is induced in the presence of Aβ. MMPs activation results in micro vascular inflammation as a consequent effect neuronal dysfunction due to BBB damage in AD.
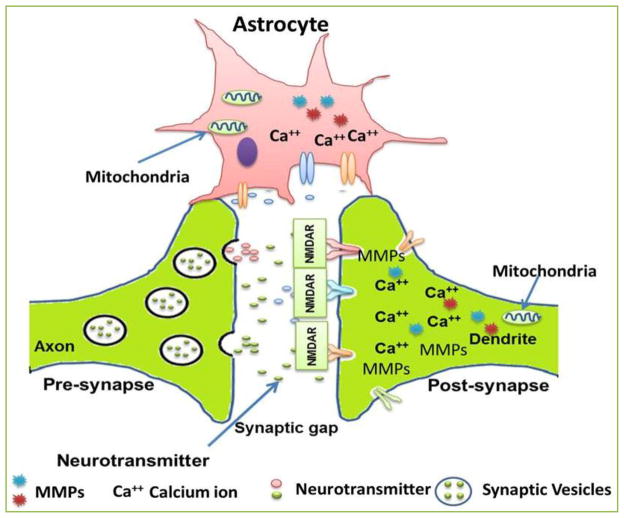
Fig 2. Schematic representation of the astrocyte mediated presynaptic and postsynaptic modifications.
Synapse is a specialized communication junction between a pre-synaptic cell (usually a neuron) that sends out a signal and a post-synaptic cell that receives the signal. Neurotransmitter molecules diffuse across the synapses and bind to their specific receptors on the post-synaptic cell. This recreates the action potential in the post-synaptic cell. When a postsynaptic action potential develops it leads to Ca2+ influx into the postsynaptic neuron. This is followed by a presynaptic action potential, leading to activation of mGluRs and astrocytes, which cause release of Ca2+ from intracellular stores. The resultant Ca2+ triggers vesicular release of glutamate from the astrocyte, which in turn activates presynaptic NMDARs. Astrocytes could act as an important factor in maintaining neurovascular unit. The channel which governs physiological regulation across synapse usually gets damaged or lost in neurodegenerative diseases.
Acknowledgments
Financial support to SS and SR from Council of Scientific and Industrial Research (CSIR), New Delhi, India and financial support to PK and NT from National Institute of Health, USA is gratefully acknowledged.
Footnotes
Conflicting interests: None
References
- 1.Del Bigio MR, Tchelingerian JL, Jacque CM. Expression of extracellular matrix degrading enzymes during migration of xenografted brain cells. Neuropathology and applied neurobiology. 1999;25:54–62. doi: 10.1046/j.1365-2990.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 2.Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. The Journal of neuroscience. 1998;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh LY, Larsen PH, Krekoski CA, Edwards DR, Donovan F, Werb Z, et al. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. The Journal of neuroscience. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uhm JH, Dooley NP, Oh LY, Yong VW. Oligodendrocytes utilize a matrix metalloproteinase, MMP-9, to extend processes along an astrocyte extracellular matrix. Glia. 1998;22:53–63. doi: 10.1002/(sici)1098-1136(199801)22:1<53::aid-glia5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Kurzepa J, Bartosik-Psujek H, Suchozebrska-Jesionek D, Rejdak K, Stryjecka-Zimmer M, Stelmasiak Z. Role of matrix metalloproteinases in the pathogenesis of multiple sclerosis. Neurologiaineurochirurgiapolska. 2005;39:63–67. [PubMed] [Google Scholar]
- 6.Romi F, Helgeland G, Gilhus NE. Serum levels of matrix metalloproteinases: implications in clinical neurology. European neurology. 2012;67:121–128. doi: 10.1159/000334862. [DOI] [PubMed] [Google Scholar]
- 7.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. Journal of cerebral blood flow and metabolism. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. Journal of cerebral blood flow and metabolism. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, et al. Cell-cell signaling in the neurovascular unit. Neurochemical research. 2007;32:2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 10.McCarty JH. Integrin-mediated regulation of neurovascular development, physiology and disease. Cell adhesion & migration. 2009;3:211–215. doi: 10.4161/cam.3.2.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rascher G, Fischmann A, Kroger S, Duffner F, Grote EH, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Actaneuropathologica. 2002;104:85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- 12.Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends in neurosciences. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 13.Kamat PK, Rai S, Swarnkar S, Shukla R, Nath C. Molecular and Cellular Mechanism of Okadaic Acid (OKA)-Induced Neurotoxicity: A Novel Tool for Alzheimer's Disease Therapeutic Application. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8699-4. [DOI] [PubMed] [Google Scholar]
- 14.Kamat PK, Rai S, Swarnkar S, Shukla R, Nath C. Mechanism of synapse redox stress in Okadaic acid (ICV) induced memory impairment: Role of NMDA receptor. Neurochemistry international. 2014;76C:32–41. doi: 10.1016/j.neuint.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Ricci S, Fuso A, Ippoliti F, Businaro R. Stress-induced cytokines and neuronal dysfunction in Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2012;28:11–24. doi: 10.3233/JAD-2011-110821. [DOI] [PubMed] [Google Scholar]
- 16.Rai S, Kamat PK, Nath C, Shukla R. Glial activation and post-synaptic neurotoxicity: the key events in Streptozotocin (ICV) induced memory impairment in rats. Pharmacology, biochemistry, and behavior. 2014;117:104–117. doi: 10.1016/j.pbb.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiology of aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Attems J, Lintner F, Jellinger KA. Amyloid beta peptide 1–42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Actaneuropathologica. 2004;107:283–291. doi: 10.1007/s00401-004-0822-6. [DOI] [PubMed] [Google Scholar]
- 19.Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. [DOI] [PubMed] [Google Scholar]
- 20.Wang LN, Wang W, Zhang XH, Ma L, Yin H, Li DJ. An interventional study on amnestic mild cognitive impairment with small dose donepezil. Zhonghuaneikezazhi. 2004;43:760–763. [PubMed] [Google Scholar]
- 21.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological reviews. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 22.Swarnkar S, Goswami P, Kamat PK, Gupta S, Patro IK, Singh S, et al. Rotenone-induced apoptosis and role of calcium: a study on Neuro-2a cells. Archives of toxicology. 2012;86:1387–1397. doi: 10.1007/s00204-012-0853-z. [DOI] [PubMed] [Google Scholar]
- 23.Swarnkar S, Singh S, Goswami P, Mathur R, Patro IK, Nath C. Astrocyte activation: a key step in rotenone induced cytotoxicity and DNA damage. Neurochemical research. 2012;37:2178–2189. doi: 10.1007/s11064-012-0841-y. [DOI] [PubMed] [Google Scholar]
- 24.John GR, Lee SC, Song X, Rivieccio M, Brosnan CF. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- 25.Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. Journal of neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Swarnkar S, Goswami P, Nath C. Astrocytes and microglia: responses to neuropathological conditions. The International journal of neuroscience. 2011;121:589–597. doi: 10.3109/00207454.2011.598981. [DOI] [PubMed] [Google Scholar]
- 27.Shaftel SS, Kyrkanides S, Olschowka JA, Miller JN, Johnson RE, O'Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. The Journal of clinical investigation. 2007;117:1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand-activated dendritic cells. Clinical immunology. 2010;134:178–187. doi: 10.1016/j.clim.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Experimental neurology. 2002;173:46–62. doi: 10.1006/exnr.2001.7834. [DOI] [PubMed] [Google Scholar]
- 30.Kyrkanides S, Olschowka JA, Williams JP, Hansen JT, O'Banion MK. TNF alpha and IL-1beta mediate intercellular adhesion molecule-1 induction via microglia-astrocyte interaction in CNS radiation injury. Journal of neuroimmunology. 1999;95:95–106. doi: 10.1016/s0165-5728(98)00270-7. [DOI] [PubMed] [Google Scholar]
- 31.McGeer PL, McGeer EG. The possible role of complement activation in Alzheimer disease. Trends in molecular medicine. 2002;8:519–523. doi: 10.1016/s1471-4914(02)02422-x. [DOI] [PubMed] [Google Scholar]
- 32.Jantzen PT, Connor KE, Di Carlo G, Wenk GL, Wallace JL, Rojiani AM, et al. Microglial activation and beta -amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. The Journal of neuroscience. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murai KK, Nguyen LN, Koolpe M, Mc Lennan R, Krull CE, Pasquale EB. Targeting the EphA4 receptor in the nervous system with biologically active peptides. Molecular and cellular neurosciences. 2003;24:1000–1011. doi: 10.1016/j.mcn.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. The European journal of neuroscience. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 36.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of neuroscience. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nature reviews. Neurology. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 40.Fiske BK, Brunjes PC. Microglial activation in the developing rat olfactory bulb. Neuroscience. 2000;96:807–815. doi: 10.1016/s0306-4522(99)00601-6. [DOI] [PubMed] [Google Scholar]
- 41.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. The Journal of neuroscience. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rai S, Kamat PK, Nath C, Shukla R. A study on neuroinflammation and NMDA receptor function in STZ (ICV) induced memory impaired rats. Journal of neuroimmunology. 2013;254:1–9. doi: 10.1016/j.jneuroim.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Masliah E, Sisk A, Mallory M, Games D. Neurofibrillary pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Journal of neuropathology and experimental neurology. 2001;60:357–368. doi: 10.1093/jnen/60.4.357. [DOI] [PubMed] [Google Scholar]
- 44.Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, et al. Differential loss of synaptic proteins in Alzheimer's disease: implications for synaptic dysfunction. Journal of Alzheimer's disease: JAD. 2005;7:103–117. doi: 10.3233/jad-2005-7203. [DOI] [PubMed] [Google Scholar]
- 45.Scheff SW, Price DA. Alzheimer's disease-related alterations in synaptic density: neocortex and hippocampus. Journal of Alzheimer's disease: JAD. 2006;9:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 46.Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiology of aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological reviews. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez MR, Pena JD, Selvidge JA, Salvador-Silva M, Yang P. Hydrostatic pressure stimulates synthesis of elastin in cultured optic nerve head astrocytes. Glia. 2000;32:122–136. doi: 10.1002/1098-1136(200011)32:2<122::aid-glia20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 49.Thon N, Haas CA, Rauch U, Merten T, Fassler R, Frotscher M, et al. The chondroitin sulphate proteoglycan brevican is upregulated by astrocytes after entorhinal cortex lesions in adult rats. The European journal of neuroscience. 2000;12:2547–2558. doi: 10.1046/j.1460-9568.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 50.Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer's disease. Neurobiology of aging. 2003;24:321–331. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 51.Kanninen K, Goldsteins G, Auriola S, Alafuzoff I, Koistinaho J. Glycosylation changes in Alzheimer's disease as revealed by a proteomic approach. Neuroscience letters. 2004;367:235–240. doi: 10.1016/j.neulet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, et al. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain research. Molecular brain research. 2002;100:103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 53.Deb S, Wenjun Zhang J, Gottschall PE. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain research. 2003;970:205–213. doi: 10.1016/s0006-8993(03)02344-8. [DOI] [PubMed] [Google Scholar]
- 54.Kalani A, Kamat PK, Familtseva A, Chaturvedi P, Muradashvili N, Narayanan N, et al. Role of microRNA29b in blood-brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism. Journal of cerebral blood flow and metabolism. 2014;34:1212–1222. doi: 10.1038/jcbfm.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–319. doi: 10.1016/j.neuroscience.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Chintala SK. Influence of interleukin-1 beta induction and mitogen-activated protein kinase phosphorylation on optic nerve ligation-induced matrix metalloproteinase-9 activation in the retina. Experimental eye research. 2004;78:849–860. doi: 10.1016/j.exer.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nature reviews. Neuroscience. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorkiewicz T, Szczuraszek K, Wyrembek P, Michaluk P, Kaczmarek L, Mozrzymas JW. Matrix metalloproteinase-9 reversibly affects the time course of NMDA-induced currents in cultured rat hippocampal neurons. Hippocampus. 2010;20:1105–1108. doi: 10.1002/hipo.20736. [DOI] [PubMed] [Google Scholar]
- 59.Stawarski M, Rutkowska-Wlodarczyk I, Zeug A, Bijata M, Madej H, Kaczmarek L, et al. Genetically encoded FRET-based biosensor for imaging MMP-9 activity. Biomaterials. 2014;35:1402–1410. doi: 10.1016/j.biomaterials.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 60.Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, et al. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. The Journal of biological chemistry. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain: a journal of neurology. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 62.Ulrich R, Gerhauser I, Seeliger F, Baumgartner W, Alldinger S. Matrix metalloproteinases and their inhibitors in the developing mouse brain and spinal cord: a reverse transcription quantitative polymerase chain reaction study. Developmental neuroscience. 2005;27:408–418. doi: 10.1159/000088455. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Alloza M, Prada C, Lattarulo C, Fine S, Borrelli LA, Betensky R, et al. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. Journal of neurochemistry. 2009;109:636–1647. doi: 10.1111/j.1471-4159.2009.06096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. The Journal of neuroscience. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nature reviews. Neuroscience. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 66.Knapska E, Lioudyno V, Kiryk A, Mikosz M, Gorkiewicz T, Michaluk P, et al. Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. The Journal of neuroscience. 2013;33:14591–14600. doi: 10.1523/JNEUROSCI.5239-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes P, et al. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. Journal of neurochemistry. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- 68.Staubli U, Vanderklish P, Lynch G. An inhibitor of integrin receptors blocks long-term potentiation. Behavioral and neural biology. 1990;53:1–5. doi: 10.1016/0163-1047(90)90712-f. [DOI] [PubMed] [Google Scholar]
- 69.Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. The Journal of neuroscience. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin B, Arai AC, Lynch G, Gall CM. Integrins regulate NMDA receptor-mediated synaptic currents. Journal of neurophysiology. 2003;89:2874–2878. doi: 10.1152/jn.00783.2002. [DOI] [PubMed] [Google Scholar]
- 71.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nature neuroscience. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 72.Szklarczyk A, Conant K. Matrix metalloproteinases, synaptic injury, and multiple sclerosis. Frontiers in psychiatry. 2010;1:130. doi: 10.3389/fpsyt.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron. 2004;44:1021–1030. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]