Abstract
The cell wall of the yeast form of the dimorphic fungus Paracoccidioides brasiliensis is enriched with α1,3-glucans. In Cryptococcus neoformans, α1,3-glucans interact with glucuronoxylomannan (GXM), a hetero polysaccharide that is essential for fungal virulence. In this study, we investigated the occurrence of P. brasiliensis glycans sharing properties with cryptococcal GXM. Protein database searches in P. brasiliensis revealed the presence of sequences homologous to those coding for enzymes involved in the synthesis of GXM and capsular architecture in C. neoformans. In addition, monoclonal antibodies (mAbs) raised to cryptococcal GXM bound to P. brasiliensis cells. Using protocols that were previously established for extraction and analysis of C. neoformans GXM, we recovered a P. brasiliensis glycan fraction composed of mannose and galactose, in addition to small amounts of glucose, xylose and rhamnose. In comparison with the C. neoformans GXM, the P. brasiliensis glycan fraction components had smaller molecular dimensions. The P. brasiliensis components, nevertheless, reacted with different GXM-binding mAbs. Extracellular vesicle fractions of P. brasiliensis also reacted with a GXM-binding mAb, suggesting that the polysaccharide-like molecule is exported to the extracellular space in secretory vesicles. An acapsular mutant of C. neoformans incorporated molecules from the P. brasiliensis extract onto the cell wall, resulting in the formation of surface networks that resembled the cryptococcal capsule. Coating the C. neoformans acapsular mutant with the P. brasiliensis glycan fraction resulted in protection against phagocytosis by murine macrophages. These results suggest that P. brasiliensis and C. neoformans share metabolic pathways required for the synthesis of similar polysaccharides and that P. brasiliensis yeast cell walls have molecules that mimic certain aspects of C. neoformans GXM. These findings are important because they provide additional evidence for the sharing of antigenically similar components across phylogenetically distant fungal species. Since GXM has been shown to be important for the pathogenesis of C. neoformans and to elicit protective antibodies, the finding of similar molecules in P. brasiliensis raises the possibility that these glycans play similar functions in paracoccidiomycosis.
Keywords: Paracoccidioides brasiliensis, Polysaccharide, Cryptococcus neoformans, Glucuronoxylomannan
1. Introduction
Paracoccidioidomycosis (PCM) is the most prevalent mycosis in Latin America (Queiroz-Telles and Escuissato, 2011). The importance of PCM, however, has been long underestimated, probably because of the geographic distribution of its etiologic agent, Paracoccidioides brasiliensis (Teles and Martins, 2011), which limits it to South America. In Brazil, about 50% of the deaths caused by systemic mycoses between 1996 and 2006 were due to P. brasiliensis (Prado et al., 2009). Although there have been advances in methods of diagnosis and disease prevention, there is no consensus on the best diagnostic and preventative approaches (reviewed in Teles and Martins (2011)). Moreover, treatment of PCM requires months to years of antimicrobial therapy and relapses are common (Morejon et al., 2009).
Polysaccharides influence physiology and pathogenesis of fungal species through multiple mechanisms. In fungi, these molecules are required for cell wall architecture, interaction with host cells, modulation of immunological responses, and virulence (reviewed in Fukazawa et al. (1995); Pirofski (2001); Rodrigues et al. (2011a); San-Blas et al. (2000); Taylor and Roberts (2005); Zaragoza et al. (2009)). Because of their structural and functional particularities, fungal polysaccharides are often utilized in diagnostic tests, including therapeutic monitoring, in mycoses patients (Fukazawa et al., 1995; Lopes et al., 2011). Furthermore, fungal polysaccharides have been shown to elicit protective antibody responses when incorporated into conjugate vaccines (Torosantucci et al., 2005). However, the current literature clearly indicates that many aspects related to structure and functions of polysaccharides remain unknown (Rodrigues et al., 2011a).
Glucuronoxylomannan (GXM) is a heteropolysaccharide produced by fungal pathogens belonging to the Cryptococcus and Trichosporon genera (Fonseca et al., 2009a; Zaragoza et al., 2009). This α1,3 mannan with β-xylosyl and glucuronyl -substitutions is an active immunomodulator that is essential to the pathogenesis of C. neoformans and C. gattii, the causative agents of human cryptococcosis (Zaragoza et al., 2009). Antibodies to GXM have been shown to alter the course of experimental animal cryptococcosis to the benefit of the host (Feldmesser and Casadevall, 1998; Pirofski, 2001), supporting the notion that GXM-binding antibodies might be useful in preventing and treating human cryptococcosis (Larsen et al., 2005). In addition, mimetic peptides sharing immunological properties with GXM are protective in animal models of vaccination (Pirofski, 2001). In this context, GXM represents an attractive target for antifungals and vaccine design in the Cryptococcus and Trichosporon models. Currently, it is not known if other pathogens can synthesize GXM-like molecules.
In C. neoformans and C. gattii, GXM is both cell-wall associated and released to the extracellular space (Zaragoza et al., 2009). GXM association to the cell wall in C. neoformans requires α1,3-glucans (Reese and Doering, 2003; Reese et al., 2007). In fact, a C. neoformans mutant lacking expression of the gene coding for α1,3-glucan synthase displayed an acapsular phenotype despite ongoing synthesis of capsular components (Reese and Doering, 2003). Disruption of the α1,3-glucan synthase gene also resulted in increased sensitivity to temperature and in reduced levels of cell division (Reese et al., 2007). Loss of α1,3-glucan was accompanied by a compensatory increase in chitin/chitosan and a redistribution of β glucan between cell wall fractions (Reese et al., 2007). These observations clearly indicated that metabolism and cellular distribution of polysaccharides are integrated and required for fungal virulence. They also established a consistent connection between glucan synthesis and GXM anchoring to the cell wall in C. neoformans and, possibly, other fungi. In fact, capsular material from C. neoformans binds to isolates of Histoplasma capsulatum containing cell wall-associated α1,3-glucan, but not to H. capsulatum strains lacking synthesis of this glucan (Reese and Doering, 2003).
Synthesis of α1,3-glucan by P. brasiliensis yeast cells is associated with fungal virulence (San-Blas and San Blas, 1977; San-Blas and Vernet, 1977). The presence of a polysaccharide with the ability to anchor C. neoformans GXM in the parasitic forms of P. brasiliensis led us to investigate whether these two pathogenic species would share metabolic events related to the syn thesis of GXM. By combining serologic, chromatographic, and microscopic approaches with a phagocytosis model of polysac charide-coated yeast cells, we observed that P. brasiliensis produces glycans that share structural, serologic and functional properties with cryptococcal GXM. These results suggest that some of the pathogenic mechanisms used by C. neoformans to damage the host and/or avoid host responses may be similar to those used by P. brasiliensis, and these observations open new avenues for the identification of therapeutic and diagnostic targets in P. brasiliensis.
2. Materials and methods
2.1. Fungal strains and growth conditions
The P. brasiliensis strain used in this study was the reference strain Pb 18, provided by Dr. Rosely Zancope-Oliveira (Fiocruz, Rio de Janeiro, Brazil). Yeast forms were cultivated in Fava Netto’s medium (proteose peptone 3 g, peptone 10 g, beef extract 5 g, sodium chloride 5 g, yeast extract 5 g, dextrose 40 g, H2O 1L) (Fava-Netto, 1955) for 7 days at 37 °C. A number of experiments in this study included C. neoformans cells; strains used were the standard serotype A isolate H99 and the acapsular mutant Cap67. C. neoformans yeast were cultivated in a minimal medium composed of 15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine and 3 μM thiamine-HCl (pH 5.5) for 2 days at 30 °C, with shaking. P. brasiliensis and C. neoformans yeast cells were obtained by centrifugation, washed in phosphate-buffered saline (PBS) and counted in a Neubauer chamber. Some of the serologic tests developed in this study required mycelial cells of P. brasiliensis (Pb 18), in addition to control systems using Candida albicans (strain IBEX 11, Tavares et al., 2008) and Saccharomyces cerevisiae (strain BY4741, provided by Dr. Marcos Pereira, Federal University of Rio de Janeiro) yeast cells. P. brasiliensis mycelia were cultivated in Sabouraud broth with shaking for 7 days at 25 °C. Filamentous cells were collected and washed by filtration as previously described (Soares et al., 1993). S. cerevisiae and C. albicans yeast cells were cultivated for 48 h in Sabouraud broth with shaking, followed by centrifugation and washing.
2.2. Protein database searches
Numerous genes related to GXM synthesis and export have been characterized in the last two decades (Chang et al., 1997, 1996, 1995; Chang and Kwon-Chung, 1998, 1999; Cottrell et al., 2007; Garcia-Rivera et al., 2004; Janbon et al., 2001; Klutts and Doering, 2008; Kmetzsch et al., 2011; Kumar et al., 2011; Moyrand et al., 2002; Panepinto et al., 2009; Sommer et al., 2003; Yoneda and Doering, 2006). Sequences of the proteins related to these processes were obtained from the Uniprot database (www.uniprot.org) and used in Blast searches (protein query/protein database) to find homologous in the P. brasiliensis proteomic data base. Hits showing the highest scores of similarity were selected and are presented in Table 1.
Table 1.
Proteins required for capsule formation, GXM synthesis and polysaccharide export in C. neoformans and their P. brasiliensis Pb18 homologs.
| Gene (references) | C. neoformans protein (UniProt accession number) |
P. brasiliensis protein (UniProt accession number) |
Identity | E-value | Protein family |
|---|---|---|---|---|---|
| Cellular events: capsule formation and/or polysaccharide synthesis | |||||
| CAP10 (Chang and Kwon-Chung, 1999) | Capsule-associated protein 10 (Q9HGG9) |
Conserved hypothetical protein (C1G0T3) |
33% | 7 × 10−16 | Glycosyltransferase |
| CAP64 (Chang et al., 1997, 1996) | Capsular-associated protein 64 (Q9HGG3) |
Not found | - | - | - |
| CAP60 (Chang and Kwon-Chung, 1998) | Capsular-associated protein 60 (Q9HGG6) |
Not found | - | - | - |
| GMT2 (Cottrell et al., 2007) | GDP-mannose transporter (P0CS04) |
GDP-mannose transporter (C1GF36) |
57% | 1 × 10−104 | Nucleotide-activated monosaccharide transporter |
| GMT1 (Cottrell et al., 2007) | GDP-mannose transporter (P0CS02) |
GDP-mannose transporter (C1GF36) |
59% | 1 × 10−104 | Nucleotide-activated monosaccharide transporter |
| CXT1 (Klutts and Doering, 2008) | Xylosyltransferase (Q5K8R6) | Conserved hypothetical protein (C1G0T3) |
28% | 3 × 10−45 | Glycosyltransferase |
| CMT1 (Sommer et al., 2003) | a1,3-Mannosyltransferase (A5D938) |
Conserved hypothetical protein (C1GGS1) |
24% | 7 × 10−6 | Glycosyltransferase |
| CAS1 (Janbon et al., 2001; Moyrand et al., 2002) | O-acetyltransferase (Q8X226) | Not found | - | - | - |
| Cellular event: GXM export | |||||
| SAV1 (Yoneda and Doering, 2009) | Rab/GTPase (E2IFI6) | GTP-binding protein (C1G092) | 73% | 2 × 10−77 | Ras-like proteins |
| SEC6 (Panepinto et al., 2009) | Vesicle fusion protein (Q5K9B3) | Conserved hypothetical protein (C1G3V8) |
26% | 3 × 10−78 | Exocyst complex component |
| CAP59 (Chang et al., 1995; Garcia-Rivera et al., 2004) | Capsular-associated protein 59 (O93823) |
Conserved hypothetical protein (C1GGS1) |
29% | 8 × 10−5 | Glycosyltransferase |
| GRASP (Kmetzsch et al., 2011) | Golgi reassembly and stacking (A6ZZA4) |
Conserved hypothetical protein (C1G8Y3) |
26% | 5 × 10−20 | GRASP55/65 PDZ-like domain |
2.3. Preparation of C. neoformans GXM
C. neoformans GXM was isolated as previously described by filtration of fungal supernatants in Amicon (Millipore, Danvers, MA) ultrafiltration cells (cutoff 100 kDa) (Nimrichter et al., 2007). After concentration of the supernatant, the viscous GXM-containing film layer was collected with a cell scraper and was transferred to plastic tubes for carbohydrate quantification. Carbohydrate contents were determined as per Dubois et al. (1951).
2.4. Preparation of fungal glycan fractions
Cellular fractions were used to search for P. brasiliensis glycans using a protocol that was previously established for extraction of C. neoformans GXM (Maxson et al., 2007b). P. brasiliensis yeast cells (2 × 109) were suspended in DMSO (15 ml) and incubated for 15 min with shaking at room temperature. Supernatants containing released glycans were collected by centrifugation and the pellet was again suspended in 15 ml DMSO for a second extraction under the same conditions. Supernatants were combined and extensively dialyzed against water for subsequent lyophilization and dry weight determination. Negative controls consisted of similar preparations obtained from C. albicans and S. cerevisiae. Glycan fractions from P. brasiliensis mycelial cells (2.5 g, wet weight) were similarly prepared for serologic tests. For light scattering analysis, the same protocol was used to extract GXM from C. neoformans cells.
2.5. Alkali treatment of fungal glycans
Removal of O-acetyl groups of GXM and similar groups potentially present in the P. brasiliensis molecule was performed by dissolving 5 mg of the glycans in 1 ml of H2O adjusted to pH 11.25 with NH4OH. The resulting solution was stirred for 24 h at 23 °C and dialyzed against water.
2.6. Fluorescence microscopy
The different components tested in this study included P. brasiliensis yeast forms, in addition to glycan-coated Cap67 cells. Yeast cells (106) were suspended in 4% paraformaldehyde cacodylate buffer (0.1 M, pH 7.2) and incubated for 30 min at room temperature. Fixed yeast cells were washed twice in PBS and incubated in 1% bovine serum albumin in PBS (PBS-BSA) for 1 h. For glycan staining, blocked cells were incubated with different monoclonal antibodies (mAbs) to cryptococcal GXM. MAbs to GXM that were available at our laboratory were generated in previous studies and included IgMs (mAbs 13F1 and 2D10) (Cleare and Casadevall, 1998; Garcia-Rivera et al., 2004) and IgG1 (mAb 18B7) (Casadevall et al., 1998). These mAbs differ in fine specificity and protective efficacy. MAb 13F1 is not protective and produces punctate immunofluorescence (Mukherjee et al., 1995, 1992). MAb 2D10 (IgM) reacts with cell wall and capsular epitopes of C. neoformans and is protective in a murine model of cryptococcosis (Garcia-Rivera et al., 2004). MAb 18B7 is a protective IgG1 that has been tested as a therapeutic tool in animals and humans (Casadevall et al., 1998; Larsen et al., 2005). This antibody reacts with all GXM sero types. After incubation in primary antibodies, yeast cells were washed and then incubated with Alexa Fluor 488-labeled goat anti-mouse (IgG or IgM) antibodies (Invitrogen). In some conditions, yeast cells were incubated with the mAbs in the pres ence of the P. brasiliensis glycan fraction (1 100 μg/ml). After washing, yeast cells were applied to slides and observed using an Axioplan 2 fluorescence microscope (Zeiss, Germany). Images were acquired using a Color View SX digital camera and were processed with the analySIS software system (Soft Imaging System) prior to processing with ImageJ software (provided by NIH, http://rsb.info. nih.gov/ij/). For controls, mAbs were replaced by irrelevant iso type-matched antibodies. Yeast grown in minimal medium alone were used as a control for the experiments examining glycancoated acapsular cells. Exposure times were similar for all conditions.
2.7. Serologic reactivity of fungal glycans with mAbs to GXM
The reactivity of the P. brasiliensis glycan fractions with mAbs to cryptococcal GXM was determined by enzyme-linked immunosorbent assays (ELISAs), using modifications of a previously described protocol for GXM detection (Casadevall et al., 1992). Polystyrene 96-well plates were coated with 0.5 μg/ml solutions of the P. brasiliensis glycan fraction and incubated for 1 h at 37 °C. Alterna tively, the plates were coated with DMSO extracts from S. cerevisiae or C. albicans (negative controls) at the same concentration. After washing to remove unbound polysaccharide, the plates were blocked with 1% bovine serum albumin, followed by addition of the mAbs to GXM. After incubation for 1 h at 37 °C, the plates were washed five times with tris-buffered saline (TBS) supple mented with 0.1% Tween 20, followed by incubation with alkaline phosphatase conjugated goat anti-mouse secondary antibodies for 1 h. Color reaction developed after the addition of p-nitrophenyl phosphate disodium hexahydrate and the absorbance was measured at 405 nm with a microplate reader (Biotek EL808 reader). Primary antibodies and alkaline phosphatase conjugated antibodies corresponded to 1 μg/ml. A comparison between the serologic reactivity of the native glycan fraction obtained from P. brasiliensis yeast cells with alkali-treated (de O-acetylated) and mycelialgly cans was also included in antibody-binding assays. Since these tests involved quantitative comparisons between chemically modified molecules with still undetermined ability to coat polystyrene plates, glycan antigens (0.03-1 μg in 5 μl PBS) in these assays were loaded onto nitrocellulose membranes for dot blot analysis (Nimrichter et al., 2007). The membranes were allowed to dry for 1 h at 37 °C and then were blocked with PBS containing 1% bovine serum albumin. Blocked membranes were incubated with mAb 18B7 at 1 μg/ml. After being washed extensively, membranes were sequentially incubated with alkaline phosphatase-conjugated goat anti-mouse IgG and p-nitrophenyl phosphate solutions. Reactions were quantified by the transfer of the soluble, colored products to the wells of 96-well plates and absorbance reading at 405 nm as described above.
2.8. Monosaccharide analysis
Monosaccharide components of the P. brasiliensis glycan fraction were determined by gas chromatography/mass spectrometry (GC/MS) analysis of the per-O-trimethylsilyl (TMS) derivatized monosaccharides. The dry sample (0.3 mg) was submitted to methanolysis in methanol/1 M HCl at 80 °C (18 22 h) for preparation of methyl glycosides. The sample was then treated with Tri-Sil (Pierce) at 80 °C (0.5 h) for per-O-trimethylsilylation. GC/MS analysis of the per-O-TMS derivatives was performed on an HP 5890 gas chromatograph interfaced to a 5970 MSD mass spectrometer, using a Supelco DB-1 fused silica capillary column (30 m × 0.25 mm ID). Carbohydrate standards included arabinose, rhamnose, fucose, xylose, glucuronic acid, galacturonic acid, mannose, galactose, glucose, mannitol, dulcitol and sorbitol.
2.9. Effective diameter determination of glycan fractions
Effective diameter and size distribution of molecules in GXM preparations were measured by Quasi elastic light scattering in a 90Plus/BI-MAS Multi Angle Particle Sizing analyzer (Brookhaven Instruments Corp., Holtsville, NY), according with the method described by Frases and colleagues (Frases et al., 2009). Multimodal size distribution analysis of polysaccharides was calculated from the values of intensity weighted sizes obtained from the non-negatively constrained least squared (NNLS) algorithm for further processing with GraphPad Prism software (version 5.0).
2.10. Glycan detection in extracellular vesicles
Vesicles were isolated from P. brasiliensis culture supernatants by sequential centrifugation as recently described (Vallejo et al., 2011, 2012a, 2012b). Vesicle pellets were lyophilized and suspended in 100 μl of absolute methanol, resulting in the immediate formation of a precipitate. The suspension was then supplemented with 900 μl of chloroform and the organic fraction was recovered by centrifugation. The residual material that was not soluble in the chloroform-methanol mixture was then dried under a nitrogen stream, suspended in PBS, and assayed for the presence of GXM-related glycans by ELISA with mAb 18B7. The organic phase, containing lipids, was recovered for analysis by high-performance thin-layer chromatography (HPTLC) aiming at the detection of sterols, which were previously characterized as extracellular vesicle lipid markers in fungi (Oliveira et al., 2010b; Rodrigues et al., 2007; Vallejo et al., 2012a). Sterol analysis by HPTLC was performed as described (Oliveira et al., 2010a, 2010b, 2009; Rodrigues et al., 2007).
2.11. Coating acapsular C. neoformans cells with fungal glycans
Acapsular C. neoformans cells (strain Cap67, 106 cells) were suspended in solutions of purified GXM or of the P. brasiliensis glycan fraction (100 μl, 10 μg/ml) and incubated for 18 h at 25 °C, fol lowed by extensive washing with PBS. Control systems consisted of Cap67 cells incubated in minimal medium.
2.12. Scanning electron microscopy (SEM)
Acapsular cells of C. neoformans or the glycan-coated mutant were washed in PBS and fixed in 0.1 M sodium cacodylate buffer containing 2.5% glutaraldehyde for 1 h and prepared for SEM as recently described (Ramos et al., 2012). Briefly, the cells were washed in a buffer containing 0.1 M sodium cacodylate, 0.2 M sucrose and 2 mM MgCl2. The samples were ?xed on coverslips coated with poly-L-lysine for 20 min. Preparations were then serially dehydrated in alcohol (30%, 50%, 70% and 100% for 5 min and 95% and 100% for 10 min), and submitted to critical point drying and metallization. The cells were observed in a scanning electron microscope (JEOL JSM-5310).
2.13. Phagocytosis
The murine macrophage cell line RAW 264.7 (American Type Culture Collection, Rockville, MD) was grown to confluence in 25 cm2 culture flasks containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), at 37 °C in a 5% CO2 atmosphere. For interaction with glycan coated or uncoated yeast cells, the macrophages were cultivated in 24 well plates, in the same conditions described above. The cell wall of fungal cells was stained with 0.5 mg/ml fluorescein isothiocyanate (FITC, Sigma) in PBS (25 °C) for 10 min. FITC reacts with amino groups on surface proteins, generating fluorescent yeast cells. This approach has been consistently used for the quantitative analysis of phagocytosis of yeast cells by flow cytometry (Barbosa et al., 2006; Nogueira et al., 2010). FITC-labeling does not impact yeast viability. Yeast suspensions were prepared in DMEM, to generate a ratio of 10 yeasts per macrophage-like cell. Interactions between fungal and host cells occurred at 37 °C in a 5% CO2 atmosphere for 18 h. After removal of non-adherent fungi by washing, yeast-host cell complexes were treated for 10 min at 25 °C with trypan blue (200 μg/ml) and washed. Trypan blue is a quenching agent of FITC-derived fluorescence and is useful to discriminate between surface-associated and intracellular yeast cells, because it is not capable of penetrating the intracellular compartment of viable cells. Thus this dye quenches the fluorescence of non-internalized cells. Cells were gently removed from the plastic surface with a cell scrapper. The cells were analyzed by flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer. Data were processed with Cyflogic software (www.cyflogic.com). The index of infection was determined as the percentage of fluorescent host cells. Control preparations were developed as described above using uninfected cells and infecting with non-stained yeast. Each experiment was repeated twice.
3. Results
3.1. The P. brasiliensis genome contains homologous genes to those involved in GXM synthesis
We first sought to evaluate whether P. brasiliensis and C. neoformans would share protein sequences related to the metabolic steps involved in GXM synthesis, capsule formation and polysaccharide export. We first selected proteins coded by genes that directly affected capsular structures in C. neoformans (Chang et al., 1997, 1996, 1995; Chang and Kwon-Chung, 1998, 1999; Cottrell et al., 2007; Janbon et al., 2001; Klutts and Doering, 2008; Kmetzsch et al., 2011; Moyrand et al., 2002; Panepinto et al., 2009; Sommer et al., 2003; Yoneda and Doering, 2009) and classified them as involved in (i) capsule formation and GXM synthesis and (ii) GXM export (Table 1), based on the literature on the generation of capsule deficient mutants. Nine of the 12 candidates we selected had homologous sequences in P. brasiliensis. Searches for proteins coded by two genes required for capsule formation in C. neoformans (CAP64 and CAP60) and by one gene required for GXM O-acetylation (CAS1) produced negative results. P. brasiliensis had homologs for all proteins required for polysaccharide export in C. neoformans and also for building the non-acetylated GXM back bone. We therefore hypothesized that P. brasiliensis may have the metabolic apparatus required for the synthesis of polysaccharides with antigenic similarity to the C. neoformans GXM, but not necessarily for the formation of a large, physical capsule.
3.2. P. brasiliensis produces glycans that share serologic and structural properties with cryptococcal GXM
The hypothesis that P. brasiliensis can synthesize molecules with serologic similarity with cryptococcal GXM was first tested by immunofluorescence using mAbs 18B7, 2D10 and 13F1 (Fig. 1A). All antibodies reacted with the surface of yeast cells in a cell wall associated punctate pattern. In addition, the GXM-binding mAbs recognized extracellular structures that accumulated in the budding neck and in intercellular spaces formed by dividing cells. Fluorescent reactions were not observed when primary antibodies were replaced with isotype-matched irrelevant antibodies. To test the specificity of the reactions between P. brasiliensis molecules and the GXM-binding antibodies, we selected mAbs 18B7 and 2D10 for immunofluorescence reactivity in the presence of varying concentrations of the Paracoccidioides glycan obtained by DMSO extraction (Maxson et al., 2007a, 2007b). Fluorescent reactions were efficiently inhibited when glycan fractions were used at 10 and 100 μg/ml (Fig. 1B). We estimated the efficacy of the glycan-mediated inhibition of antibody binding by counting the number of surface associated fluorescence spots in at least 100 yeast cells. According to this calculation, the P. brasiliensis glycan fraction inhibited mAb binding by approximately 70% and 90% at 10 and 100 μg/ml, respectively. This inhibitory profile was similar for both mAbs. Altogether, these results suggested the existence of surface structures in P. brasiliensis that share serologic properties with cryptococcal GXM.
Fig. 1.

A surface glycan of P. brasiliensis (Pb18) yeast cells shares serologic properties with cryptococcal GXM. (A) Reactivity of P. brasiliensis surface components with different monoclonal antibodies (mAbs 18B7, 2D10 and 13F1) generated to C. neoformans GXM is shown. Fluorescent reactions were not observed when primary antibodies were replaced with isotype matched irrelevant antibodies, as illustrated for IgG1 in the ‘None’ panel. Similar results were obtained with irrelevant IgMs and TRITC-labeled anti-IgM antibodies (not shown). P. brasiliensis cells yeast cells are shown under differential interferential contrast (DIC) and fluorescence (anti-GXM) modes. Image merging (‘merge’ panel) demonstrates that the molecules recognized by the anti-GXM antibodies are externally associated with the cell wall. (B) Immunofluorescence analysis of P. brasiliensis yeast cells incubated with mAbs 18B7 and 2D10 in the presence of varying concentrations of the GXM-related glycan fraction. Increasing concentrations of the glycan efficiently inhibited recognition of yeast cells by the GXM antibodies. P. brasiliensis cells yeast cells are shown under DIC and fluorescence (mAbs 18B7 and 2D10) modes. Scale bar, 20 μm.
To exclude the possibility that the results observed in Fig. 1 originated from unspecific reactivity of GXM-binding mAbs with surface P. brasiliensis structures, we extended the serologic approach for detection of GXM-related molecules to another model of antigen–antibody reactivity. Glycan fractions extracted from P. brasiliensis yeast cells were used in ELISA with different mAbs raised to cryptococcal GXM. All mAbs showed dose-dependent serologic reactivity with the P. brasiliensis extract with variable affinities (Fig. 2A), with the IgG1 18B7 having the best binding. The GXM-binding antibodies showed negligible reactivity with DMSO extracts obtained from either C. albicans or S. cerevisiae yeast cells.
Fig. 2.
Serologic reactivity of P. brasiliensis glycans with antibodies originally raised to cryptococcal GXM. (A) Dose-dependent serologic reactivity of a DMSO extract from P. brasiliensis with different monoclonal antibodies raised to cryptococcal GXM (18B7, IgG; 2D10 and 13F1, IgMs) as measured by ELISA. Negative controls included similar serologic tests with DMSO extracts obtained from S. cerevisiae or C. albicans. (B) Reactivity of fungal glycans with mAb 18B7 after de-O-acetylation by alkali treatment. De-O-acetylation affected the recognition of cryptococcal GXM (Cn glycan) by mAb 18B7. The P. brasiliensis fraction (Pb glycan) was not affected by treatment with alkali. (C) DMSO extracts from yeast cells and mycelia show similar profiles of recognition by mAb 18B7. Results in β and C are representative of three independent dot blot analyses.
O-acetylation is a serologic marker of cryptococcal GXM (Kozel et al., 2003), which led us to evaluate whether the presence of this group would affect antibody recognition of the fungal glycans. As expected, GXM de-O-acetylation caused a marked reduction in its reactivity with mAb 18B7 (Fig. 2B). The P. brasiliensis glycan fraction, however, was not affected by alkali treatment, which is in agreement with the lack of mannosyl O-acetyltransferases in this fungus (Table 1). We finally tested whether the saprophytic form of P. brasiliensis would produce similar molecules. Immunofluores cence experiments were inconclusive due to high backgrounds (data not shown), but serologic tests with DMSO extracts from mycelial cells revealed that the filamentous forms of P. brasiliensis also produce molecules that share epitopes with cryptococcal GXM (Fig. 2C).
Crude culture supernatants of P. brasiliensis were also tested for reactivity with GXM-binding mAbs, showing negative results (data not shown). This observation could imply that the glycan is not released extracellularly. However, we could not rule out the possibility that the P. brasiliensis molecules sharing serologic reactivity with GXM were present in culture supernatants at concentrations below the detection limit of our ELISA protocol. Considering that GXM is exported in extracellular vesicles in the C. neoformans model (Oliveira et al., 2009; Rodrigues et al., 2007), we then evaluated whether concentrated vesicle fractions isolated from P. brasiliensis supernatants would show reactivity with mAb 18B7, the most efficient antibody in serologic tests with the Paracoccidioides glycan. The presence of vesicles in ultracentrifugation fractions was confirmed by the positive detection of sterols in lipid extracts (Fig. 3A). ELISA with mAb 18B7 revealed a dose-dependent recognition of the extracellular vesicle fraction (Fig. 3B), suggesting that the GXM related P. brasiliensis glycan is also exported extracellu larly in secretory vesicles.
Fig. 3.

Analysis of P. brasiliensis extracellular vesicles. (A) Detection of sterols by HPTLC in extracellular vesicle fractions isolated from culture supernatants. (B) Dose-dependent recognition of P. brasiliensis vesicle (Pb vesicles) and cellular glycan (Pb glycan) fractions by mAb 18B7.
The serologic reactivity of molecules in the P. brasiliensis extract with antibodies to C. neoformans GXM was suggestive of structural similarities in glycans produced by both fungal species. To address this possibility, the carbohydrate composition of the P. brasiliensis DMSO extract was analyzed by biochemical methods. After acidic methanolysis of the sample, monosaccharide constituents were analyzed by GC. Classic criteria were used to interpret the results of the GC analysis allowing for the potential detection of four different peaks for each sugar derivative, corresponding to the a and b-forms of furanose and pyranose rings. In combination with MS analysis, each peak could be precisely identified, based on the profile of fragmentation observed. Fragmentation of TMS-derivatives of hexoses usually generates diagnostic peaks at m/z 217 and 214 (Kamerling et al., 1975; Vliegenthart and Kamerling, 1975). Pyranose rings give rise to a ratio (m/z 204)/(m/z 217) > 1, whereas furanose rings show a ratio <1. Based on the principles detailed above, peaks with retention times and fragmentation profiles corresponding to mannopyranose (63.3%; relative intensity) and anomers of galactose (30.3%; relative intensity), glucose (5.2%; relative intensity), xylose (1%; relative intensity) and rhamnose (0.3%; relative intensity) were identified (Fig. 4). Therefore, the glycan nature of the P. brasiliensis DMSO extract was confirmed, suggesting the presence of polysaccharides constituted primarily of mannosyl and galactosyl units and smaller amounts of glucose, xylose and rhamnose.
Fig. 4.
Monosaccharide composition of the polysaccharide fraction obtained from P. brasiliensis Pb18 by DMSO extraction. Methanolysis and derivatization of the components extracted with DMSO followed by separation by gas chromatography (A) revealed the presence of different peaks (1–9) corresponding to carbohydrate units. Mass spectrometry analysis of each of these peaks (B) resulted in the identification of abundant mannose and galactose, as well as of trace amounts of glucose, xylose and rhamnose. The relative molar distribution of each monosaccharide in the DMSO extract is shown in C.
3.3. Molecular dimensions of C. neoformans and P. brasiliensis glycans
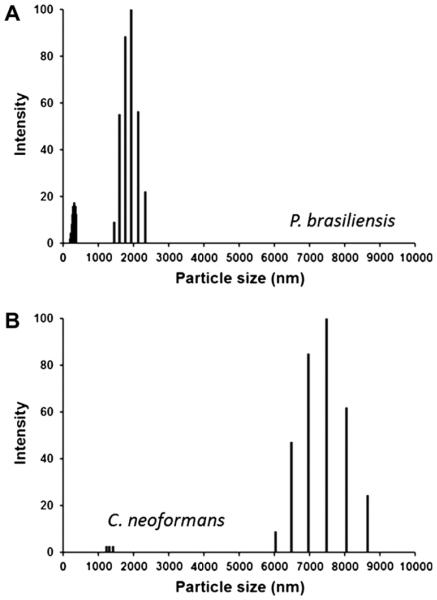
The fact that P. brasiliensis lacks a true capsule, but has the ability to synthesize molecules that are similar to cryptococcal GXM, led us to evaluate whether the dimensions of glycans from this fungus and C. neoformans GXM were comparable. Glycans from both species were analyzed by dynamic light scattering. P. brasiliensis produced glycan fractions with a size distribution ranging from 1500 to 2500 nm, while the C. neoformans molecules ranged from 6000 to 9000 nm (Fig. 5). This finding is consistent with the surface morphological features of each pathogen in the sense that larger fibrils are used to form the cryptococcal capsule (Frases et al., 2009).
Fig. 5.
Size distribution of glycan fractions obtained after DMSO extraction of P. brasiliensis Pb18 (A) and encapsulated C. neoformans H99 (B) cells.
3.4. Binding of P. brasiliensis glycans to the cell surface of an acapsular mutant of C. neoformans
The Cap67 mutant of C. neoformans is believed to lack a capsule due to the inability to export GXM but retains the ability to incorporate exogenously provided capsular components onto its cell wall (Garcia-Rivera et al., 2004; Reese and Doering, 2003). Based on this property, the ability of the P. brasiliensis glycans to interact with surface components of C. neoformans was tested in an immunofluorescence model. Incubation of acapsular C. neoformans cells in the presence of the P. brasiliensis glycan fraction extracted with DMSO resulted in binding to the cryptococcal surface (Fig. 6). Glycan incorporation by acapsular cryptococci differed when purified GXM or the P. brasiliensis extract were used. The use of mAbs 18B7 and 2D10 as detection probes revealed that the acapsular mutant bound to GXM to form an annular pattern of surface coating. In contrast, mAb 13F1 produced a punctate pattern of antibody, which is in agreement with a previous report (Cleare and Casadevall, 1998). However, punctate binding occurred with all of the mAbs when incubated with Cap67 cells coated with the P. brasiliensis glycan fraction. These results indicate that, although cryptococcal cell wall components interact with polysaccharide fractions obtained from both C. neoformans and P. brasiliensis, this process varies depending on what glycan is used, possibly suggesting structural differences.
Fig. 6.

Incorporation of P. brasiliensis Pb18 (Pb) molecules by an acapsular mutant (Cap67) of C. neoformans. Cap67 cells were treated with the Pb extract or with GXM purified from C. neoformans H99 cultures followed by incubation with mAbs raised to GXM. Positive reactions were observed for all antibodies, but the pattern of reactivity depended on which polysaccharide fraction was used to coat Cap67 cells. Scale bar, 20 μm.
In the vast majority of the coating experiments, the P. brasiliensis material was found to be tightly associated to the cell wall of Cap67 cells (Fig. 6). However, different patterns of interaction of the P. brasiliensis glycans with acapsular cryptococci were occasionally observed. In these rare cases, DIC images suggested the formation of extracellular aggregates that were not closely attached to the cell wall of the acapsular C. neoformans mutant (Fig. 7). Analysis of these cells by immunofluorescence with mAb 18B7 revealed the existence of surface structures that resemble a capsular network, which was confirmed by SEM. Although most of the cells did not contain these surface structures, we found that approximately 5% of the cells formed surface networks that resembled fungal capsules. We therefore concluded that the P. brasiliensis glycan is recognized by components of the cryptococcal cell wall with possible formation of glycan networks that may be related to fungal capsules.
Fig. 7.
Coating of Cap67 C. neoformans cells with the P. brasiliensis Pb18 extract results in the formation of capsule-like structures. (A and B) Analysis of the C. neoformans acapsular mutant after incubation in the absence of polysaccharide fractions (control) followed by exposure to mAb 18B7 by fluorescence microscopy. No serologic reactivity was observed. Incubation of Cap67 cells with the DMSO extract from P. brasiliensis results in the formation of extracellular aggregates (C, arrows) that resembled capsular structures when observed by fluorescence microscopy (D). Differential interferential contrast (A and C) and fluorescence (B and D) modes are shown. Results shown in C–D were confirmed by scanning electron microscopy of control (E) or glycan-coated (F) Cap 67 cells. Scale bars correspond to 20 μm in panels for A–D, 1 μm in E and 2 μm in F.
3.5. Binding to P. brasiliensis glycans reduces the efficacy of association of the acapsular mutant of C. neoformans with host phagocytes
Based on the results presented in Figs. 6 and 7, we asked whether coating Cap67 cells with the P. brasiliensis glycan fraction would affect the interaction of the mutant with macrophages, since protection against phagocytosis is one of the primary functions of cryptococcal GXM. We then compared the ability of uncoated Cap67 cells to interact with phagocytic cells relative to that observed for acapsular cells coated with the P. brasiliensis glycan fraction. As a control, we used Cap67 cells coated with C. neoformans H99 GXM. As expected, GXM-coated Cap67 cells were about 40% less efficient in their interactions with phagocytic cells relative to the uncoated acapsular cells (Fig. 8A). Coating of the mutant with the P. brasiliensis glycan resulted in 70% reduction in the ability of the C. neoformans mutant to associate with RAW macrophages, in comparison with uncoated yeast cells (Fig. 8B). Therefore, in quantitative terms, the P. brasiliensis glycan fraction was approximately 50% more effective than GXM in inhibiting the interaction of C. neoformans with RAW phagocytes (Fig. 8C).
Fig. 8.
Coating of C. neoformans Cap67 (Cn) acapsular cells with P. brasiliensis Pb18 (Pb) molecules renders yeast cells less efficient in their ability to associate with macrophage-like cells. To measure the association of the Cap67 mutant with host cells, fungi were stained with FITC and incubated with the phagocytes, which were then analyzed by flow cytometry. (A) Comparison between the indices of association of macrophages with control Cap67 cells (no coating) or with the GXM-coated mutant. (B) Coating of Cap67 cells with the P. brasiliensis glycan fraction caused a decrease in the index of association of the acapsular mutant with macrophages. (C) Analysis of the efficacy of coating Cap67 cells with C. neoformans GXM or with the P. brasiliensis glycan fraction to inhibit the association of yeast cells with phagocytes. To differentiate adhered and internalized yeast cells, infected macrophages were treated with trypan blue, an extinguisher of extracellular FITC-derived fluorescence (D–F). The shift of histograms to regions of lower fluorescence after exposure to trypan blue denotes the presence of extracellularly-associated yeast cells. This figure is representative of three different experiments producing similar results.
To compare indices of uncoated and glycan-coated Cap67 adhesion to and internalization by phagocytes, infected macrophages were treated with trypan blue after infection with C. neoformans. Trypan blue extinguishes extracellular fluorescence, which implies that the relative fluorescence reduction of the macrophages after exposure to this dye will correspond to the percentage of yeast cells that adhered to but were not internalized by the macrO-phages. Fluorescence intensity was reduced by about 20% after exposure of macrophages that were infected with uncoated Cap67 cells to trypan blue (Fig. 8D), which is in agreement with the observation that acapsular variants of C. neoformans are efficiently phagocytized (Kozel and Gotschlich, 1982). When macrO-phages that were infected with GXM-coated Cap67 cells were treated with trypan blue, a decrease in fluorescence intensity in the range of 30% was observed (Fig. 8E). In similar systems where the acapsular mutant was coated with the P. brasiliensis glycan, fluorescence reduction corresponded to 13% (Fig. 8F). Therefore, in all cases most of the fungal cells were internalized after incubation in the presence of host phagocytes, although the variation in fluorescence reduction fluctuated in the three different systems.
4. Discussion
Polysaccharides are key regulators of fungal pathogenesis. In different fungal pathogens, neutralization of polysaccharides with antibodies, as well as the induction of immune responses to poly saccharides, results in modification of the course of infection in favor of the host (Casadevall et al., 1998; Casadevall and Pirofski, 2006; Pirofski, 2001). In this context, it seems clear that understanding the role of polysaccharides in fungal infections is a key strategy for the design of new therapeutics.
α1,3 Glucans are found in the cell walls of several pathogenic fungi, including C. neoformans (Reese and Doering, 2003; Reese et al., 2007), H. capsulatum (Klimpel and Goldman, 1988), Blastomyces dermatitidis (Hogan and Klein, 1994) and P. brasiliensis (San-Blas and Vernet, 1977). In H. capsulatum, gene depletion studies have demonstrated that the virulence of certain strain types requires α1,3 glucan (Rappleye et al., 2004). Both RNA interference (RNAi)-mediated reduction in the synthase for α1,3-glucan (AGS1) and traditional allelic replacement substantially reduced the ability of the fungus to proliferate and kill macrophages in culture and decreased lung fungal burden in a murine infection model. In P. brasiliensis, a similar relationship between virulence and α1,3-glucan has been described, as concluded from the observation that spontaneous loss of the polysaccharide correlated with decreased virulence (San-Blas and San-Blas, 1977). In C. neoformans, the lack of α1,3-glucan synthesis correlates with an acapsular phenotype, which was accompanied by disorganization of the cell wall, altered polysaccharide composition, and an avirulent phenotype (Reese and Doering, 2003; Reese et al., 2007). The lack of visible capsules in the strain lacking α1,3-glucan was not a consequence of altered GXM synthesis, since cells with altered α1,3-glucan synthesis continued to shed capsule material into the environment (Reese and Doering, 2003). This observation led to the conclusion that α1,3-glucan was responsible for anchoring surface GXM. In fact, capsule material from C. neoformans efficiently bound only to α1,3-glucan-producing H. capsulatum strains (Reese and Doering, 2003). These cells showed a blurred and very intense staining with a monoclonal antibody to GXM, resembling capsular structures (Reese and Doering, 2003). Apparently, no direct staining of H. capsulatum with antibodies to GXM was performed by the authors, implying that the possibility that H. capsulatum also produces GXM-like polymers could not be ruled out at present.
P. brasiliensis is a non-encapsulated fungal pathogen. An early report, however, indicates the occurrence of cell wall-associated fibrilar material in this species (Carbonell, 1967; Carbonell et al., 1970). Nevertheless, it is inaccurate to state that P. brasiliensis synthesizes a true capsule. Our results, however, suggest that a P. brasiliensis glycan fraction shares structural, functional and serologic properties with C. neoformans GXM. The P. brasiliensis glycan sharing epitopes with cryptococcal GXM was surface-associated, but its detection in DMSO extracts raised additional possibilities. Considering that (i) concentrated DMSO is very efficient in damaging the plasma membrane by solubilizing lipids and (ii) C. neoformans GXM is synthesized intracellularly and then exported to the extra-cellular space (Rodrigues et al., 2007, 2011b), we cannot rule out the possibility that the GXM-related components of the P. brasiliensis glycan are also found in intracellular compartments.
Glycans sharing serologic properties with C. neoformans GXM were detected in both mycelia and yeast cells. Given that α1, 3-glucan is found exclusively at the cell wall of yeast forms of P. brasiliensis (San-Blas and Vernet, 1977), this observation suggests that other molecules interact with the GXM-related structures in Paracoccidioides yeast cells, as was previously described for chitin in C. neoformans and T. asahii (Fonseca et al., 2009a, 2009b; Ramos et al., 2012; Rodrigues et al., 2008a). The molecular dimensions of the P. brasiliensis glycan were smaller than those reported for C. neoformans H99 GXM and similar to those from hypocapsular C. neoformans (Kmetzsch et al., 2011), which is consistent with the acapsular morphology of P. brasiliensis. Nevertheless, acapsular C. neoformans cells were able to use the P. brasiliensis glycans to construct surface structures that resembled capsular networks, indicating that both species used similar polysaccharides to protect themselves against the immune defense and environmental predators. In fact, several mechanisms of pathogenicity and immunological escape are shared by C. neoformans and P. brasiliensis, including pigmentation (Gomez et al., 2001; Wang et al., 1995), production of extracellular matrix-degrading proteases (Puccia et al., 1998; Rodrigues et al., 2003), sialylation of surface glycoproteins (Rodrigues et al., 2002; Soares et al., 1998), synthesis of immunogenic glycosylceramides (Bertini et al., 2007; Rodrigues et al., 2000), ability to attach to host cells in the lung (Barbosa et al., 2006; Gonzalez et al., 2008) and vesicular secretion of virulence factors (Eisenman et al., 2009; Rodrigues et al., 2008b, 2007; Vallejo et al., 2011, 2012b). Our results are indeed suggestive that extracellular vesicles are involved in export of the currently studied P. brasiliensis glycan. In C. neoformans and P. brasiliensis, extra-cellular vesicles have been demonstrated to be immunologically active (Oliveira et al., 2010a; Rodrigues et al., 2008b; Vallejo et al., 2011), which is likely influenced by the presence of GXM and other glycans in these secretory compartments.
Our analysis of the P. brasiliensis homologs corresponding to proteins involved in C. neoformans GXM synthesis, capsule formation and polysaccharide export was consistent with our experimental findings and with the literature in the field. For instance, two of the three proteins involved in capsule formation in C. neo formans (Chang and Kwon-Chung, 1998; Chang et al., 1996) were not found in P. brasiliensis, which is consistent with the lack of a capsule in this pathogen and with the reduced dimensions of its glycans that have antigenic similarities to GXM. In fact, polysaccharide enlargement was demonstrated to be essential for capsule growth in C. neoformans (Frases et al., 2009). On the other hand, P. brasiliensis had homologs for the genes required for C. neoformans GXM synthesis and export, except O-acetylation. These observations are in agreement with serologic, biochemical and microscopic data suggesting that P. brasiliensis produces a surface glycan likely representing a substituted mannan. The synthesis of mannans does not necessarily translate to capsule formation, since it is well known that, in C. neoformans, capsular assembly depends also on the interaction of GXM with other components, including glucuronoxylomannogalactan (GXMGal) (De Jesus et al., 2009). This observation may be also be related to the fact that some of the acapsular C. neoformans cells used the P. brasiliensis glycan fraction to form surface networks that resembled fungal capsules. The acapsular mutant used in this study is known to be an efficient pro ducer of GXMGal (Vaishnav et al., 1998), which could interact with the P. brasiliensis glycan to form capsule-like structures. Based on our current data, it is impossible to affirm that these structures form true capsules, but the possibility that P. brasiliensis formed extracellular aggregates that would then be incorporated onto the cryptococcal cell surface with consequent formation of capsule-like structures cannot be ruled out, considering the ability of GXM to self-aggregate (Nimrichter et al., 2007). Another important issue particularly related to this model is the suggestion that, despite the similarities detected in our studies, P. brasiliensis and C. neoformans glycans are likely to differ in certain structural aspects, as concluded from the different patterns of serologic reactivity after incorporation into the surface of acapsular cells, as well as lack of glucuronic acid as a monosaccharide constituent and ab sence of O acetylation.
The complete structure of the P. brasiliensis glycan still remains to be elucidated. The abundance of mannose and galactose suggests a galactomannan, which has been previously described in this fungus (San-Blas and San-Blas, 1982) and characterized in detail in Aspergillus species (Latge, 2009). Interestingly, preliminary data from our laboratory shows that A. fumigatus conidia were efficied ciently labeled by mAb 18B7 (P.C. Albuquerque, E. BarretO-Bergter and M.L. Rodrigues, unpublished). H. capsulatum glycans are also recognized by GXM-binding antibodies (A. Guimarães, unpub lished) and this fungus also produces antigens that cross-react with galactomannan (Zancope-Oliveira et al., 1994). These obser vations are all consistent with the notion that the P. brasiliensis GXM-related glycan identified in this study is a galactomannan. However, the detection of glucose, xylose and rhamnose may suggest a more complex polysaccharide or, more likely, the presence of different polysaccharides in the same fraction. Improved methods for purification of theses P. brasiliensis molecules and complete structural elucidation are underway in our laboratory. Serologic epitopes in the P. brasiliensis glycan also remain to be determined. Using a set of mAbs that were not related to those used in this study, Kozel and colleagues demonstrated that GXM de-O-acetylation affected the reactivities of five of seven anti-GXM antibodies (Kozel et al., 2003). In addition, loss of xylosylation in C. neoformans produced a substantive alteration in the binding behavior of one mAb. These results demonstrated that polysaccharide O-acetylation and xylosylation are determinant for the serologic reactivity of cryptococcal GXM and support the hypothesis that additional epitopes exist in the P. brasiliensis glycan, considering the lack of genes required for mannose O-acetylation, resistance to alkali treatment and the trace amounts of xylose found in the glycan fraction obtained from this fungus. Regardless, it seems plausible to suggest, based on our current data, that the synthesis of GXM-related polymers is not restricted to pathogenic Cryptococcus species. In fact, different reports demonstrate that fungal species that include C. liquefaciens (Araujo Gde et al., 2012) and Trichosporon asahii (Fonseca et al., 2009a) produce GXM-like polysaccharides that share structural, antiphagocytic and serologic properties with the polysaccharides produced by C. neoformans and C. gattii.
Protection against phagocytosis is a principal function of cryptococcal GXM (Kozel and Gotschlich, 1982). In our study, coating acapsular C. neoformans cells with exogenous P. brasiliensis glycans suggested that the latter molecules could also impact P. brasiliensis yeast cell interactions with host effector cells. The C. neoformans polysaccharide has diverse additional functions, most of them related to modulation of the immune response (Zaragoza et al., 2009). GXM is severely deleterious to a number of host’s immuno logical mechanisms but, depending on structural particularities and molecular dimensions, the polysaccharide may exert paradoxical functions to stimulate the host response (Fonseca et al., 2010; Rodrigues et al., 2011a). The clear relevance of GXM for disease progress and the uniqueness in its structure and functions make this polysaccharide an attractive target for antifungal therapy. The observation that, as previously described for Trichosporon species (Fonseca et al., 2009a), molecules with properties that resembled those described for cryptococcal GXM are found in non-Cryptococcus fungal species suggest that antibodies to GXM and/or similar tools may be therapeutic in different models of fungal infections. Since the ability of the P. brasiliensis glycan fraction to stimulate the immune response is still unknown, its use in vaccine preparations could be also possible.
Our results add P. brasiliensis to the group of fungal pathogens producing GXM-related molecules. Considering the major impact that this polysaccharide exerts in infections caused by C. neoformans, C. gattii and, possibly, Trichosporon species, the possibility that the currently identified glycan impacts the pathogenesis of PCM is intriguing from an evolutionary standpoint as well as representing potential targets for diagnostics and therapeutics. Significantly, the use of mimetic peptides and passively administered protective antibodies to prevent and treat animal and human PCM may represent new promising alternatives to control this fungal infection.
Acknowledgments
P.C.A, C.L.R, R.P., K.M., L.N., A.J.G and M.L.R are supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nìvel Superior (CAPES, Brazil), Conselho Nacional de Desenvolvimento Cientì?co e Tecnológico (CNPq, Brazil), Fundação de Amparo a Pes quisa do Estado de São Paulo (FAPESP, Brazil) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil). AC is supported by NIH Grants AI033142, AI033774, AI052733, and HL059842 and the Center for AIDS Research at Einstein. M.L.R, L.N. and K.M. are CNPq research fellows. A.J.G., L.N. and P.C.A. were also partially supported by the Interhemispheric Research Training Grant in Infectious Diseases, Fogarty International Center (NIH D43-TW007129). We are thankful to Prof. Eliana Barreto Bergter for helpful comments and suggestions. Carbohydrate analyses were performed at the Complex Carbohydrate Research Center, University of Georgia (Atlanta), which is supported in part by the Department of Energy-funded Center for Plant and Microbial Complex Carbohydrates (DE-FG-9-93ER-20097).
References
- Araujo Gde S, et al. Capsules from pathogenic and non-pathogenic Cryptococcus spp. manifest significant differences in structure and ability to protect against phagocytic cells. PLoS One. 2012;7:e29561. doi: 10.1371/journal.pone.0029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FM, et al. Glucuronoxylomannan-mediated interaction of Cryptococcus neoformans with human alveolar cells results in fungal internalization and host cell damage. Microbes Infect. 2006;8:493–502. doi: 10.1016/j.micinf.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Bertini S, et al. Expression of antibodies directed to Paracoccidioides brasiliensis glycosphingolipids during the course of paracoccidioidomycosis treatment. Clin. Vaccine Immunol. 2007;14:150–156. doi: 10.1128/CVI.00285-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell LM. Cell wall changes during the budding process of Paracoccidioides brasiliensis and Blastomyces dermatitidis. J. Bacteriol. 1967;94:213–223. doi: 10.1128/jb.94.1.213-223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell LM, et al. Chemical morphology of glucan and chitin in the cell wall of the yeast phase of Paracoccidioides brasiliensis. J. Bacteriol. 1970;101:636–642. doi: 10.1128/jb.101.2.636-642.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Pirofski LA. Polysaccharide-containing conjugate vaccines for fungal diseases. Trends Mol. Med. 2006;12:6–9. doi: 10.1016/j.molmed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Casadevall A, et al. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- Casadevall A, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 1998;66:2230–2236. doi: 10.1128/iai.66.5.2230-2236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 1999;181:5636–5643. doi: 10.1128/jb.181.18.5636-5643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, et al. Further analysis of the CAP59 locus of Cryptococcus neoformans: structure defined by forced expression and description of a new ribosomal protein-encoding gene. Gene. 1995;167:179–183. doi: 10.1016/0378-1119(95)00640-0. [DOI] [PubMed] [Google Scholar]
- Chang YC, et al. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 1996;64:1977–1983. doi: 10.1128/iai.64.6.1977-1983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, et al. Structure and biological activities of acapsular Cryptococcus neoformans 602 complemented with the CAP64 gene. Infect. Immun. 1997;65:1584–1592. doi: 10.1128/iai.65.5.1584-1592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare W, Casadevall A. The different binding patterns of two immunoglobulin M monoclonal antibodies to Cryptococcus neoformans serotype A and D strains correlate with serotype classification and differences in functional assays. Clin. Diagn. Lab. Immunol. 1998;5:125–129. doi: 10.1128/cdli.5.2.125-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell TR, et al. The pathogenic fungus Cryptococcus neoformans expresses two functional GDP-mannose transporters with distinct expression patterns and roles in capsule synthesis. Eukaryot. Cell. 2007;6:776–785. doi: 10.1128/EC.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus M, et al. Capsular localization of the Cryptococcus neoformans polysaccharide component galactoxylomannan. Eukaryot. Cell. 2009;8:96–103. doi: 10.1128/EC.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, et al. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Eisenman HC, et al. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155:3860–3867. doi: 10.1099/mic.0.032854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava-Netto C. Estudos quantitativos sobre a fixação de complemento na blastomicose sul-americana, com antìgeno polissacarìdico. Arq. Cir. Clin. Exp. São Paulo. 1955;18:197–254. [PubMed] [Google Scholar]
- Feldmesser M, Casadevall A. Mechanism of action of antibody to capsular polysaccharide in Cryptococcus neoformans infection. Front. Biosci. 1998;3:d136–d151. doi: 10.2741/a270. [DOI] [PubMed] [Google Scholar]
- Fonseca FL, et al. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet. Biol. 2009a;46:496–505. doi: 10.1016/j.fgb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca FL, et al. Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot. Cell. 2009b;8:1543–1553. doi: 10.1128/EC.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca FL, et al. Immunomodulatory effects of serotype β glucuronoxylomannan from Cryptococcus gattii correlate with polysaccharide diameter. Infect. Immun. 2010;78:3861–3870. doi: 10.1128/IAI.00111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frases S, et al. Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc. Natl. Acad. Sci. USA. 2009;106:1228–1233. doi: 10.1073/pnas.0808995106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, et al. Cell wall polysaccharides of pathogenic yeasts. Curr. Top. Med. Mycol. 1995;6:189–219. [PubMed] [Google Scholar]
- Garcia-Rivera J, et al. Cryptococcus neoformans CAP59 (or Cap59p) is involved in the extracellular trafficking of capsular glucuronoxylomannan. Eukaryot. Cell. 2004;3:385–392. doi: 10.1128/EC.3.2.385-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez BL, et al. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect. Immun. 2001;69:5760–5767. doi: 10.1128/IAI.69.9.5760-5767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, et al. Paracoccidioides brasiliensis conidia recognize fibronectin and ?brinogen which subsequently participate in adherence to human type II alveolar cells: involvement of a specific adhesin. Microb. Pathog. 2008;44:389–401. doi: 10.1016/j.micpath.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Hogan LH, Klein BS. Altered expression of surface alpha-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect. Immun. 1994;62:3543–3546. doi: 10.1128/iai.62.8.3543-3546.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G, et al. Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Mol. Microbiol. 2001;42:453–467. doi: 10.1046/j.1365-2958.2001.02651.x. [DOI] [PubMed] [Google Scholar]
- Kamerling JP, et al. Characterization by gas–liquid chromatography–mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem. J. 1975;151:491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel KR, Goldman WE. Cell walls from avirulent variants of Histoplasma capsulatum lack alpha-(1,3)-glucan. Infect. Immun. 1988;56:2997–3000. doi: 10.1128/iai.56.11.2997-3000.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klutts JS, Doering TL. Cryptococcal xylosyltransferase 1 (Cxt1p) from Cryptococcus neoformans plays a direct role in the synthesis of capsule polysaccharides. J. Biol. Chem. 2008;283:14327–14334. doi: 10.1074/jbc.M708927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmetzsch L, et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol. Microbiol. 2011;81:206–218. doi: 10.1111/j.1365-2958.2011.07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Gotschlich EC. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J. Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- Kozel TR, et al. Antigenic and biological characteristics of mutant strains of Cryptococcus neoformans lacking capsular O acetylation or xylosyl side chains. Infect. Immun. 2003;71:2868–2875. doi: 10.1128/IAI.71.5.2868-2875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, et al. Emerging themes in cryptococcal capsule synthesis. Curr. Opin. Struct. Biol. 2011;21:597–602. doi: 10.1016/j.sbi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen RA, et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 2005;49:952–958. doi: 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge JP, Galactofuranose containing molecules in Aspergillus fumigatus. Med. Mycol. 2009;47(Suppl. 1):S104–S109. doi: 10.1080/13693780802258832. [DOI] [PubMed] [Google Scholar]
- Lopes LC, et al. Glycoconjugates and polysaccharides from the Scedosporium/Pseudallescheria boydii complex: structural characterisation, involvement in cell differentiation, cell recognition and virulence. Mycoses. 2011;54(Suppl. 3):28–36. doi: 10.1111/j.1439-0507.2011.02105.x. [DOI] [PubMed] [Google Scholar]
- Maxson ME, et al. The volume and hydration of the Cryptococcus neoformans polysaccharide capsule. Fungal Genet. Biol. 2007a;44:180–186. doi: 10.1016/j.fgb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Maxson ME, et al. Radial mass density, charge, and epitope distribution in the Cryptococcus neoformans capsule. Eukaryot. Cell. 2007b;6:95–109. doi: 10.1128/EC.00306-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morejon KM, et al. Paracoccidioidomycosis in patients infected with and not infected with human immunodeficiency virus: a case-control study. Am. J. Trop. Med. Hyg. 2009;80:359–366. [PubMed] [Google Scholar]
- Moyrand F, et al. Isolation and characterization of capsule structure mutant strains of Cryptococcus neoformans. Mol. Microbiol. 2002;45:837–849. doi: 10.1046/j.1365-2958.2002.03059.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, et al. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, et al. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one β cell. J. Exp. Med. 1995;181:405–409. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrichter L, et al. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot. Cell. 2007;6:1400–1410. doi: 10.1128/EC.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira SV, et al. Paracoccidioides brasiliensis enolase is a surface protein that binds plasminogen and mediates interaction of yeast forms with host cells. Infect. Immun. 2010;78:4040–4050. doi: 10.1128/IAI.00221-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, et al. Cryptococcus neoformans cryoultramicrotomy and vesicle fractionation reveals an intimate association between membrane lipids and glucuronoxylomannan. Fungal Genet. Biol. 2009;46:956–963. doi: 10.1016/j.fgb.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, et al. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect. Immun. 2010a;78:1601–1609. doi: 10.1128/IAI.01171-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One. 2010b;5:e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto J, et al. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol. Microbiol. 2009;71:1165–1176. doi: 10.1111/j.1365-2958.2008.06588.x. [DOI] [PubMed] [Google Scholar]
- Pirofski LA. Polysaccharides, mimotopes and vaccines for fungal and encapsulated pathogens. Trends Microbiol. 2001;9:445–451. doi: 10.1016/s0966-842x(01)02134-5. [DOI] [PubMed] [Google Scholar]
- Prado M, et al. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem. Inst. Oswaldo Cruz. 2009;104:513–521. doi: 10.1590/s0074-02762009000300019. [DOI] [PubMed] [Google Scholar]
- Puccia R, et al. Exocellular proteolytic activity of Paracoccidioides brasiliensis: cleavage of components associated with the basement membrane. Med. Mycol. 1998;36:345–348. [PubMed] [Google Scholar]
- Queiroz-Telles F, Escuissato DL. Pulmonary paracoccidioidomycosis. Semin. Respir. Crit. Care Med. 2011;32:764–774. doi: 10.1055/s-0031-1295724. [DOI] [PubMed] [Google Scholar]
- Ramos CL, et al. Chitin-like molecules associate with Cryptococcus neoformans glucuronoxylomannan to form a glycan complex with previously unknown properties. Eukaryot. Cell. 2012 doi: 10.1128/EC.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, et al. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol. Microbiol. 2004;53:153–165. doi: 10.1111/j.1365-2958.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- Reese AJ, Doering TL. Cell wall alpha-1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol. Microbiol. 2003;50:1401–1409. doi: 10.1046/j.1365-2958.2003.03780.x. [DOI] [PubMed] [Google Scholar]
- Reese AJ, et al. Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 2007;63:1385–1398. doi: 10.1111/j.1365-2958.2006.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 2000;68:7049–7060. doi: 10.1128/iai.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Sialylglycoconjugates and sialyltransferase activity in the fungus Cryptococcus neoformans. Glycoconj. J. 2002;19:165–173. doi: 10.1023/A:1024245606607. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Cleavage of human fibronectin and other basement membrane-associated proteins by a Cryptococcus neoformans serine proteinase. Microb. Pathog. 2003;34:65–71. doi: 10.1016/s0882-4010(02)00195-x. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell. 2007;6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot. Cell. 2008a;7:602–609. doi: 10.1128/EC.00307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell. 2008b;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Fungal polysaccharides: biological activity beyond the usual structural properties. Front. Microbiol. 2011a;2:171. doi: 10.3389/fmicb.2011.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ML, et al. Vesicular transport systems in fungi. Future Microbiol. 2011b;6:1371–1381. doi: 10.2217/fmb.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Blas G, San-Blas F. Paracoccidioides brasiliensis: cell wall structure and virulence. A review. Mycopathologia. 1977;62:77–86. doi: 10.1007/BF01259396. [DOI] [PubMed] [Google Scholar]
- San-Blas G, San-Blas F. Variability of cell wall composition in Paracoccidioides brasiliensis: a study of two strains. Sabouraudia. 1982;20:31–40. doi: 10.1080/00362178285380061. [DOI] [PubMed] [Google Scholar]
- San-Blas G, Vernet D. Induction of the synthesis of cell wall alpha-1,3-glucan in the yeastlike form of Paracoccidioides brasiliensis strain IVIC Pb9 by fetal calf serum. Infect. Immun. 1977;15:897–902. doi: 10.1128/iai.15.3.897-902.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Blas G, et al. Fungal morphogenesis and virulence. Med. Mycol. 2000;38(Suppl. 1):79–86. [PubMed] [Google Scholar]
- Soares RM, et al. Identification of sialic acids on the cell surface of hyphae and yeast forms of the human pathogen Paracoccidioides brasiliensis. FEMS Microbiol. Lett. 1993;108:31–34. doi: 10.1016/0378-1097(93)90483-i. [DOI] [PubMed] [Google Scholar]
- Soares RM, et al. Anionogenic groups and surface sialoglycoconjugate structures of yeast forms of the human pathogen Paracoccidioides brasiliensis. Microbiology. 1998;144:309–314. doi: 10.1099/00221287-144-2-309. Pt. 2. [DOI] [PubMed] [Google Scholar]
- Sommer U, et al. An alpha-1,3-mannosyltransferase of Cryptococcus neoformans. J. Biol. Chem. 2003;278:47724–47730. doi: 10.1074/jbc.M307223200. [DOI] [PubMed] [Google Scholar]
- Tavares PM, et al. In vitro activity of the antifungal plant defensin RsAFP2 against Candida isolates and its in vivo efficacy in prophylactic murine models of candidiasis. Antimicrob. Agents Chemother. 2008;52:4522–4525. doi: 10.1128/AAC.00448-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CM, Roberts IS. Capsular polysaccharides and their role in virulence. Contrib. Microbiol. 2005;12:55–66. doi: 10.1159/000081689. [DOI] [PubMed] [Google Scholar]
- Teles FR, Martins ML. Laboratorial diagnosis of paracoccidioidomycosis and new insights for the future of fungal diagnosis. Talanta. 2011;85:2254–2264. doi: 10.1016/j.talanta.2011.07.099. [DOI] [PubMed] [Google Scholar]
- Torosantucci A, et al. A novel glycO-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnav VV, et al. Structural characterization of the galactoxylomannan of Cryptococcus neoformans Cap67. Carbohydr. Res. 1998;306:315–330. doi: 10.1016/s0008-6215(97)10058-1. [DOI] [PubMed] [Google Scholar]
- Vallejo MC, et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot. Cell. 2011;10:343–351. doi: 10.1128/EC.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. 7:e39463. doi: 10.1371/journal.pone.0039463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo MC, et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J. Proteome Res. 2012b;11:1676–1685. doi: 10.1021/pr200872s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart JF, Kamerling JP. Mass spectrometry of carbohydrates. Biochem. Soc. Trans. 1975;3:460–462. doi: 10.1042/bst0030460. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect. Immun. 1995;63:3131–3136. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Doering TL. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol. Biol. Cell. 2006;17:5131–5140. doi: 10.1091/mbc.E06-08-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Doering TL. An unusual organelle in Cryptococcus neoformans links luminal pH and capsule biosynthesis. Fungal Genet. Biol. 2009;46:682–687. doi: 10.1016/j.fgb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zancope-Oliveira RM, et al. Immunochemical analysis of the H and M glycoproteins from Histoplasma capsulatum. Clin. Diagn. Lab. Immunol. 1994;1:563–568. doi: 10.1128/cdli.1.5.563-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, et al. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]