Abstract
Background:
Diabetes mellitus is a real pandemic of the modern world and the incidence of the disease is increasing at a tremendous rate with a number of complications involving major systems of the human body. The renin angiotensin system (RAS) is considered to be involved in most of the pathological processes that result in diabetic nephropathy and retinopathy.
Aim:
The study was designed to evaluate and compare effects of ramipril (angiotensin-converting enzyme inhibitor-ACEI) and telmisartan (angiotensin II receptor blocker - ARBs) combinations on the progression of retinopathy and nephropathy in the streptozotocin (STZ) induced diabetic model.
Materials and Methods:
Diabetic state in rats was induced by chemical method using STZ 55 mg/kg intraperitoneally. Diabetic renal tubulopathy and interstitial inflammatory changes were done. Diabetic retinopathy manifested in the form of vacuolar changes in the inner plexiform and the ganglionic layers of the retina was observed.
Results:
Treatments with ACEI and ARBs reduced the incidence of the occurrence of cataract. The effect of combinational drugs of ACEI (ramipril) and AT1 receptor blocker (Telmisartan) was evaluated. The drugs used in combinations showed improvement in the histopathological and biochemical changes of the diabetic animals, both for the retina and kidney.
Conclusion:
The efficacy of the drugs suggests a pivotal role of the local RAS system in the pathogenesis of tubulopathy in the kidney and neuronal damage in the retina of the diabetic animals.
Keywords: Diabetes, Diabetic nephropathy, Dual block, Nephropathy, Retinopathy, Renin angiotensin system, Ramipril, Telmisartan, STZ
Introduction
Diabetes mellitus (DM) is a real pandemic of the modern world and the incidence of this disease is increasing at a tremendous rate.[1] It is a disease with a number of complications involving most major systems of the human body.[2] Long-term impairment of the microcirculation in diabetes, largely through the adverse actions of hyperglycemia and hypertension, leads to loss of nutritive blood flow and damage within the eye, the kidney and the nerves in particular.[3] The renin angiotensin system (RAS) is considered to be involved in most of the pathological processes that result in diabetic nephropathy.[4] The elevated expression of angiotensin II (AT-II) in diabetes systemically is also involved in the retinopathy and its progression.[5] Studies regarding the dual inhibition of RAS including angiotensin-converting enzyme (ACE) and angiotensin receptor in diabetic nephropathy were done, but the effect of this over the retinopathy is not yet published in literature.[6] In a human study, it has been shown that the ACE inhibition or AT-II (AT-1 subtype) receptor blocking helps in prevention of progression of retinopathy than what is actually expected, in its prevention in nephropathy in type I diabetes.[7] To the best of our knowledge the progression status of diabetic retinopathy in combinational treatment using ACE inhibitor and AT-II receptor blocker is unknown. As diabetic retinopathy is a common diabetic complication that we face in day-to-day clinical practice, generation of such knowledge will definitely help to develop appropriate therapeutics of diabetic retinopathy and has the potential to aid in the prevention of diabetic related blindness in the days to come.
Therefore, in the present study, we propose to study the therapy effect of ACE inhibitor (ramipril) and AT-II receptor blocker (telmisartan) and its combinational therapy in type 1 rat model of diabetes mellitus and compare its effect on the progression of diabetic retinopathy and nephropathy.
Materials and Methods
Animals, drugs and chemicals
Adult male Wistar rats, weighing between 200 to 250 g were procured from the central animal house of the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. They were kept individually in an environmentally-controlled room with free access to dietary chow and water. Rats were fed with a standard diet. After a week of acclimatization, the experiments were carried out as per the guidelines of the committee for the purpose of control and supervision of experiment in animals (CPCSEA) for the use of animals in research. The study was initiated following approval of the Institute's animal ethics committee (IAEC NO: 296, AH-71).
The STZ and ramipril powders were purchased from Himedia laboratories private limited, Mumbai. Pure powder of Telmisartan was purchased from the Themis Medicare Limited, Mumbai as an active pharmaceutical ingredient (API) and NPH insulin (Insugen), from Biocon Limited, Bangalore. All other reagents and chemicals used in the study were of the highest purity and quality and procured from the institution PGIMER. Strict asepsis was maintained throughout the study.
Experimental groups
The adult male Wistar rats of 200-250g were divided into the following groups: Control group (maintained on normal diet and no drug)-C; diabetic group (induced by STZ injection and insulin maintained)-D; diabetic group treated with ramipril (0.9 mg/kg/d)-D1; diabetic group treated with ramipril (0.45 mg/kg/d)-D2; diabetic group treated with telmisartan (3.5 mg/kg/d)-D3; diabetic group treated with telmisartan (1.75 mg/kg/d)-D4; diabetic group treated with combination of ramipril (0.9 mg/kg/d) and telmisartan (3.5 mg/kg/d)-D5; diabetic group treated with combination of ramipril (0.45 mg/kg/d) and telmisartan (1.75 mg/kg/d)-D6; Diabetic group treated with combination of ramipril (0.3 mg/kg/d) and telmisartan (1.2 mg/kg/d)-D7.
Induction of diabetic state
After an adaptation period of 1 week, the animals were given an injection of streptozotocin (55 mg/kg) in 0.05 M sodium citrate, pH 4.5 after an overnight fast through the peritoneum. Diabetes was confirmed by blood glucose on the sixth day after streptozotocin (STZ) injection. Diabetic rats were included in the study if the glucose value exceeded 300 mg/dl. Once diabetes was confirmed, the rats were randomly divided into eight groups with diabetic control and diabetic drug-treated groups. The drugs, namely ramipril and telmisartan were administered orally in various doses and combinations till 60 days from the time of randomization. All the diabetic rats irrespective of the treatment group were given 1-2 IU of insulin on alternate days after 6 days of induction of diabetes to control mortality, ketonuria and to stabilize body weight.[8]
Biochemical parameters
The change in body weight was noted at different points of time. The blood was drawn from the tail vein of the diabetic Wistar albino rats; under light ether anesthesia on the respective days and random blood glucose, glycated hemoglobin, serum creatinine and the blood urea nitrogen were measured. Urine was collected and analyzed for spot albumin creatinine ratio.
Histopathology of kidney and retina
All the animals were sacrificed at the end of the study period (i.e. on day 60). The viscera like kidneys (right and left), eyes (right and left) were dissected out and fixed in buffered formalin (pH = 7.4) for a week for histopathological analysis. The slides were stained with hematoxylin and eosin (H&E) using standard histology procedures. Four sections per organ per animal in each group were made for staining and histological studies and scoring by a blinded qualified pathologist. The whole field of the cortex of the kidney and retina was examined under high power magnification (400×).
Renal tubulointerstitium was evaluated semiquantitatively using a grading system with a score of 0 to 4+ on the basis of tubular dilatation, tubular atrophy and interstitial inflammatory cell infiltrates: 0 - no lesion (normal kidney); 1+ - single small focal lesion with very minimal tubular change and interstitial inflammatory cell infiltration; 2+ to 4+ indicated increased severity of tubular lesions and interstitial inflammatory cell infiltrations. A score of 4+ was arbitrarily assigned when approximately 50% or more renal parenchyma and interstitium was involved.[9]
Statistical analysis
All values were expressed as mean ± SD. Multiple comparisons were made between the groups for all parametric data by using one way analysis of variance (ANOVA) followed by Tukey's post-hoc analysis. For categorical data, Fisher's exact test was used. For comparison of scores for renal tubulointerstitial lesions between groups Wilcoxon sign rank test was used. The P value of <0.05 was considered statistically significant.
Results
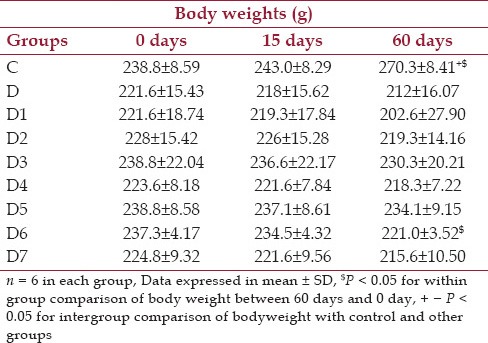
Effect of drugs on body weights
From Table 1 it is seen that in the control group (C) and in the group that received half doses of the combinational drugs (D6), the weights vary significantly over the baseline value within their respective groups.
Table 1.
Changes in body weights (g) at baseline, day 15 and day 60 for control, diabetic and diabetic treated groups of rats
Effect of drugs on random blood glucose levels
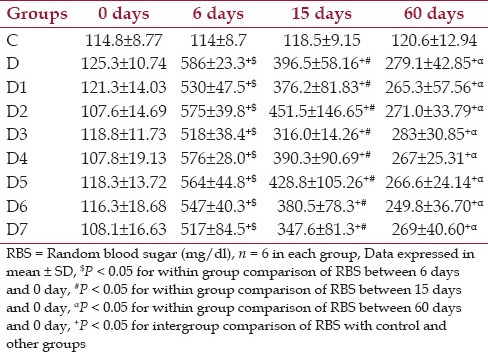
From Table 2 the baseline mean RBS values were comparable among the different groups. Also, it was seen that in all diabetic and diabetic treated groups there is a significant increase in blood glucose values from 0, 6, 15 and 60 days. From 6-day values there was reduction in 15-day and 60-day value. When the RBS values are compared between control and diabetic groups, there was huge margin of increase in blood glucose, which was highly significant (P < 0.001). When the treated groups were compared with the diabetic control group, all treated groups showed a reduction in blood glucose values over the period from 15 days to 60 days, but none had achieved statistical significance. The same scenario was also seen between the diabetic treated groups. During the course of the study it was also noticed among the different groups, the mean value of RBS was maintained between 200-300 mg/dl.
Table 2.
Changes in random blood glucose level (mg/dl) at baseline, day 15 and day 60 for control, diabetic and diabetic treated groups of rats
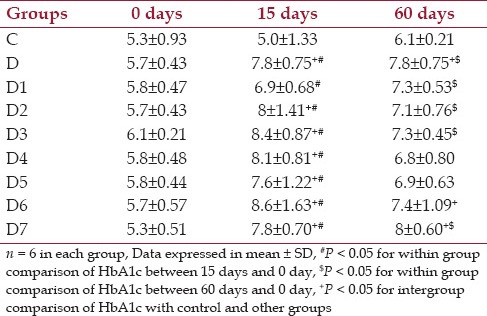
Effect of drugs on glycated hemoglobin HbA1c (%) in different groups of animal
From Table 3 it was shown that the mean glycated hemoglobin values at baseline were comparable among the control, diabetic and the diabetic treated groups and within the normal range values. At 15 days, the mean values in diabetic (D) and other diabetic treated groups were increased above normal range. At 60 days the glycated hemoglobin value decreased but not in the normal range.
Table 3.
Changes in glycated hemoglobin (HbA1c) % at baseline, day 15, day 60 for control, diabetic and diabetic treated groups of rats
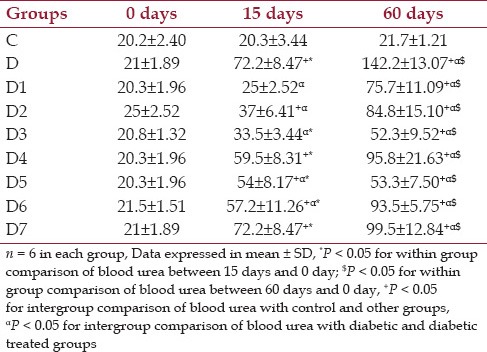
Effect of drugs on blood urea in different groups of animal
From Table 4 it was shown that the blood urea, which is a marker for renal involvement is comparable among different groups at the baseline. It was shown that the blood urea values between 0 and 15 and 60 days was increased in all diabetic and diabetic treated groups and found statistically significant (P < 0.05). On day 15 when the control and the other groups were compared, the group D, D2, D4, D5, D6 and D7 had shown increased urea values which were significant and the comparison between the diabetic and drug-treated diabetic groups at day 15 showed the groups D1, D2, D3, D5 and D6 showed decrease in blood urea values which were statistically significant. At 60 days when the control and the other groups were compared there was a statistically significant increase in the values between them and there was a statistically significant reduction in values between the diabetic and the diabetic drug-treated groups. It was seen that the groups D3 and D5 showed a major decline in levels when compared with the diabetic control showing that the drugs have some effects in the prevention of progression of diseases. Between the diabetic treated groups it was shown that the group D5, showed a remarkable reduction in blood urea values from the diabetic groups which were highly significant (P < 0.001).
Table 4.
Changes in blood urea (mg/dl) at baseline, 15 days and 60 days control, diabetic and diabetic treated groups of rats
Effect of drugs on serum creatinine, urine spot albumin creatinine ratio (ACR) in different groups of animal
The serum creatinine values and spot ACR were comparable among different groups at the baseline. It was also seen that the mean values of serum creatinine and spot ACR over the different groups at different time points was also within the normal range indicating no involvement of glomerulus. This was also evident from the histological pictures of normal glomerulus.
Histopathological examination of kidney in different groups of animal
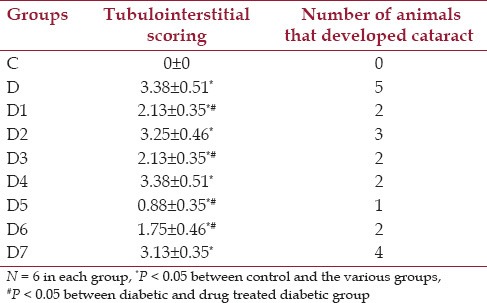
There was no gross morphological change in the kidneys of any group of the animals. No change in the glomerulus could be detected in any of the groups of animals [Figure 1]. In contrast to the control group, the untreated diabetic rat kidneys at the end of 60 days showed extensive tubular dilatations, tubular atrophy with severe interstitial inflammations lymphocytic cellular infiltrates and normal glomerulus where in the control group have normally filled tubules, normal interstitium without any cellular infiltrate and normal glomerulus [Figure 1] and This picture was also correlated with the biochemical renal parameter changes. It was shown that in group D1, D3, D6 moderate tubular dilatation with mild to moderate interstitial inflammations and with normal glomerulus was seen. In D5 group, mild tubular dilatations, mild interstitial inflammations and normal glomerulus were noticed. The highest Reno protective effect was observed with D5 group. The histological score for the renal changes were commensurate to the histopathological changes as mentioned above and documented in Table 5. The score of tubulointerstitial lesions was significantly less in groups D5 and D6, compared to that of the untreated diabetic group. However the scores were significantly higher for all the diabetic groups compared to the control group of animals.
Figure 1.
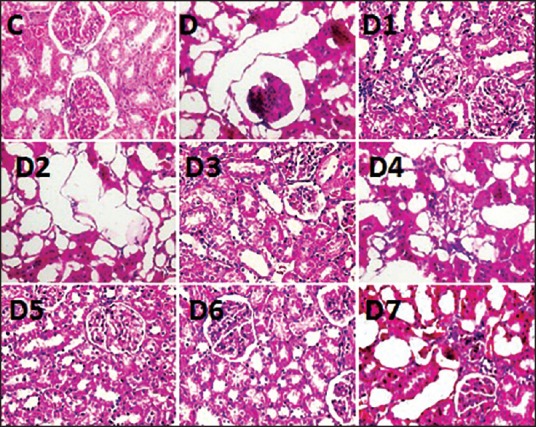
H&E staining of kidney structure containing tubules, interstitium and glomerulus. H&E staining of kidney structure containing tubules, interstitium and glomerulus. C - control with normal tubules, interstitium and glomerulus. D - diabetic control with extensive tubular dilatation showing epithelial cell atrophy severe interstitial inflammations (not shown) and normal glomerulus. D1, D3, D6 shows moderate tubular dilatation with mild to moderate interstitial inflammations (not shown) and normal glomerulus. D5 photograph shows only mild tubular dilatation with mild interstitial inflammations and normal glomerulus. (magnification 400×)
Table 5.
Effect of drugs on cataract and scoring of renal tubulointerstial lesions in STZ treated animals
Histopathological examination of retina in different groups of animal
In the diabetic rats it was found that there was lots of vacuolations seen in the inner plexiform layer and the ganglionic layer of the retina which was marked as a neuronal diabetic retinopathy changes [Figure 2]. Other late changes which were supposed to be in the diabetic eye (endothelial injury, basement membrane thickening, neovascularisation etc.) were not seen. After treatment with drugs, there was improvement shown, by the reduction in vacuolations, in the group (D5) which received a full dose of combinational drug treatment.
Figure 2.

H&E staining of layers of retina of control (C), diabetic (D) and the drug treated diabetic groups. H&E staining of layers of retina of control (C), diabetic (D) and the various drugs treated diabetic groups. The control group showed the orderly arrangement of all the cell layers of retina without any vacuolations. Diabetic group and D1, D2, D3, D4, D6 and D7 show extensive vacuolations in the inner plexiform layers and the ganglionic layer of the retina. In diabetic treated group (D5) vacuolations markedly reduced. (Magnification 400×)
Morphological changes in eye
The diabetic rat, over the course of the study developed lens changes. It was observed that cataract [Table 5] development occurred after 3-4 weeks of STZ injection. Although a large number of animals in the untreated diabetic group had developed cataract compared to the drug treated diabetic groups, yet, the values did not reach statistical significance, due to, smaller number of animals. The group of animals receiving full combinational doses (D5) showed best protection against the development of cataract.
Discussion
In a human study, it was reported that the ACE inhibition or AT-II (AT-1 subtype) receptor blocking helps in the prevention of progression of retinopathy in type I DM.[7] However, there is no published document on the effect of combinational treatment with ACEI and AT-II (AT-1 subtype) receptor antagonist on the progression of diabetic retinopathy either in animals or in human beings.
It was reported earlier that there was 17-20% mortality following STZ injection.[10] Accordingly, the model of STZ induced DM was, thus, standardized with a view to obtain persistent diabetic state without mortality to the animals. In order to achieve the goal, 5% sucrose[11] was added for first 48 hours and NPH insulin was injected, s.c.at a dose of 1-2 units throughout the study period, depending on the level of blood glucose.[12] The changes in body weight are considered as one of the markers for diabetic condition.[13] The diabetic state was confirmed on day 6 with blood sugar and HbA1c levels remained persistently higher (blood sugar in the range of 200-300 mg/dl) throughout the study. It was found that the study drugs had no significant effect on the RBS or HbA1c level compared to the untreated diabetic group either on the 15th or 60th day following STZ injection.[14] There is no published literature documenting the effect of ACEI or angiotensin receptor blockers on blood sugar level or HbA1c level in the animal model of diabetic state except one report[14] which documents that telmisartan and benzipril had no effect on the blood sugar level compared to untreated diabetic control group. The glycated hemoglobin values were also evaluated and these values depict glycemic control over the past few months.[15] Significant difference in HbA1c level between the control group and D5 group was observed on day 15 suggesting thereby that prolonged treatment with the combination of telmisartan and ramipril might beneficially influence the HbA1c level. The blood urea was significantly increased in the untreated diabetic group compared to the values of the control animals. Further, it was observed the blood urea level in an untreated diabetic group significantly increased from the baseline value, thereby indicating progressive renal tubular damage caused by persistent diabetes in animals. Blood urea levels showed progressive increase on day 15 and day 60 compared to the baseline in the entire drug treated diabetic groups. However the levels of the blood urea in all of the drug-treated diabetic groups were significantly lower compared to the untreated diabetic group on day 15 and day 60. It suggested the protective effect of ACEI and ARBs in the progression of renal damage. However, the protection offered by the full dose of ramipril and telmisartan combination was maximum, since the increase in blood urea level in this group of animals was least among these entire drug-treated groups on day 15 and day 60.
Incidentally, it was observed that there was no change in the serum creatinine values in the control group, untreated diabetic group or the different drug treated diabetic groups of animals. Further, there was no change in urinary spot ACR in any of the groups of the animals. The precise reason for isolated increase in blood urea is not clearly known. It might be due to diabetes associated catabolic states of animals and the involvement of renal proximal tubules, since renal proximal tubules are involved in urea handling[16] whereas glomerulopathy is associated with creatinine clearance and the appearance of albumin in the urine. It might be possible that the diabetic animals were suffering from renal tubulopathy which precede glomerulopathy.[17] Keeping in view the fact that the increase in blood urea levels for the drug treated groups of animals were significantly lower compared to untreated diabetic groups, it might be possible that ACEI, angiotensin receptor blocker and particularly their combinations have renal tubule protective effects against diabetes induced tubulopathy. The effect of diabetes and the drugs on blood urea levels as observed in the present study corroborate similar finding reported in the literature. Previous studies[17] had documented that the renal tubulopathy precede micro albuminuria resulting on account of diabetic glomerulopathy. It was also found in the present study that there was extensive tubular dilatation with renal interstitial inflammation in the histopathology of the kidney in the diabetic animals. The tubular involvement and the interstitial inflammation were significantly lower in the drug treated diabetic animals compared to untreated diabetic group.
Diabetic nephropathy is classically understood as a glomerular disease. However, this understanding is currently being contested with recent studies suggesting a pivotal role of the tubulointerstitium in the initiation and progression of DN.[18] The literature suggests that chronic hyperglycemia may orchestrate several structural and functional abnormalities of the tubulointerstitium before significant glomerular changes.[18]
It was also mentioned in the literature that tubular cells are not only affected secondary to glomerular injury, but also the primary targets for the pathological influences. It was said that in renal disease in diabetes, there was involvement of severe tubular lesions without involvement of the glomerulus in about one third of the diabetic population. The pathologic reactions which lead to diabetic nephropathy may first occur in the peritubular microcirculations which cause oxidative injury and the subsequent tubular damage. The tubular cells are the direct targets for the enhanced glucose levels present in diabetes. Glucose uptake is independent of the action of insulin and the intracellular glucose level of the tubular cells parallel plasma glucose concentration. Raised plasma glucose concentration resulted in augmented glomerular filtrate levels of glucose, which in its turn resulted in increased proximal tubular glucose reabsorption independent of the action of insulin. Tubular cells are direct targets for enhanced glucose levels present in diabetes. In addition, excess glucose in the glomerular filtrate leads to enhanced proximal tubular glucose efflux within the proximal tubule. On exposure to glucose, tubular cells secrete vasoactive hormones like AT-II, transforming growth factor β and matrix proteins. Glucose-dependent metabolic pathways and vasoactive hormones may directly influence tubular and interstitial cells, leading to renal dysfunction caused by non-glomerular mechanisms.[19]
In the STZ model of diabetes, the tubulointerstitial injury was due to the combined action of the hyperglycemia and STZ.[20,21] At the optimal diabetogenic dose of STZ, diabetic animals developed progressive histological changes typical of diabetic nephropathy. It was shown in the present study that the drugs causing inhibition of the RAS system protected the kidney of the diabetic animals from the tubulointerstial damage. The findings of the present study are at variance to the reported studies in the literature in which it was documented that early diabetic nephropathy occurred by one month with mesangial expansion and basement membrane thickening.[22]
In the present study it was observed that fewer number of animals in the drug treated group developed cataract in 60 days against the untreated diabetic group. The results showed a definite trend of ACEI and ARBs exerting beneficial effects in preventing the development of cataract in the diabetic animals. The results of the present study are in confirmation of such previously reported study of ACEI and ARBs on cataract development in diabetic animals.[23] The results of several studies suggest that the oxidative stress is a major determinant in diabetic complications.[24] It is also known that ACEIs remove free radicals.[25] The level of these free radicals increases in diabetes and along with hyperglycemia, is considered as a cause of accelerated cataractogenesis. Apart from hyperglycemia, increased amount of free radicals[26], the attenuated endogenous antioxidant mechanisms[27] also contribute to cataract development. Drugs like ACEI had shown some effect on the prevention of cataract. The molecular mechanisms underlying the inhibitory action of ACEIs on diabetic cataracts could be related to various reductions in non-enzymatic glycosylation, the polyol pathway, and free radical production. In the present study the use of ACEI or ARBs showed protection in the development of cataract. This delay in the genesis of cataract was also mentioned in previous studies[28,29] suggesting the role of ACEI in preventing the development of cataract. Keeping in view the results of the present study and the postulated mechanism mentioned above[28,29] further studies are warranted to find out the anticataract effect of ACEI and ARBs.
The histological changes that develops into a full blown diabetic retinopathy includes decreased number of neurons, especially ganglionic cells, a narrow layer of rods and cones, atrophy of pigmented epithelium, attenuation of the inner nuclear layer and accumulation of eosinophil substances between photoreceptor outer segments and retinal epithelium.[30,31] However, in the early phase of diabetic retinopathy such changes do not occur, but there are vacuolations seen in the different cell layers including disorderly arrangement of cells in different layers of the retina.[32] As with earlier studies[32], in the present study it was observed that the untreated diabetic group developed neuronal changes of diabetic retinopathy manifested by extensive violations of the inner plexiform layer and ganglionic cell layer. This model shows apoptosis of the inner retinal neurons, such as ganglion cells and amacrine cells, and the activation of the Muller glial cells in the retina[33,34], which can all contribute to the inner retinal dysfunction.[35] It has been found that the treatment with ACEI and ARBs markedly reduced the vacuolar changes as observed in the untreated diabetic group. It has been reported that tissue RAS system is present in the retina.[36,37] The AT-II has been reported to adversely influence the vascular component of the retina.[38] However, recent studies using the STZ-induced diabetes model have begun to reveal the influence of AT-II on neural cells.[39] The activation of the local RAS in STZ-induced diabetes model cause damage to the inner cell layer of the retina leading to functional impairment.[40] The above mentioned facts highlight the activation of local RAS system in the retina causing damage which was observed in the present study. Further it explains the protective role of ARBs and ACEI on the development of retinal inflammations and the change in the inner granular layer of the retina which were observed in the untreated diabetic group. However, long term studies are warranted to observe and document more florid retinopathy changes[30,31] and the effect of drugs on it.
Conclusion
The efficacy of ACEI (ramipril) and ARBs (telmisartan) combinations in three-dose ratios were evaluated against the microvascular complications and was found that the combination of ramipril and telmisartan showed improvement in the biochemical parameter and in the histopathological improvement of renal tubulopathy, renal interstitial inflammatory changes and neuronal changes in the retinopathy in the diabetic animals. ACEI and ARBs showed delayed development of cataract in their full dose combination. These combinations might act through the inhibition of the locally activated renin-angiotensin system triggered by persistent hyperglycaemia. Thus ARB and ACEI have shown a trend in protecting the animals from early renal tubulopathy, neuronal changes in the retina and cataract formation caused by STZ-induced diabetes mellitus.
Acknowledgement
We thank Dr. Hariharan Balasubramanian and Dr. Sajitha for their valuable contributions throughout our manuscripts.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Narayan KM, Zhang P, Kanaya AM, Williams DE, Engelgau MM, Imperatore G, et al. Diabetes: The Pandemic and Potential Solutions. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Disease Control Priorities in Developing Countries. 2nd ed. Washington (DC): World Bank; 2006. pp. 591–603. [PubMed] [Google Scholar]
- 2.Nazimek-Siewniak B, Moczulski D, Grzeszczak W. Risk of macrovascular and microvascular complications in Type 2 diabetes: Results of longitudinal study design. J Diabetes Complications. 2002;16:271–6. doi: 10.1016/s1056-8727(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 3.Wiernsperger NF, Bouskela E. Microcirculation in insulin resistance and diabetes: More than just a complication. Diabetes Metab. 2003;29:6S77–87. doi: 10.1016/s1262-3636(03)72791-8. [DOI] [PubMed] [Google Scholar]
- 4.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27:144–52. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi N. Modulation of the renin-angiotensin system and retinopathy. Heart. 2000;84:i29–31. doi: 10.1136/heart.84.suppl_1.i29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit AJ, Lutgers HL. The clinical relevance of advanced glycation end products (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem. 2004;11:2767–84. doi: 10.2174/0929867043364342. [DOI] [PubMed] [Google Scholar]
- 8.Sucheta BK, Narsingh S, Kisan MK, Vithal MK, Subhash LB, Vikram SG. VMNS2e: A Potential biphenyl PTP1B inhibitor, suppresses the development of diabetic retinopathy in STZ induced diabetic rats. Pharmacologia. 2011;2:237–45. [Google Scholar]
- 9.Velasquez MT, Striffler JS, Abraham AA, Michaelis OE, 4th, Scalbert E, Thibault N. Perindopril ameliorates glomerular and renal tubulointerstitial injury in the SHR/N-corpulent rat. Hypertension. 1997;30:1232–7. doi: 10.1161/01.hyp.30.5.1232. [DOI] [PubMed] [Google Scholar]
- 10.Hongmei C, Brahmbhatt S, Gupta A, Sharma AC. Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phosphorylation in renal and vascular dysfunction. Cardiovasc Diabetol. 2005;4:1–13. doi: 10.1186/1475-2840-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesch GH, Allen TJ. Rodent models of streptozotocin induced diabetic nephropathy. Nephrology (Carlton) 2007;12:261–6. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 12.Davis BJ, Johnston CI, Burrell LM, Burns WC, Kubota E, Cao Z, et al. Renoprotective effects of vasopeptidase inhibition in an experimental model of diabetic nephropathy. Diabetologia. 2003;46:961–71. doi: 10.1007/s00125-003-1121-9. [DOI] [PubMed] [Google Scholar]
- 13.Hi-Lin-Tian, Li S, Zhong-sxin X, Ru-Tong Z, Dong-Ling J, Jin-Sheng G. Correlations between blood glucose level and Diabetes signs in Streptozotocin induced diabetic mice. Global J Pharmacol. 2010;4:111–6. [Google Scholar]
- 14.Singh J, Budhiraja S, Lal H, Arora BR. Renoprotection by telmisartan versus benazepril in streptozotocin induced diabetic nephropathy. Iran J Pharmacol Ther. 2006;5:135–9. [Google Scholar]
- 15.Albright AL, Johnson PR, Greene S, Stern JS. Use of glycated hemoglobin to assess Glycemic control in Wistar diabetic fatty rats and Zucker fatty rats. Obes Res. 1994;2:535–9. doi: 10.1002/j.1550-8528.1994.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 16.Schrier RW. Blood urea nitrogen and serum creatinine: Not married in heart failure. Circ Heart Fail. 2008;1:2–5. doi: 10.1161/CIRCHEARTFAILURE.108.770834. [DOI] [PubMed] [Google Scholar]
- 17.Morcos M, Sayed AA, Bierhaus A, Yard B, Waldherr R, Merz W, et al. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51:3532–44. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- 18.Singh DK, Farrington K. The tubulointerstitium in early diabetic nephropathy: Prime target or bystander? Int J Diabetes Dev Ctries. 2010;30:185–90. [Google Scholar]
- 19.Jones SC, Saunders HJ, Pollock CA. High glucose increase growth and collagen synthesis in cultured human tubulointerstitial cells. Diabet Med. 1999;16:932–8. doi: 10.1046/j.1464-5491.1999.00174.x. [DOI] [PubMed] [Google Scholar]
- 20.Tay YC, Wang Y, Kairaitis L, Rangan GK, Zhang C, Harris DC. Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int. 2005;68:391–8. doi: 10.1111/j.1523-1755.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 21.Kraynak AR, Storer RD, Jensen RD, Kloss MW, Soper KA, Clair JH, et al. Extent and persistence of streptozotocin induced DNA damage and cell proliferation in rat kidney as determined by in vivo alkaline elution and BrdUrd labeling assays. Toxicol App Pharmacol. 1995;135:279–86. doi: 10.1006/taap.1995.1234. [DOI] [PubMed] [Google Scholar]
- 22.Al-Awadi FM, al-Adnani MS. Structural changes in glomeruli and proteinuria in streptozotocin diabetic rats. Histol Histopath. 1989;4:129–35. [PubMed] [Google Scholar]
- 23.Jablecka A, Czaplicka E, Olszewski J, Bogdanski P, Krauss H, Smolarek I. Influence of selected angiotensin- converting enzyme inhibitors on alloxan -induced diabetic cataract in rabbits. Med Sci Monit. 2009;15:BR334–8. [PubMed] [Google Scholar]
- 24.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 25.Reguli J, Misik V. Superoxide scavenging by thiol/copper complex of captopril - An EPR spectroscopy study. Free Radic Res. 1995;22:123–30. doi: 10.3109/10715769509147534. [DOI] [PubMed] [Google Scholar]
- 26.Hegde KR, Varma SD. Combination of glycemic and oxidative stress in lens: Implications in augmentation of cataract formation in diabetes. Free Radic Res. 2005;39:513–7. doi: 10.1080/10715760400013755. [DOI] [PubMed] [Google Scholar]
- 27.Wolf N, Penn P, Pendergrass W, Van Remmen H, Bartke A, Rabinovitch P, et al. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp Eye Res. 2005;81:276–85. doi: 10.1016/j.exer.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Romana K, Chandra D, Wills NK, Bhatnagar A, Srivastava SK. Oxidative stress-induced up-regulation of chloride channel and Na+/Ca+ exchanger during cataractogenesis in diabetic rats. J Diabetes Complications. 2004;18:177–82. doi: 10.1016/S1056-8727(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 29.Zusma R. Angiotensin -converting enzyme inhibitors; more different than alike? Am J Cardiol. 1993;71:25–31. doi: 10.1016/0002-9149(93)91052-j. [DOI] [PubMed] [Google Scholar]
- 30.Zarebska A, Czerny K, Bakiera K, Cichacz-Kwiatkowska B, Lis-Sochocka M, Ki G, et al. Histological changes in the retina in experimental alloxan-induced diabetes in rabbits? Ann Univ Mariae Curie Sklodowska Med. 2001;56:81–4. [PubMed] [Google Scholar]
- 31.Bek T. Transretinal histopathological changes in capillary-free areas of diabetic retinopathy. Acta Ophthalmol (Copenh) 1994;72:409–15. doi: 10.1111/j.1755-3768.1994.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 32.Sun HQ, Zhou ZY. Effect of ginsenoside -Rg3 on the expression of VEGF and TNF-α in retina with diabetic rats. Int J Opthalmol. 2010;3:220–3. doi: 10.3980/j.issn.2222-3959.2010.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes: Early onset and effect of insulin. J Clin Invest. 1998;102:783–91. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, et al. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47:815–20. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- 35.Yoko O, Toshihide K, Mariko S, Norimitsu B, Kenya Y, Shunsuke K, et al. Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model. Exp Diabet Res. 2011:1–7. doi: 10.1155/2011/108328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sramek SJ, Wallow IH, Tewksbury DA, Brandt CR, Poulsen GL. An ocular renin-angiotensin system: Immune histochemistry of angiotensinogen. Invest Ophthalmol Vis Sci. 1992;33:1627–32. [PubMed] [Google Scholar]
- 37.Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, et al. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: Evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996;80:159–63. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson-Berka JL. Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol. 2006;38:752–65. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher EL, Phipps JA, Ward MM, Vessey KA, Wilkinson-Berka JL. The renin-angiotensin system in retinal health and disease: Its influence on neurons, glia and the vasculature. Prog Retin Eye Res. 2010;29:284–311. doi: 10.1016/j.preteyeres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–50. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]


