Abstract
Brain tumors represent a vexing clinical problem in oncology due to their increasing incidence, difficulties in treatment and high rates of recurrence. It is especially challenging to evaluate the posttreatment disease status because differentiation of recurrence from treatment-induced changes (radiation necrosis) is not possible with the use of magnetic resonance imaging, the most commonly used imaging method in this setting. Various functional imaging methods, including positron emission tomography and single photon emission computed tomography (SPECT) have been employed in this context. SPECT with 99m-technetium (99mTc)-glucoheptonate (GHA) has shown promising results for differentiation of recurrent brain tumor from radiation necrosis. In this review, we have discussed in details the basics of 99mTc-GHA SPECT imaging in brain tumor along with the available literature in this regard.
Keywords: 99m-technetium-glucoheptonate, brain tumor, single photon emission computed tomography, recurrence
INTRODUCTION
Brain tumor is the second most common childhood malignancy, and it is a common cause of cancer-related deaths in middle-aged adults.[1,2] Glioma is the most common primary brain tumor (50-60%), followed by meningioma (20%) in adults.[3] Diagnosis of brain tumors is based on the clinical features, neurological examination, and neuroimaging. Magnetic resonance imaging (MRI) is the most relied upon neuroimaging technique for the diagnosis of brain tumors, not only for the anatomic details that can be obtained because its high soft tissue contrast and resolution, but also increasingly for functional information that MRI can provide. Standard T1- and T2-weighted MRI sequences detect brain tumors with high sensitivity with regard to its size and localization, as well as associated mass effect, edema, hemorrhage, necrosis, and signs of elevated intracranial pressure. Biopsy is confirmative and is used to determine the accurate histopathological nature including tumor grade that is very important for treatment planning and prognosis.[4,5] Unfortunately, it is difficult to evaluate disease status with MRI in patients who have been treated for brain tumor. Treatment induced changes, such as radiation necrosis, can be difficult to distinguish from recurrent tumor clinically as well as on MRI.[6,7] This is becoming a more critical issue nowadays as concurrent chemoradiation and stereotactic radiosurgery are being used more extensively, which increases the prevalence of necrosis. Furthermore, dexamethasone has been shown to induce a reduction in tumor size on MRI.[8] 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) can help in this regard by demonstrating the metabolic activity of a suspicious lesion so that the lesion can be characterized accordingly.[9] The main limitation of 18F-FDG PET is its high false negative rate due to low lesion to background contrast, as glucose is also a normal substrate for the brain.[10] Single photon emission computed tomography (SPECT) with various radiotracers has been employed for brain tumor imaging. In contrast to PET, SPECT is more widely available and cheaper. This makes it an attractive alternative, especially in developing countries. SPECT with 99m-technetium (99mTc)-glucoheptonate (GHA) is one such excellent brain-scanning radiopharmaceutical. Though initially introduced as a renal imaging agent during 70's, it emerged as a brain tumor agent very soon, and there was a lot of interest in this agent during 70's and 80's. However, advancement in structural imaging of the brain by computed tomography (CT) and MRI reduced its importance in brain imaging later on. In recent years, there is a resurgence of interest among researchers regarding its role in the management of patients treated for brain tumor - for detection of residual tumor after initial surgery/radiotherapy or detecting recurrence after complete resection. In this review, we have discussed in details the basics of 99mTc-GHA SPECT imaging in brain tumor along with the available literature in this regard.
STRUCTURE
The 99mTc-GHA complex can be described as an oxobis (glucoheptonato) technetate (V) anion. In the liquid phase, it shows one oxo group and two GHA groups per molecule. A GHA molecule can coordinate to the 99mTc center with one or two oxygen atoms (mono- or bidentate). The GHA is bound to 99mTc by the carboxyl oxygen. pH titrations show release of one proton per GHA in the complex formation and hence one of the hydroxyl oxygens must also be coordinated to 99mTc, with the release of a proton. The two GHA molecules form bidentate chelates to 99mTc [Figure 1]. The compound does not crystallize from an aqueous solution after the addition of large cations (e.g. allyl-ammonium salts) and/or organic solvents.[11,12]
Figure 1.
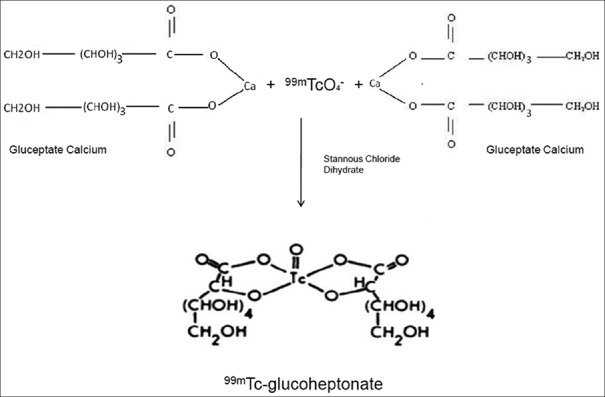
Chemical structure of 99m-technetium-glucoheptonate and outline of its labeling procedure
LABELING
Labeling of GHA with 99mTc is easy; it can be prepared in-house and is very cost effective. 99mTc-GHA can be obtained by using commercially available GHA labeling kit, with 95-100% of 99mTc labeling efficiency. In this kit, both GHA and Sn (II) are present in excess to which nanomolar amount of 99mTc-pertechnetate added. The Sn (II) excess is necessary, to overcome partial oxidation in the solution buffered at pH 5.6 by the GHA excess. Partial hydrolysis of Sn (II) at pH 5.6 does not influence the reduction capacity. Available kits contain Gluceptate Calcium and Stannous Chloride Dihydrate in lyophilized form. The final pH is adjusted to 5.0-5.5 with HCl or NaOH prior to lyophilization.[13] The kit can also be prepared in-house from commercially available GHA powder. You need to adjust the pH by adding hydrochloric acid and sodium hydroxide before lyophilization. To make 99mTc-GHA injection for imaging, it has to reconstituted from the freeze-dried kit using freshly eluted 99mTc-pertechnetate solution containing a maximum of 100 mCi (3.7 GBq) of activity, followed by stirring for 1 min and can be used after 5 min. The 99mTc-GHA labeled in this manner should be stable for over 6 h after labeling. The 99mTc-labeling reaction depends on maintaining the stannous ion in the reduced state. Hence, sodium pertechnetate containing oxidants should not be employed. Using proper shielding, the vial should be visually inspected to ensure that the solution is clear and free of particulate matter before proceeding further.[14] The solution should not be used if it is cloudy. 99mTc-GHA solution should be stored at 2°C-8°C and discarded 6 h after reconstitution.
BIODISTRIBUTION
When injected intravenously, 99mTc-GHA is rapidly cleared from the blood. The blood clearance curve is triexponential with the two faster components accounting for more than 90% of the injected dose. In patients with normal renal function, less than 15% of the initial activity remains in the blood after 1 h. About 40% of the injected dose is excreted in the urine in 1 h, whereas about 70% is excreted in 24 h. In patients with renal disease, the blood clearance and urinary excretion of the radiopharmaceutical are delayed. Up to 15% of the injected dose is localized in the kidney by 3 h and retained in the kidneys.[11] The renal retention is greater in the cortex than in the medulla. The radiopharmaceutical may be bound to the proximal convoluted tubules, which are located primarily in the renal cortex. 99mTc-GHA tends to accumulate in intracranial lesions that are associated with excessive neovascularity or an altered blood-brain barrier (BBB). It does not accumulate in the choroid plexus or salivary glands.
MECHANISM OF 99M-TECHNETIUM-GLUCOHEPTONATE UPTAKE IN BRAIN TUMOR
Various authors have recognized the following principal factors involved in the molecular radiopharmacokinetics of brain tumors: (a) Vascularity, (b) interstitial fluid, (c) capillary permeability, and (d) intracellular uptake.[15,16] Biochemical and electron-microscope studies have shown that in almost all brain tumors there is much more interstitial fluid than in normal brain.[17,18] The results with 99mTc-gluconate complex as obtained by Boyd et al. suggest rapid filling into the extracellular fluid compartment and a subsequent release from it.[19] The extracellular fluid compartment in the brain tumor could be responsible for the initial faint appearance of an abnormal zone that would represent an intermediate step between the optimal time for tumor visualization and rapid blood clearance of 99mTc-GHA. The different results obtained with the 99mTc-GHA and 99mTc-pertechnetate undermines the importance of BBB breakdown as the only mechanism of 99mTc-GHA uptake in brain tumors. Léveillé et al. opined that 99mTc-GHA contributes significantly to the detection of brain tumors because: (a) 99mTc-GHA molecular dynamics are different from those of Tc-pertechnetate in neoplastic brain tissue, but similar in infarcted brain tissue; and (b) there is a gradual uptake of the 99mTc-GHA over a period of many hours in brain tumors in spite of very rapid blood clearance.[15] The results obtained in animals[19] and humans[20] showed rapid diffusion into the extracellular fluid of the whole body, with subsequent release from the extracellular compartment. While vascularity and capillary permeability are surely among the earliest and most critical factors, the low blood level of the 99mTc-GHA observed after an hour excludes any passive uptake mechanism, so the gradual accumulation in the brain tumors, probably involves an active transport mechanism. Being glucose analog, 99mTc-GHA is probably taken up as an energy substrate by the tumor tissue and thus it can reflect the metabolic activity of the lesion.[15,21] Furthermore, being a blood-brain barrier agent, 99mTc-GHA gives high lesion to background ratio with good quality images for lesion visualization. The uptake of 99mTc-GHA in viable tumor increases with time whereas it clears rapidly from inflammatory lesions.[22]
RADIATION DOSIMETRY
Based on the data of the International Commission on Radiological Protection Publication 53,[23] total effective dose to the patients injected with 99mTc-GHA radiopharmaceutical is 0.0090 mSv/MBq (0.033 rem/mCi). Bladder wall gets the highest radiation absorbed dose as an individual organ, i.e. 0.056 mGy/MBq (0.21 rad/mCi). Next are the kidneys with radiation absorbed dose of 0.049 mGy/MBq (0.18 rad/mCi). Other pelvic organs and large intestine receive very minimal radiation dose.
IMAGING AND INTERPRETATION
The usual recommended dose of 99mTc-GHA for adult and adolescents is 20–25 mCi (740-925 MBq). Though there is no uniform postinjection waiting period for brain tumor imaging, an uptake period of 1 h is good enough to obtain a good quality image with high tumor to background contrast. As there is rapid clearance of the tracer from circulation, many studies are performed even after half an hour waiting period.[24] However since some studies have suggested that delayed scanning shows increasing tracer uptake in the tumor tissue with time,[25,26] we are in favor of at least 1 h waiting period. Some researchers have also proposed for early and delayed scanning (at 1 h and 3 h) to calculate retention index. Imaging could be performed with any SPECT system, preferably a dual head gamma camera. Planar imaging is not required, and only SPECT acquisition is sufficient. At the time of the imaging head should be fixed and kept as close to the collimator as possible.[26] Images should be properly reconstructed (tomographic reconstruction: Filtered back projection or ordered subset expectation maximization) after excluding motion abnormality. Reconstructed images should be reviewed in three axes (axial, coronal, and sagittal) and three-dimensional view.
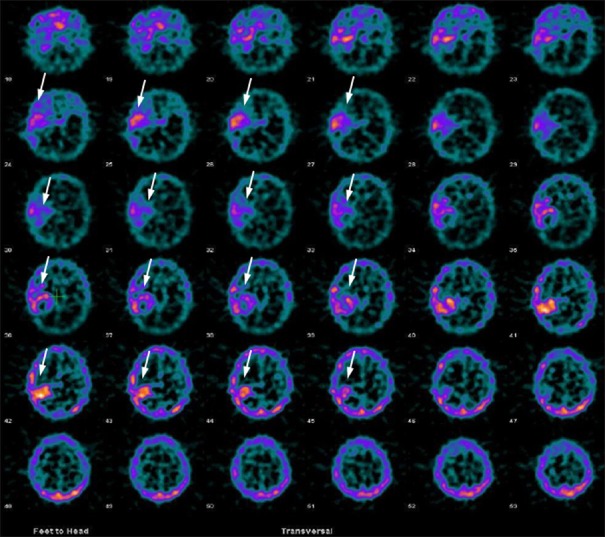
Normal 99mTc-GHA brain scans show no abnormal radiotracer uptake within the brain parenchyma except for low background activity. Abnormal 99mTc-GHA uptake in brain parenchyma is, usually, interpreted as positive for tumor. However, presence of radiotracer accumulation in the venous sinuses should be kept in mind while interpreting the images [Figure 2]. Use of hybrid SPECT/CT or side by side viewing with MRI/CT, improves reader confidence as well as specificity. For quantifying the radiotracer uptake in the tumor or severity of BBB breakage, lesion to background ratios (to the scalp, surrounding brain tissue, and nasopharynx) can be calculated. Some of these ratios used in different studies are T/B uptake ratio (tumor activity/normal brain parenchymal activity), T/S uptake ratio (tumor activity/scalp activity), and T/NP uptake ratio (tumor activity/nasopharyngeal activity).[27,28,29] In our previous studies, we found that a viable brain tumor usually has 99mTc-GHA uptake more than double of the normal brain background (T/B > 2) and similar to scalp radiotracer uptake (T/S ≈ 1).[28,29]
Figure 2.
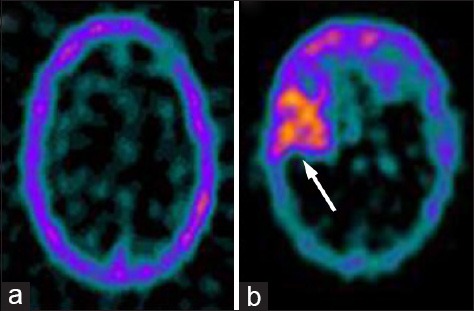
Representative images of a normal tumor negative transaxial 99m-technetium (99mTc)-glucoheptonate (GHA) brain single photon emission computed tomography (SPECT) (a) and a tumor positive 99mTc-GHA brain SPECT (b, arrow)
REVIEW OF LITERATURE
99mTc-GHA was introduced as a brain-scanning agent in mid-1970. Waxman et al. demonstrated the advantages of 99mTc-GHA over 99mTc-pertechnetate as a brain-scanning agent for brain tumors.[30] Based upon previous reports of 99mTc-GHA accumulation in acutely infracted myocardium, Rollo et al. postulated that the improved detection of brain lesions was due to a combination of increased binding to abnormal tissue and a rapid blood clearance of the agent. In a comprehensive study comparing 99mTc-GHA with 99mTc-pertechnetate and 99mTc-diethylenetriamine penta-acetic acid as brain-scanning agents, they concluded that 99mTc-GHA was the radiotracer of choice for detection of central nervous system abnormalities.[31] Léveillé et al., in their study also demonstrated the superiority of 99mTc-GHA over 99mTc-pertechnetate for the detection of CNS tumors. In addition, they concluded that compared with early brain scans, delayed studies frequently provided improved lesion detection, and they observed progressive accumulation of 99mTc-GHA in primary and metastatic brain tumor at 4 h and even up to 9 h after injection. The authors proposed that 99mTc-GHA functions as a substrate for the highly metabolic brain tumor tissue, which results in an enhanced lesion uptake.[15] Tanasescu et al. analyzed 859 consecutive 99mTc-GHA brain scans and found a tumor detection rate of 94%. They reported that a combination of several factors such as enhanced blood clearance, effective penetration of the BBB, active uptake by tumoral tissue and tissue binding forms the basis of brain tumor imaging with 99mTc-GHA.[22] Barai et al. in their study found 99mTc-GHA SPECT to be an effective imaging modality for detecting recurrent glioma in patients treated with radiotherapy. In their series SPECT was true positive in 51/55 and true negative in 17/21 patients.[27] Jaiswal et al. in a recent study evaluated intracranial space occupying lesions with 99mTc-GHA brain SPECT and observed a high specificity for neoplastic Tissues. No 99mTc-GHA concentration was noted in any of the inflammatory lesions, with a sensitivity of 97.62% and specificity of 100% for brain tumors.[24] Perfusion tracers like Thallium-201 (201Tl), 99mTc-sestamibi and 99mTc-tetrofosmin have been used as the brain tumor imaging agents with the assumption that their uptake is independent of BBB disruption and depends on their active intracellular uptakes. Barai et al. in their subsequent studies compared 201Tl and 99mTc-tetrofosmin SPECT, with 99mTc-GHA SPECT for the evaluation of recurrent brain tumors. They concluded that 99mTc-GHA is an accurate agent for SPECT imaging of recurrent brain tumors, may provide significant information about the location of the tumor margin and is more advantageous because of its specific uptake.[32,33]
In one of our studies (2011), we compared the efficacies of 99mTc-GHA SPECT and contrast-enhanced MRI for detection of recurrent glioma. Eighty-five patients with histopathologically proven glioma with a clinical suspicion of recurrence were evaluated using 99mTc-GHA SPECT and MRI. The sensitivity, specificity and accuracy of 99mTc-GHA SPECT were 86.5%, 96.5% and 89.4%, respectively, whereas those of MRI were 94.6%, 24.1% and 70.5%, respectively. Thirty patients had intermodality discordance, with 99mTc-GHA SPECT being correct in 23 of them.[28] In another study (2011) we compared the efficacies of 99mTc-GHA SPECT and 18F-FDG PET/CT for detection of recurrence in patients with glioma. The sensitivity, specificity, and accuracy of 99mTc-GHA SPECT were 85%, 97%, and 89%, respectively, whereas those of 18F-FDG PET/CT were 70%, 97%, and 80%, respectively. On histopathological subgroup analysis, 99mTc-GHA SPECT performed better than 18F-FDG PET/CT in all grades except for grade II gliomas, where both were equally effective. In all, 15 patients in this series had intermodality discordance, with 99mTc-GHA SPECT being correct in 13 of them.[29] In recent study Karunanithi et al.[34] compared the diagnostic accuracies of 99mTc-GHA SPECT/CT with 18F-Fluorodopa (18F-FDOPA) PET/CT in 30 patients with suspicion of recurrent glioma. Sensitivity, specificity, and accuracy were 86.4%, 62.5%, and 80% for 99mTc-GHA SPECT/CT and 100%, 87.5%, and 96% for 18F-FDOPA PET/CT, respectively. No significant difference was found between them overall (P = 1.00), as well as for low-grade (P = 0.250) or high-grade tumors (P = 0.50). The authors concluded that 99mTc-GHA SPECT/CT can be used as a low cost alternative to 18F-FDOPA PET/CT for recurrent glioma.[34] It must also be highlighted that 99mTc-GHA SPECT has limitations in imaging of posterior fossa tumors due to high background activity in this region. Barai et al. in their study found that brain SPECT is not sensitive enough in detecting recurrence of infratentorial brain tumors.[35]
CRITICAL APPRAISAL
99mTc-GHA SPECT is routinely performed at some centers such as ours for brain tumor evaluation. Its uptake is predominantly dependent on BBB disruption. BBB disruption or capillary leak is variable in different grades of glioma, being higher in high-grade tumors and limited in low-grade tumors. Hence, low-grade primary brain tumors are not very much 99mTc-GHA avid. 99mTc-GHA SPECT is a good agent for detection of residual tumor after primary treatment with surgery/radiotherapy and also for detecting recurrence [Figures 3–5]. Since recurrent tumors, usually, transform to higher grades, 99mTc-GHA is even useful in recurrent low-grade tumors. Early detection and optimal treatment of the residual/recurrent tumors may have lifesaving impact. All of the studies reported earlier including our own observation support the usefulness of 99mTc-GHA SPECT in this setting.[28,29] 99mTc-GHA also overcomes several limitations of other SPECT agents used for brain tumor imaging. SPECT with 201Tl has the disadvantage of low energy emission (69-83 keV) unsuitable for gamma camera, dependence on clearance of background activity and its inability to differentiate tumor from other slow clearance sites (such as infection, inflammation). 99mTc-sestamibi has the disadvantage of accumulation in the choroids plexus, which makes it less preferable for some tumors. In addition, massive endothelial injury in postoperative case also limits its utility.[33,35] Active tumoral uptake of 99mTc-GHA with no or minimal background activity is a strong point in its favor. It is clearly superior to MRI for differentiation of active tumor and radiation necrosis [Figures 6 and 7]. It is also better than 18F-FDG PET/CT in some situations, where the latter is false negative due to high physiological uptake in normal brain [Figure 8]. Limitations of 99mTc-GHA SPECT in the grade I brain tumors because of intact BBB and in posterior fossa tumor because of high background activity are well-known and the results from our previous studies confirm this. Other causes of false negative 99mTc-GHA SPECT are small lesion size and proximity of the lesion to scalp or nasopharynx where normal tracer uptake is very high. Small lesion cannot be picked because of limited resolution capability of the SPECT system. Proximity to high background areas (scalp/nasopharynx) overshadows the lesion.[28,29,35] For the purpose of quantification of tumoral tracer uptake, different ratios have been used by various authors. Among all these 99mTc-GHA uptake ratios, in our opinion “tumor to normal brain background” (T/B) ratio is most useful for quantification purposes. From our experience, T/B ratio should be more than 2 to declare the tracer uptake as positive for tumor.[27,28,29] Some authors have suggested the term of “99mTc-GHA index.” It is be calculated by drawing region of interest over the tumor and on the contralateral brain parenchyma, and calculating the ratio of average counts/pixel of both the regions.
Figure 3.
A 27-year-old male patient with perisylvian oligodendroglioma, postsurgery and radiotherapy, with suspicion of local recurrence in magnetic resonance imaging. 99m-technetium-glucoheptonate single photon emission computed tomography was negative for recurrence in this patient
Figure 5.
A 35-year-old female patient with right temporal grade II astrocytoma, primarily treated with surgery and radiotherapy, was evaluated for suspected recurrence. 99m-technetium-glucoheptonate brain single photon emission computed tomography was positive for local recurrence (arrows)
Figure 6.

A 38-year-old female with right temporal glioma, postsurgery and radiotherapy with clinically suspected recurrence. She was positive for recurrence on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) (a, arrow), magnetic resonance imaging (MRI) (b, arrow) and 99m-technetium (99mTc)-glucoheptonate (GHA) single photon emission computed tomography (SPECT) (c, arrow). However, the lesion on MRI (a) covered both viable tumor and radionecrotic area. 99mTc-GHA SPECT image is showing only tracer uptake at viable tumor area (c, arrow), well correlated with the positive tumor area delineated by PET/CT (a, arrow)
Figure 7.
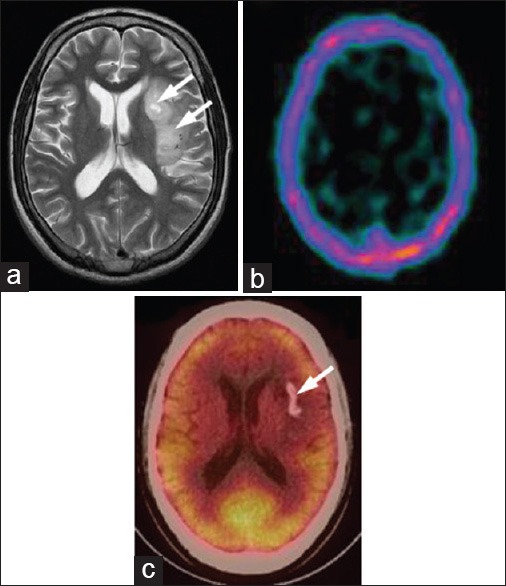
A 27-year-old male with left temporoparietal oligodendroglioma, primarily treated with surgery and radiotherapy. Recuurence was suspected on magnetic resonance imaging (MRI) (a, arrows). 99m-technetium (99mTc)- glucoheptonate (GHA) single photon emission computed tomography (SPECT) was negative for recurrence (b). 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) (c) showed calcification (arrow) but no recurrent tumor. MRI was false positive while 99mTc-GHA SPECT and 18F-FDG PET/CT were true negative in this patient
Figure 8.
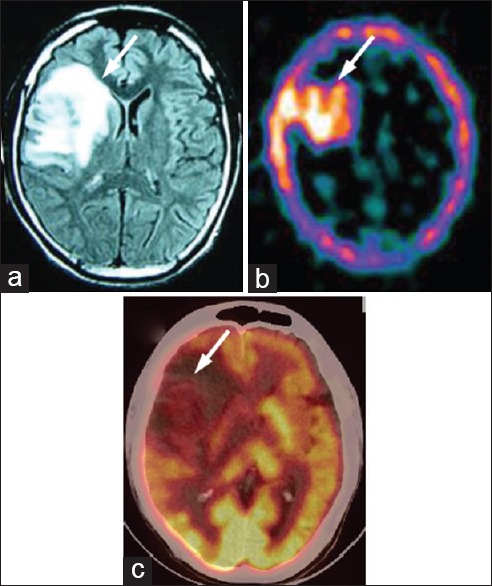
A 30-year-old male with right frontal grade II astrocytoma, primarily treated with radiotherapy, presented with severe headache. Magnetic resonance imaging (a) was positive for residual/recurrent tumor (arrow). 99m-technetium-glucoheptonate single photon emission computed tomography (b) was also strongly positive for recurrent/residual tumor (arrow). However, on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (c) the recurrent/residual lesion was hypometabolic (arrow)
Figure 4.
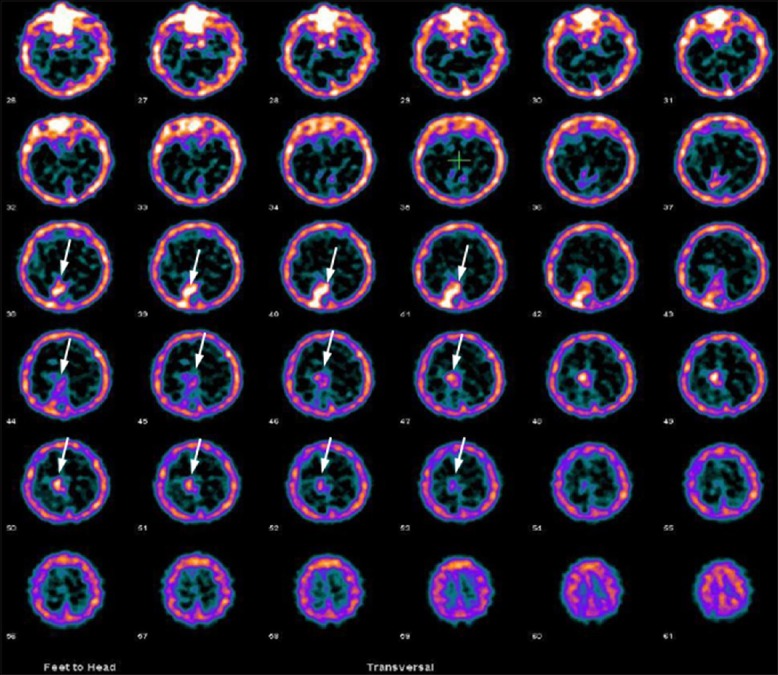
A 44-year-old male patient with right occipital grade II oligodendroglioma, primarily treated with surgery, radiotherapy and temozolamide was evaluated for residual tumor. 99m-technetium-glucoheptonate brain single photon emission computed tomography was positive for residual tumor in this patient (arrows)
CONCLUSIONS
99mTc-GHA is a time tested, low-cost radiopharmaceutical agent for brain tumor imaging. In view of structural clarity of brain MRI, 99mTc-GHA SPECT has not much additional value in primary brain tumors. However, it can be is strongly recommended for detecting residual tumor after surgery/radiotherapy and also for detection of recurrent tumors. It shows comparable, sometimes better diagnostic performance than other SPECT and PET tracers and therefore can be used as a low-cost alternative.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al. Bethesda, MD: United States SEER Program 1975-1995, National Cancer Institute, SEER Program, NIH Pub; 1999. Cancer Incidence and Survival among Children and Adolescents; pp. 99–4649. [Google Scholar]
- 3.Chamberlain MC, Kormanik PA. Practical guidelines for the treatment of malignant gliomas. West J Med. 1998;168:114–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Berger MS, Wilson CB. 1st ed. Vol. 17. St Louis: W. B. Saunders Company; 1998. The Gliomas; pp. 172–84. [Google Scholar]
- 5.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–31. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 6.Ricci PE. Imaging of adult brain tumors. Neuroimaging Clin N Am. 1999;9:651–69. [PubMed] [Google Scholar]
- 7.Nelson SJ. Imaging of brain tumors after therapy. Neuroimaging Clin N Am. 1999;9:801–19. [PubMed] [Google Scholar]
- 8.Ostergaard L, Hochberg FH, Rabinov JD, Sorensen AG, Lev M, Kim L, et al. Early changes measured by magnetic resonance imaging in cerebral blood flow, blood volume, and blood-brain barrier permeability following dexamethasone treatment in patients with brain tumors. J Neurosurg. 1999;90:300–5. doi: 10.3171/jns.1999.90.2.0300. [DOI] [PubMed] [Google Scholar]
- 9.Kim EE, Chung SK, Haynie TP, Kim CG, Cho BJ, Podoloff DA, et al. Differentiation of residual or recurrent tumors from post-treatment changes with F-18 FDG PET. Radiographics. 1992;12:269–79. doi: 10.1148/radiographics.12.2.1561416. [DOI] [PubMed] [Google Scholar]
- 10.Davis WK, Boyko OB, Hoffman JM, Hanson MW, Schold SC, Jr, Burger PC, et al. [18F] 2-fluoro-2-deoxyglucose-positron emission tomography correlation of gadolinium-enhanced MR imaging of central nervous system neoplasia. AJNR Am J Neuroradiol. 1993;14:515–23. [PMC free article] [PubMed] [Google Scholar]
- 11.de Kieviet W. Technetium radiopharmaceuticals: Chemical characterization and tissue distribution of Tc-glucoheptonate using Tc-99m and carrier Tc-99. J Nucl Med. 1981;22:703–9. [PubMed] [Google Scholar]
- 12.Chi SL, Hoag SG, Yanchick VA. Electrolytic complexing of glucoheptonate and technetium-99m. J Nucl Med. 1978;19:520–4. [PubMed] [Google Scholar]
- 13.Rockville, MD: United States Pharmacopeia 30, USP Convention; 2006. United States Pharmacopeia Convention, Technetium (Tc-99m) Glucoheptate Injection; pp. 3278–9. [Google Scholar]
- 14.International Atomic Energy Agency. Vienna: International Atomic Energy Agency; 2008. 99mTc Radiopharmaceuticals: Manufacture of kits; p. 24. Technical reports series, ISSN 0074-1914; no. 466. [Google Scholar]
- 15.Léveillé J, Pison C, Karakand Y, Lemieux R, Vallières BJ. Technetium-99m glucoheptonate in brain-tumor detection: An important advance in radiotracer techniques. J Nucl Med. 1977;18:957–61. [PubMed] [Google Scholar]
- 16.Tator CH. Radiopharmaceuticals for tumor localization with special emphasis on brain tumors. In: Subramanian G, Rhodes BA, Cooper JF, Soddv J, editors. Radiopharmaceuticals. New York: Society of Nuclear Medicine; 1975. pp. 474–81. [Google Scholar]
- 17.Aleu FP, Edelman FL, Katzman R, Scheinberg LC. Ultrastructural and biochemical analysis in cerebral edema associated with experimental mouse gliomas. J Neuropathol Exp Neurol. 1964;23:253–63. doi: 10.1097/00005072-196404000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Raimondi AJ, Mullan S, Evans JP. Human brain tumors: An electron-microscopic study. J Neurosurg. 1962;19:731–53. doi: 10.3171/jns.1962.19.9.0731. [DOI] [PubMed] [Google Scholar]
- 19.Boyd RE, Robson J, Hunt FC, Sorby PJ, Murray IP, McKay WJ. 99Tcm gluconate complexes for renal scintigraphy. Br J Radiol. 1973;46:604–12. doi: 10.1259/0007-1285-46-548-604. [DOI] [PubMed] [Google Scholar]
- 20.Arnold RW, Subramanian G, McAfee JG, Blair RJ, Thomas FD. Comparison of 99mTc complexes for renal imaging. J Nucl Med. 1975;16:357–67. [PubMed] [Google Scholar]
- 21.Ziesman HA, O’Malley JP, Thrall JH. 3rd ed. Missouri: Elsevier Mosby; 2006. Nuclear Medicine: The Requisites in Radiology; pp. 419–49. [Google Scholar]
- 22.Tanasescu DE, Wolfstein RS, Waxman AD. Critical evaluation of 99mTcglucoheptonateas a brain scanning agent. J Nucl Med. 1977;18:630. [PubMed] [Google Scholar]
- 23.Task Group of Committee 2 of the International Commission on Radiological Protection. New York: ICRP Publication 53, Pergamon Press; 1988. Annals of the ICRP. Radiation dose to Patients from Radiopharmaceuticals; p. 194. [PubMed] [Google Scholar]
- 24.Jaiswal S, Barai S, Rajkumar, Gambhir S, Ora M, Mahapatra AK. Evaluation of intracranial space-occupying lesion with Tc99m-glucoheptonate brain single photon emission computed tomography in treatment-naïve patients. J Postgrad Med. 2009;55:180–4. doi: 10.4103/0022-3859.57397. [DOI] [PubMed] [Google Scholar]
- 25.Tanasescu DE, Wolfstein RS, Brachman MB, Waxman AD. Early and delayed Tc-99m glucoheptonate brain scintigraphy: Are routine early images indicated? J Nucl Med. 1979;20:287–90. [PubMed] [Google Scholar]
- 26.Mittal BR, Bhattacharya A, Singh B. 99mTc-GHA Brain SPECT in the Evaluation of Brain Tumors. 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23:S1549. [Google Scholar]
- 27.Barai S, Bandopadhayaya GP, Julka PK, Naik KK, Haloi AK, Kumar R, et al. Role of Tc-glucoheptonic acid brain single photon emission computed tomography in differentiation of recurrent brain tumour and post-radiation gliosis. Australas Radiol. 2004;48:296–301. doi: 10.1111/j.0004-8461.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- 28.Santra A, Sharma P, Kumar R, Bal C, Kumar A, Julka PK, et al. Comparison of glucoheptonate single photon emission computed tomography and contrast-enhanced MRI in detection of recurrent glioma. Nucl Med Commun. 2011;32:206–11. doi: 10.1097/MNM.0b013e328341c3e9. [DOI] [PubMed] [Google Scholar]
- 29.Santra A, Kumar R, Sharma P, Bal C, Julka PK, Malhotra A. Detection of recurrence in glioma: A comparative prospective study between Tc-99m GHA SPECT and F-18 FDG PET/CT. Clin Nucl Med. 2011;36:650–5. doi: 10.1097/RLU.0b013e318217aee0. [DOI] [PubMed] [Google Scholar]
- 30.Waxman AD, Tanacescu D, Siemsen JK, Wolfstein RS. Technetium-99m-glucoheptonate as a brain-scanning agent: Critical comparison with pertechnetate. J Nucl Med. 1976;17:345–8. [PubMed] [Google Scholar]
- 31.Rollo FD, Cavalieri RR, Born M, Blei L, Chew M. Comparative evaluation of 99mTc GH, 99mTcO4, and 99mTc DTPA as brain imaging agents. Radiology. 1977;123:379–83. doi: 10.1148/123.2.379. [DOI] [PubMed] [Google Scholar]
- 32.Barai S, Rajkamal, Bandopadhayaya GP, Pant GS, Haloi AK, Malhotra A, et al. Thallium-201 versus Tc99m-glucoheptonate SPECT for evaluation of recurrent brain tumours: A within-subject comparison with pathological correlation. J Clin Neurosci. 2005;12:27–31. doi: 10.1016/j.jocn.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Barai S, Bandopadhayaya GP, Julka PK, Malhotra A, Bal CS, Dhanpathi H. Imaging using Tc99m-tetrofosmin for the detection of the recurrence of brain tumour: A comparative study with Tc99m-glucoheptonate. J Postgrad Med. 2004;50:89–93. [PubMed] [Google Scholar]
- 34.Karunanithi S, Bandopadhyaya GP, Sharma P, Kumar A, Singla S, Malhotra A, et al. Prospective comparison of (99m) Tc-GH SPECT/CT and (18) F-FDOPA PET/CT for detection of recurrent glioma: A pilot study. Clin Nucl Med. 2014;39:e121–8. doi: 10.1097/RLU.0b013e318279bcd8. [DOI] [PubMed] [Google Scholar]
- 35.Barai S, Bandopadhayaya GP, Naik K, Haloi AK. Is brain SPECT a suitable modality for evaluation of postradiotherapy posterior fossa brain tumours. A comparative evaluation with contrast enhanced computed tomography? J Indian Med Assoc. 2004;102:477–9. 486. [PubMed] [Google Scholar]