Abstract
Introduction:
Targeted therapeutic agents are indicated in metastatic lung cancers. These being receptor specific therapies, manifestation of response can be best assessed by estimating the metabolic activity of tumor, rather than the size. This retrospective analysis studied metabolic and morphological response on Positron Emission Tomography (PET) and Computed Tomography (CT), respectively to these agents.
Materials and Methods:
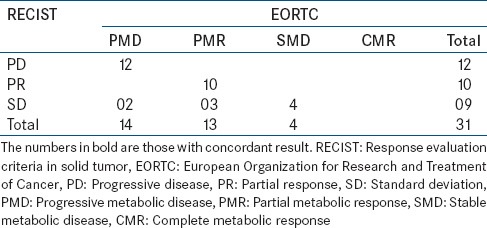
Thirty-one patients (23 males, 8 females with an age range of 42–77 years) with Epidermal Growth Factor Receptor (EGFR) positive metastatic lung cancer on Gefitinib, who underwent PET/CT, at baseline and at 4–6 weeks, were assessed by Response Evaluation Criteria In Solid Tumors [RECIST] 1.1 and European Organization for Research and Treatment of Cancer (EORTC) criteria.
Results:
Concordance between RECIST 1.1 and EORTC was seen in 26 (83.7%) patients. Discordance was seen in 5 (16.3%) patients. In patients with discordance, the results were confirmed by follow-up imaging. Metabolic EORTC criteria changed the disease status from stable disease to partial response (3 out of 5) and progressive disease (2 out of 5) in these five patients.
Conclusions:
Metabolic criteria using PET/CT could accurately predict response as well as disease progression early in the course of targeted therapy, compared to morphologic criteria. In addition, early metabolic response assessment can predict refractoriness of therapy.
Keywords: Computed Tomography, Epidermal growth factor receptor, European organization for research and treatment of cancer, Gefitinib, Lung cancer, Response, Response evaluation criteria in solid tumors, Fluoro-deoxy glucose positron emission tomography
INTRODUCTION
PET/CT is the recommended modality for staging of lung cancers. Standard approach towards patients with metastatic lung cancers is palliative oral chemotherapy with targeted agents. Treatment response to these agents is routinely assessed by comparing baseline and post-treatment CT scans, based on Response Evaluation Criteria In Solid Tumors ([RECIST] 1.1) criteria. However, these being receptor specific therapies, manifestation of response can be best assessed by estimating the metabolic activity of tumor, rather than the size. Based on this principle, we aimed at assessing the treatment response on metabolic imaging using FDG PET and comparing it with morphological criteria on CT.
MATERIALS AND METHODS
Patients with metastatic non small cell lung carcinoma (NSCLC) of lung on targeted therapy who were referred to our department for a PET CT study prior to start of treatment and for a response evaluation after 4–6 weeks, between June 2010 and June 2013 were included in this retrospective evaluation. Written informed consent was obtained from all patients.
Patient selection
31 patients (23 males, 8 females) with metastatic (NSCLC) of lung who tested positive for epidermal growth factor receptor (EGFR) were included. These patients received EGFR tyrosine kinase (TK) inhibitor Gefitinib, 250 mg, orally. Independent evaluation was done by - contrast-enhanced CT component of PET/CT and PET component of fused 18 F-FDG PET/CT studies, at baseline and at 4–6 weeks post-therapy. Patient Characteristics are given in Table 1.
Table 1.
Patient characteristics

Molecular analysis
EGFR mutation diagnostics were performed in all patients with samples suitable for molecular analysis.
Patient preparation
Patients were asked to fast for 4–6 h prior to the study and blood glucose levels were checked and confirmed to be <150 mg/dl. The studies were performed 60–90 min following intravenous administration of 5 MBq/kg of 18F-FDG.
Image acquisition protocol
Imaging was performed on a Discovery ST PET-CT system (GE Medical), equipped with a 16 slice CT scanner with a dedicated PET (BGO crystal, dimensions 3.8 mm × 3.8 mm × 3.8 cm). CT was performed over 5 to 8 eight bed positions from skull base to mid-thigh. CT parameters included 140 kV, 110–210 mA, 0.8 s/rotation, pitch of 1.75:1, field of view [FOV] of 50 cm, length of scan 1.0–1.6 m, 0.625 spatial resolution and slice thickness of 3.75 mm. Intravenous and oral contrast was routinely administered in all patients, after confirming the serum creatinine levels. PET data was acquired sequentially in the same anatomic locations with 15.4 cm axial FOV acquired in 3D (three-dimensional) mode with 3 min/bed position.
Image reconstruction and interpretation
Images reconstruction was done using a standard reconstruction algorithm with ordered subset expectation maximization (OSEM). Image fusion was performed using coordinate based fusion software and transferred to the workstation that provided multi-planar reformatted images and displayed PET, CT, and PET-CT fusion images. Images were independently interpreted by a nuclear medicine physician and a radiologist who were blinded to each other's results. CT findings were analyzed using the RECIST 1.1 and the PET findings by European Organization for Research and Treatment of Cancer Criteria (EORTC) criteria.
Response criteria as per RECIST 1.1[1]
Complete response (CR) indicates disappearance of all target lesions, partial response (PR) as 30% or more decrease in sum of diameters of target lesions, progressive disease (PD) as 20% or more increase in sum of diameters of target lesions and also an absolute increase of at least 5 mm and/or appearance of one or more new lesions, stable disease (SD) – who did not qualify for either sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
Response criteria as per EORTC criteria[2]
Complete resolution of FDG uptake within the measurable target lesion so that it is indistinguishable from surrounding background, with the appearance of no new lesion, was labeled as complete metabolic response (CMR). Reduction of minimum of 15–25% of standardized uptake value (SUV) max in the target volume in the same lesion as the baseline measurement was grouped under partial metabolic response (PMR). Progressive metabolic disease (PMD) was a more than 25% increase in the SUV max of the FDG uptake or appearance of FDG avid new lesion/s that is/are morphologically typical of cancer. Stable metabolic disease (SMD) was disease which did not qualify for CMR, PMR, or PMD.
RESULTS
31 patients of metastatic lung cancers, on targeted therapy underwent contrast enhanced PET CT were assessed using RECIST 1.1 New RECIST 1.1and EORTC criteria, respectively [Table 2].
Table 2.
Results
Concordance
Concordance [Figure 1] in response to targeted therapy was seen in 26 (83.4%) out of 31 patients, on metabolic and morphological criteria. Of these 26 patients, 4 patients showed PR/PMR, 10 patients showed SD/SMD and 12 patients showed PD/PMD.
Figure 1.
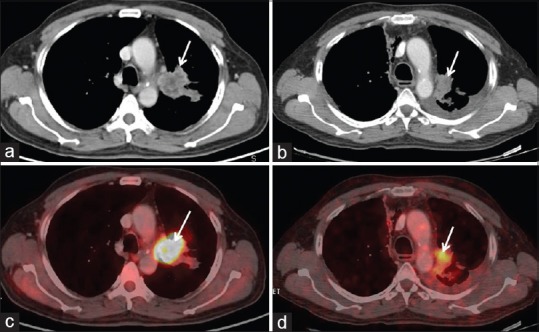
Concordance on metabolic and morphological imaging. There is significant reduction in size (1a and, b – arrow) and metabolic activity (max SUV) (1c, and d – arrow) seen on both axial CT component and axial fused PET/CT component, respectively; thus, partial response (PR) on RECIST is concordant with partial metabolic response (PMR), according to EORTC criteria
Discordance
Discordance in response to targeted therapy was seen in 5 (16.6%) patients.
Of these, three showed SD on RECIST 1.1 and PMR on EORTC criteria. All the three patients showed complete metabolic and morphological regression in metastatic sites, namely in left supraclavicular nodes [Figure 2] in two patients and contralateral nodule in one patient. However, at the primary site, CT images showed no significant change in size – SD on RECIST 1.1. But on corresponding PET images, significant regression was seen in SUV max values – PMR on EORTC criteria. Follow-up imaging either with CT or PET/CT after 2 months showed no further change in disease status.
Figure 2.
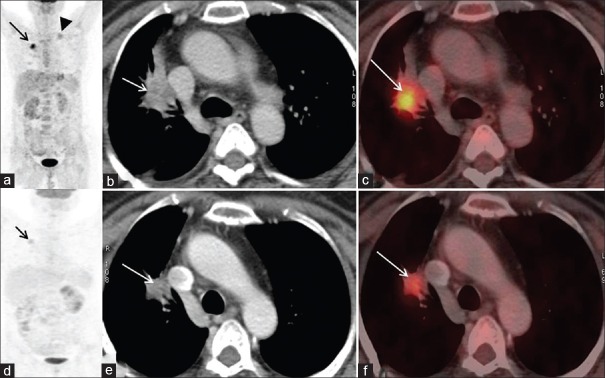
Discordance on metabolic and morphological imaging. There is no significant interval change in size on axial CT images (2b and e – arrow). However, coronal maximum intensity projection (MIP) (2a and d – arrow) and axial fused PET/CT (2c and f– arrow) images show significant regression in metabolic activity Coronal MIP (2a – arrowhead) also demonstrates tracer uptake in left supraclavicular node (arrowhead), which completely regresses on post-treatment MIP image. Thus stable disease (SD) on RECIST is discordant with EORTC criteria, which show partial metabolic response (PMR)
Two patients showed SD on RECIST 1.1 and PMD on EORTC metabolic criteria. New focal FDG avid marrow lesions [Figure 3] were seen in both these cases, whereas CT images were unremarkable, thus making the disease status as SD on RECIST 1.1 and PMD on EORTC criteria.
Figure 3.
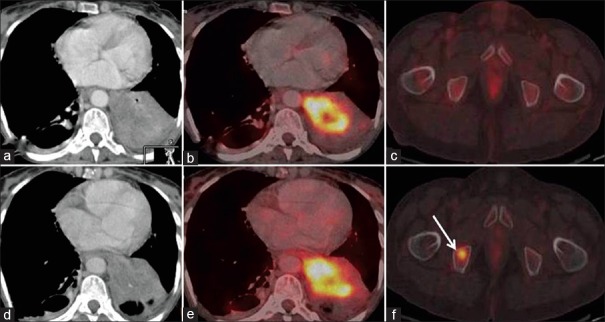
Discordance on metabolic and morphological imaging. Baseline and post Gefitinib axial CT (3a and d) and fused PET/CT (3b and e) images in soft tissue window show no interval change in size and metabolic activity of primary lung mass. Focal FDG uptake in the marrow of right pubic bone (3f – arrow) is a new finding However, axial CT - baseline and post-treatment images in bone window show no demonstrable lesion, thus patient has stable disease (SD) by RECIST. However, new hypermetabolic metastatic marrow lesion is suggestive of progressive metabolic disease (PMD) on EORTC criteria
Follow-up imaging either with CT or PET/CT after 2 months in these patients showed further metabolic and morphological disease progression.
DISCUSSION
Targeted therapy has evolved as a critical therapeutic strategy in treatment of metastatic NSCLC. TK inhibitors like Gefitinib targeting the EGFR can improve progression-free survival (PFS) and overall survival (OS) in receptor positive patients with these cancers.[3,4,5] Moreover, translational studies have shown that 18F-FDG uptake decreased within 2 h in H3255 cell lines having EGFR mutations after incubation with 0.2 mM of Gefitinib. This was due to translocation of glucose transporters from the cell membrane to the cytoplasm. Similar results were seen on 18F-FDG tumor uptake in animal experiments after only two doses of Gefitinib.[6] This forms the molecular basis of the utility of FDG PET for assessing response to targeted therapy, especially in receptor positive lung cancers.
It is well-known that metabolic response demonstrated by FDG PET-CT well precedes the anatomic response and has been well-documented in the existing literature.[7] Metabolic response on PET is manifested by decrease in the glycolytic activity of tumor, whereas anatomic response criteria which are based on size of the tumor, lag weeks and months behind the metabolic response.[8] Conventionally, NSCLC is an FDG avid tumor and PET/CT is routinely indicated for staging this cancer.[9,10] Hence, metastatic burden can be assessed and depending on EGFR status, metastatic cancers can be subjected to targeted therapy. The clinical end point of the targeted agents is to achieve a prolonged stable disease rather than tumor shrinkage.[11] Thus, lack of progression is associated with good improvement in outcome, even in the absence of partial or complete response.[2] As a result, standard size-based assessment of response to these therapies using RECIST underestimates therapeutic activity. This forms the basis of use of metabolic parameters using PET for treatment response assessment.
Criteria for metabolic response assessment have been evolving and improving – with PET Response Criteria in Solid Tumors (PERCIST) criteria being followed in all recent studies. Skougaard et al. compared EORTC and PERCIST criteria for response assessment in metastatic colorectal cancer, concluding that both criteria gave similar response and survival outcomes with good agreement.[12]
Metabolic response on PET also impacts the survival statistics in patients with metastatic lung cancer; patients with progressive metabolic disease (PMD) on post-treatment PET study showing shorter time to progression and overall survival (OS) compared to patients with SMD or with partial PMR or complete CMR metabolic response.[13] Our data, though in a small cohort of three patients with PMD on FDG PET showed similar results. Further metabolic and morphological progression seen on follow-up imaging.
In addition, early assessment on FDG PET is also a predictor of response to targeted therapy; PMD suggestive of refractoriness of targeted agents in spite of EGFR receptor positivity.[14] Early prediction of therapeutic failure not only warrants change of treatment, but also avoids unnecessary expenditure and potential toxicity.
Our study highlights the utility of metabolic imaging in metastatic lung cancers, not only for treatment effectiveness of therapy, but also for early assessment of refractoriness.
Though the number of patients is limited, results obtained warrant further assessment with a larger cohort. Furthermore, in addition to follow-up imaging, survival statistics would make the study more robust.
CONCLUSION
Metabolic criteria using PET/CT could accurately predict response as well as disease progression early in the course of targeted therapy, compared to morphologic criteria on CT scan. Hence, functional imaging with FDG PET provides a more definitive evidence of response to targeted therapy and disease status, unlike conventional anatomical imaging.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using 18F-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Su H, Bodenstein C, Dumont RA, Seimbille Y, Dubinett S, Phelps ME, et al. Monitoring tumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2006;12:5659–67. doi: 10.1158/1078-0432.CCR-06-0368. [DOI] [PubMed] [Google Scholar]
- 7.Van den Abbeele AD. The lessons of GIST – PET and PET/CT: A new paradigm for imaging. Oncologist. 2008;13(Suppl 2):8–13. doi: 10.1634/theoncologist.13-S2-8. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 9.Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koëter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 10.Weber WA, Dietlein M, Hellwig D, Kirsch CM, Schicha H, Schwaiger M. PET with (18) F-fluorodeoxyglucose for staging of non-small cell lung cancer. Nuklearmedizin. 2003;42:135–44. [PubMed] [Google Scholar]
- 11.Mileshkin L, Hicks RJ, Hughes BG, Mitchell PL, Charu V, Gitlitz BJ, et al. Changes in 18F-fluorodeoxyglucose and 18F-fluorodeoxythymidine positron emission tomography imaging in patients with non-small cell lung cancer treated with erlotinib. Clin Cancer Res. 2011;17:3304–15. doi: 10.1158/1078-0432.CCR-10-2763. [DOI] [PubMed] [Google Scholar]
- 12.Skougaard K, Nielsen D, Jensen BV, Hendel HW. Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med. 2013;54:1026–31. doi: 10.2967/jnumed.112.111757. [DOI] [PubMed] [Google Scholar]
- 13.Benz MR, Herrmann K, Walter F, Garon EB, Reckamp KL, Figlin R, et al. (18) F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J Nucl Med. 2011;52:1684–9. doi: 10.2967/jnumed.111.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skoura E, Datseris IE, Platis I, Oikonomopoulos G, Syrigos KN. Role of positron emission tomography in the early prediction of response to chemotherapy in patients with non – Small-cell lung cancer. Clin Lung Cancer. 2012;13:181–7. doi: 10.1016/j.cllc.2011.05.004. [DOI] [PubMed] [Google Scholar]


